Abstract
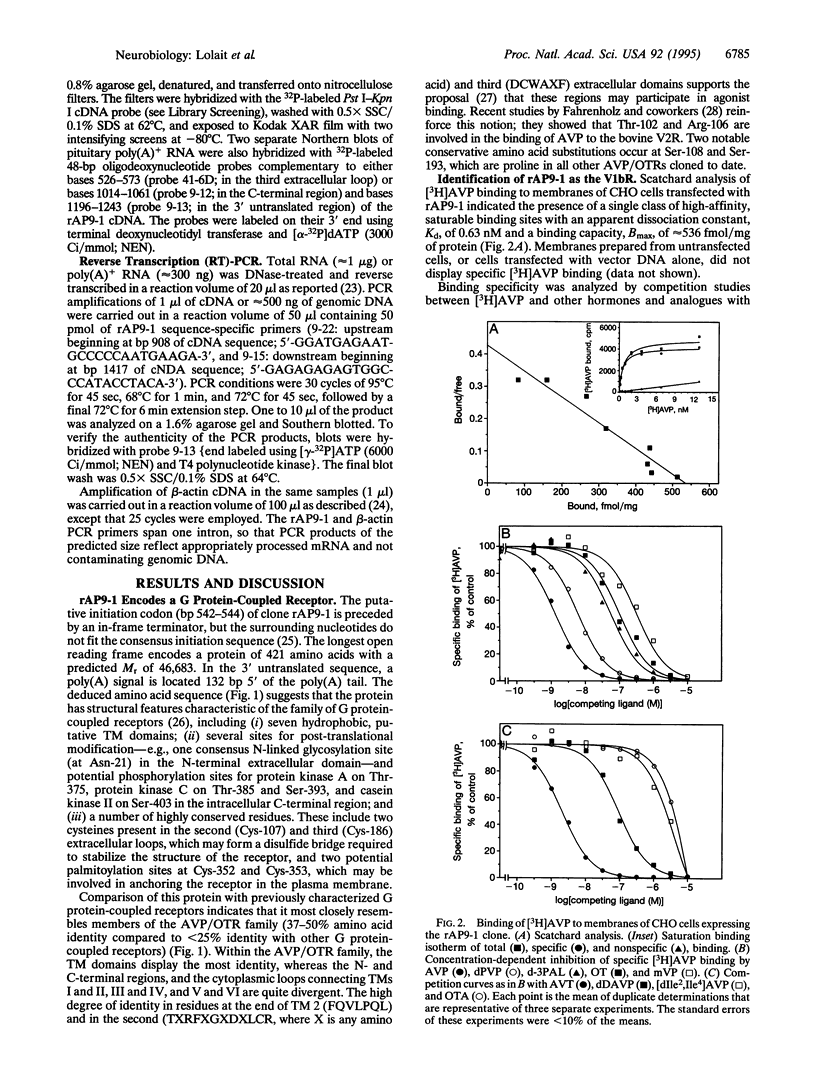
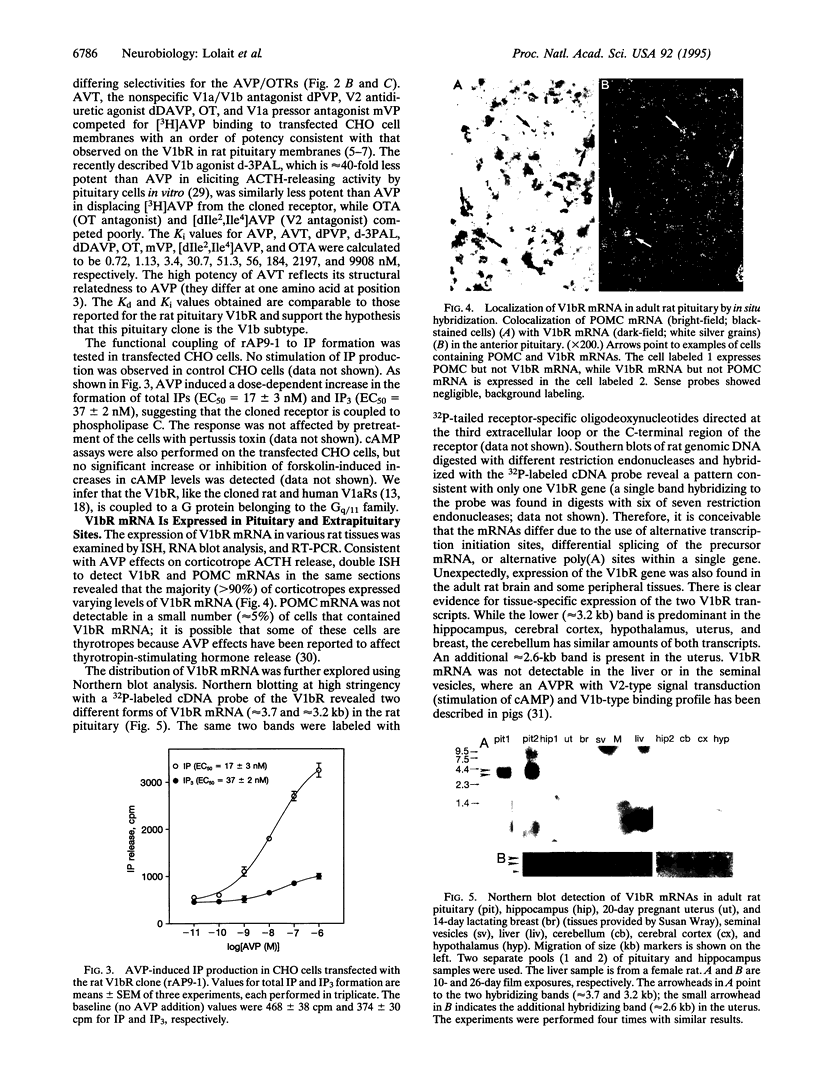
[Arg8]vasopressin (AVP) stimulates adrenocorticotropic hormone release from the anterior pituitary by acting on the V1b AVP receptor. This receptor can be distinguished from the vascular/hepatic V1a and renal V2 AVP receptors by its differential binding affinities for structural analogous of AVP. Recent studies have shown that the cloned V1a and V2 receptors are structurally related. We have isolated a clone encoding the V1b receptor from a rat pituitary cDNA library using polymerase chain reaction (PCR)-based methodology. The rat V1b receptor is a protein of 421 amino acids that has 37-50% identity with the V1a and V2 receptors. Homology is particularly high in the seven putative membrane-spanning domains of these guanine nucleotide-binding protein-coupled receptors. Expression of the recombinant receptor in mammalian cells shows the same binding specificity for AVP agonists and antagonists as the rat pituitary V1b receptor. AVP-stimulated phosphotidylinositol hydrolysis and intracellular Ca2+ mobilization in Chinese hamster ovary or COS-7 cells expressing the cloned receptor suggest second messenger signaling through phospholipase C. RNA blot analysis, reverse transcription PCR, and in situ hybridization studies reveal that V1b receptor mRNA is expressed in the majority of pituitary corticotropes as well as in multiple brain regions and a number of peripheral tissues, including kidney, thymus, heart, lung, spleen, uterus, and breast. Thus, the V1b receptor must mediate some of the diverse biological effects of AVP in the pituitary as well as other organs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer M., Seibold A., Gilbert S., Ishido M., Barberis C., Antaramian A., Brabet P., Rosenthal W. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992 May 28;357(6376):333–335. doi: 10.1038/357333a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Towle H. C., Young W. S., 3rd Spatial and temporal expression of alpha- and beta-thyroid hormone receptor mRNAs, including the beta 2-subtype, in the developing mammalian nervous system. J Neurosci. 1992 Jun;12(6):2288–2302. doi: 10.1523/JNEUROSCI.12-06-02288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley E. M., Lolait S. J., Axelrod J., Felder C. C. The cloned vasopressin V1a receptor stimulates phospholipase A2, phospholipase C, and phospholipase D through activation of receptor-operated calcium channels. Neuropeptides. 1994 Jul;27(1):63–74. doi: 10.1016/0143-4179(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Kanterman R. Y., Ma A. L., Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2187–2191. