Abstract
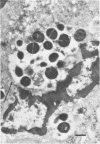
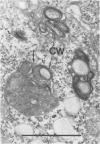
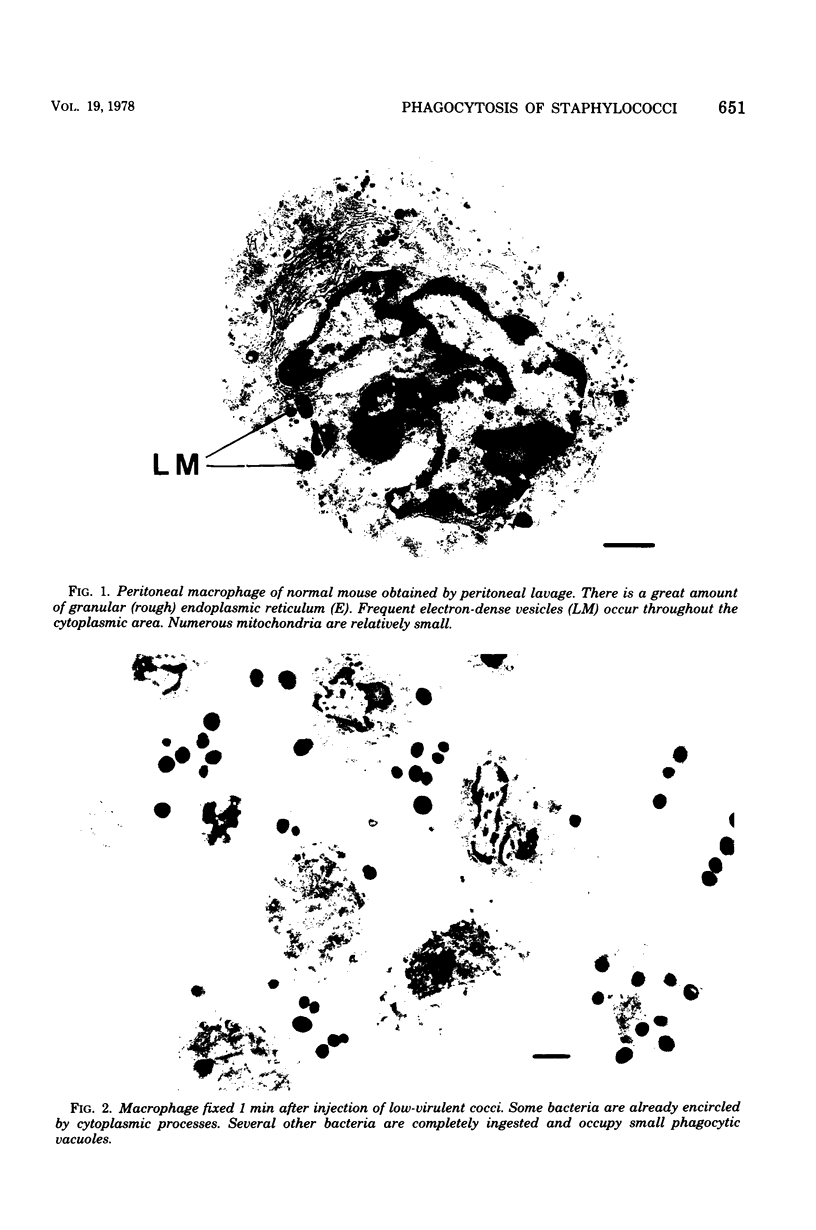
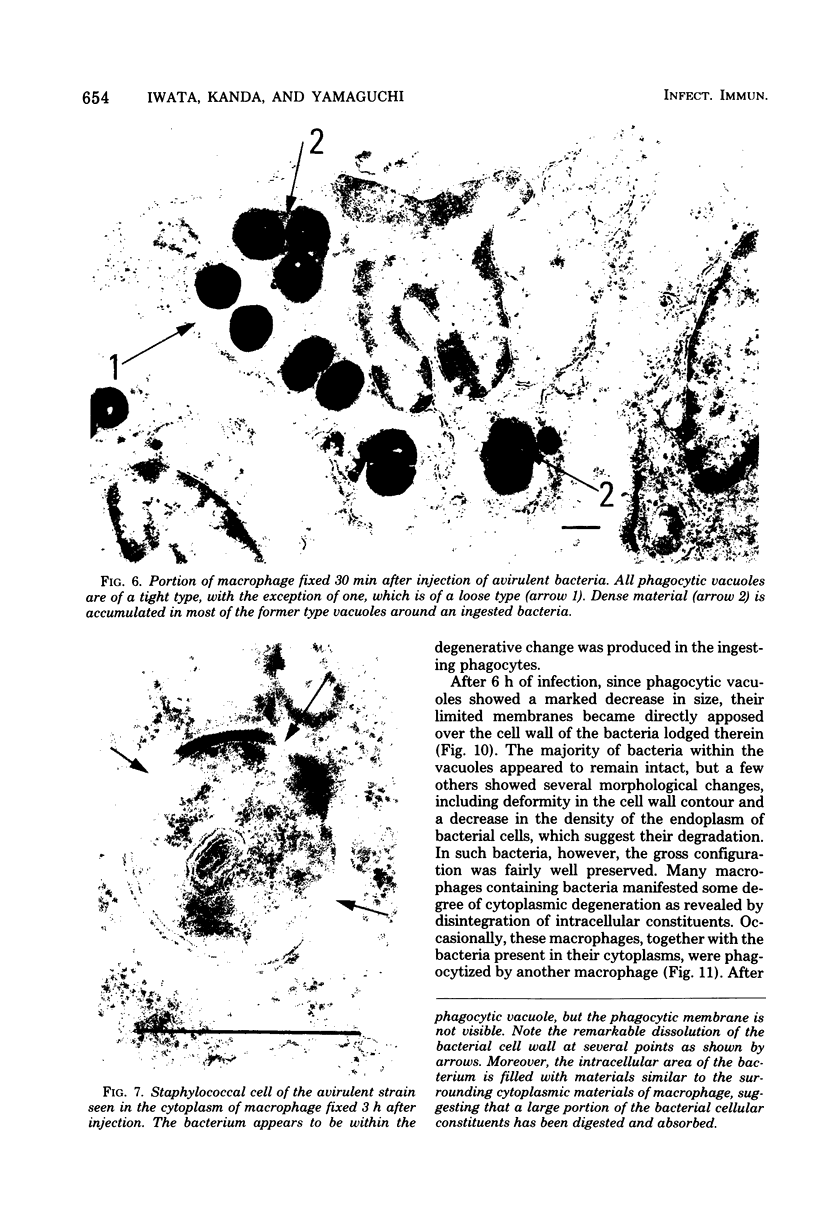
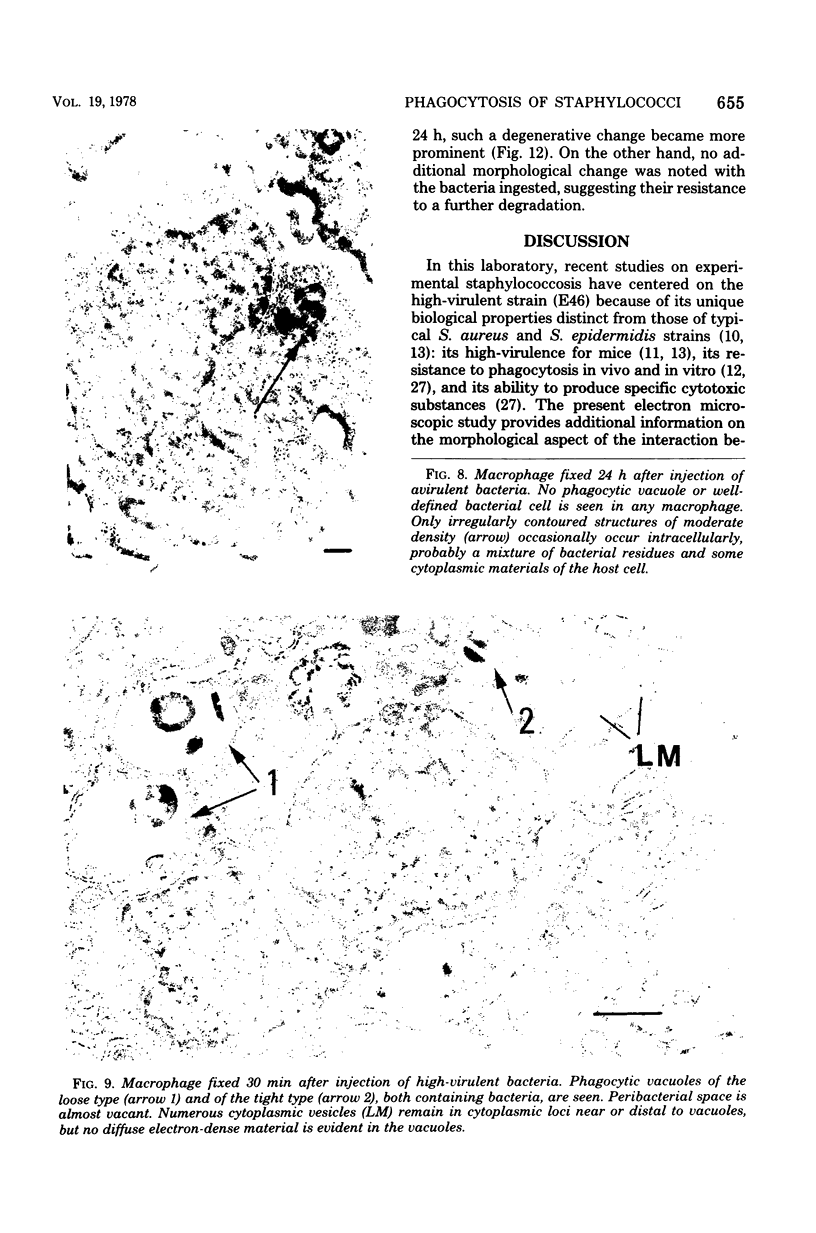
Macrophages from the mouse peritoneal cavity were examined by electron microscopy at various time intervals up to 24 h after intraperitoneal administration of each of three strains of staphylococci different in virulence for mice: high-virulent, low-virulent, and avirulent strains. After engulfment, avirulent bacteria were highly liable to intracellular digestion, resulting in almost complete degradation within 24 h after injection, whereas high-virulent bacteria were more resistant to digestion, some showing figures suggestive of a dividing process; the gross configuration of most of the ingested bacteria was relatively well preserved over the 24-h period. Time-dependent morphological changes of low-virulent bacteria were intermediate. Among the most distinct cytoplasmic responses to the ingested bacteria was the formation of phagocytic vacuoles around them, the type of which was dependent on the staphylococcal strain infected; ingestion of avirulent bacteria led to formation of vacuoles in which the bacteria were surrounded by a halo of amorphous material of moderate