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howl J., Ismail T., Strain A. J., Kirk C. J., Anderson D., Wheatley M. Characterization of the human liver vasopressin receptor. Profound differences between human and rat vasopressin-receptor-mediated responses suggest only a minor role for vasopressin in regulating human hepatic function. Biochem J. 1991 May 15;276(Pt 1):189–195. doi: 10.1042/bj2760189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jard S., Barberis C., Audigier S., Tribollet E. Neurohypophyseal hormone receptor systems in brain and periphery. Prog Brain Res. 1987;72:173–187. doi: 10.1016/s0079-6123(08)60206-x. [DOI] [PubMed] [Google Scholar]
- Jard S., Gaillard R. C., Guillon G., Marie J., Schoenenberg P., Muller A. F., Manning M., Sawyer W. H. Vasopressin antagonists allow demonstration of a novel type of vasopressin receptor in the rat adenohypophysis. Mol Pharmacol. 1986 Aug;30(2):171–177. [PubMed] [Google Scholar]
- Kimura T., Tanizawa O., Mori K., Brownstein M. J., Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992 Apr 9;356(6369):526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Kojro E., Eich P., Gimpl G., Fahrenholz F. Direct identification of an extracellular agonist binding site in the renal V2 vasopressin receptor. Biochemistry. 1993 Dec 14;32(49):13537–13544. doi: 10.1021/bi00212a020. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait S. J., O'Carroll A. M., McBride O. W., Konig M., Morel A., Brownstein M. J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992 May 28;357(6376):336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- Lumpkin M. D., Samson W. K., McCann S. M. Arginine vasopressin as a thyrotropin-releasing hormone. Science. 1987 Feb 27;235(4792):1070–1073. doi: 10.1126/science.2881350. [DOI] [PubMed] [Google Scholar]
- Maggi M., Kassis S., Malozowski S., Guardabasso V., Rodbard D. Identification and characterization of a vasopressin isoreceptor in porcine seminal vesicles. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8824–8828. doi: 10.1073/pnas.83.23.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlmann S., Meyerhof W., Hausmann H., Heierhorst J., Schönrock C., Zwiers H., Lederis K., Richter D. Structure, function, and phylogeny of [Arg8]vasotocin receptors from teleost fish and toad. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1342–1345. doi: 10.1073/pnas.91.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenberg G., Simmons W. H. Hypothermia induced by centrally administered vasopressin in rats. A structure-activity study. Neuropharmacology. 1984 Oct;23(10):1195–1200. doi: 10.1016/0028-3908(84)90239-9. [DOI] [PubMed] [Google Scholar]
- Morel A., O'Carroll A. M., Brownstein M. J., Lolait S. J. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992 Apr 9;356(6369):523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- O'Carroll A. M., Lolait S. J., König M., Mahan L. C. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol. 1992 Dec;42(6):939–946. [PubMed] [Google Scholar]
- O'Carroll A. M., Raynor K., Lolait S. J., Reisine T. Characterization of cloned human somatostatin receptor SSTR5. Mol Pharmacol. 1994 Aug;46(2):291–298. [PubMed] [Google Scholar]
- Ostrowski N. L., Lolait S. J., Young W. S., 3rd Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994 Oct;135(4):1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Ostrowski N. L., Young W. S., 3rd, Knepper M. A., Lolait S. J. Expression of vasopressin V1a and V2 receptor messenger ribonucleic acid in the liver and kidney of embryonic, developing, and adult rats. Endocrinology. 1993 Oct;133(4):1849–1859. doi: 10.1210/endo.133.4.8404628. [DOI] [PubMed] [Google Scholar]
- Rozen F., Russo C., Banville D., Zingg H. H. Structure, characterization, and expression of the rat oxytocin receptor gene. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):200–204. doi: 10.1073/pnas.92.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Derdowska I., Sobocinska M., Kupryszewski G. A potent new synthetic analog of vasopressin with relative agonist specificity for the pituitary. Endocrinology. 1991 Aug;129(2):1107–1109. doi: 10.1210/endo-129-2-1107. [DOI] [PubMed] [Google Scholar]
- Sharif M., Hanley M. R. Peptide receptors. Stepping up the pressure. Nature. 1992 May 28;357(6376):279–280. doi: 10.1038/357279a0. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Saito M., Mochizuki S., Watanabe Y., Hashimoto S., Kawashima H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J Biol Chem. 1994 Oct 28;269(43):27088–27092. [PubMed] [Google Scholar]
- Usdin T. B., Mezey E., Button D. C., Brownstein M. J., Bonner T. I. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993 Dec;133(6):2861–2870. doi: 10.1210/endo.133.6.8243312. [DOI] [PubMed] [Google Scholar]
- Yoshimura R., Kiyama H., Kimura T., Araki T., Maeno H., Tanizawa O., Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993 Sep;133(3):1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Kovács K., Lolait S. J. The diurnal rhythm in vasopressin V1a receptor expression in the suprachiasmatic nucleus is not dependent on vasopressin. Endocrinology. 1993 Aug;133(2):585–590. doi: 10.1210/endo.133.2.8344200. [DOI] [PubMed] [Google Scholar]
- de Wied D., Diamant M., Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol. 1993 Oct;14(4):251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]