density, which may be the lysosomal content. In contrast, larger vacuoles developed after ingestion of high-virulent bacteria and contained only a small quantity of such amorphous materials. Both types of phagocytic vacuoles were seen around the low-virulent bacteria ingested. Some degenerative changes were found in the macrophages ingesting high- or low-virulent bacteria, but were not in those ingesting avirulent bacteria. Thus, resistance to intracellular degradation, as well as cytotoxicity toward phagocytes of staphylococcal strains, can be correlated with their virulence.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER R. F., GOODMAN J. R., MOORE R. E. Electron microscopic study of phagocytosis of staphylococcus by human leukocytes. II. Virulent and non-virulent staphylococci. J Bacteriol. 1956 Dec;72(6):736–745. doi: 10.1128/jb.72.6.736-745.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. Determinants of infection in the peritoneal cavity. I. Response to and fate of Staphylococcus aureus and Staphylococcus albus in the mouse. Yale J Biol Med. 1962 Aug;35:12–28. [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Hirsch J. G., Fedorko M. E. The in vitro differentiation of mononuclear phagocytes. IV. The ultrastructure of macrophage differentiation in the peritoneal cavity and in culture. J Exp Med. 1966 Apr 1;123(4):747–756. doi: 10.1084/jem.123.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diengdoh J. V., Turk J. L. Immunological significance of lysosomes with lymphocytes in vivo. Nature. 1965 Sep 25;207(5004):1405–1406. doi: 10.1038/2071405a0. [DOI] [PubMed] [Google Scholar]
- Dumont A., Robert A. Electron microscopic study of phagocytosis of Histoplasma capsulatum by hamster peritoneal macrophages. Lab Invest. 1970 Sep;23(3):278–286. [PubMed] [Google Scholar]
- GARVEY J. S., EITZMAN D. V., SMITH R. T. The distribution of S35-labeled bovine serum albumin in newborn and immunologically tolerant adult rabbits. J Exp Med. 1960 Sep 1;112:533–550. doi: 10.1084/jem.112.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN J. R., MOORE R. E. Electron microscopic study of phagocytosis of Staphylococcus by human leukocytes. J Bacteriol. 1956 May;71(5):547–556. doi: 10.1128/jb.71.5.547-556.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard G. C. Electron microscopic study of the differentiation of mouse peritoneal macrophages stimulated by Corynebacterium ovis infection. Lab Invest. 1969 Oct;21(4):309–315. [PubMed] [Google Scholar]
- Horn R. G., Koenig M. G., Goodman J. S., Collins R. D. Phagocytosis of Staphylococcus aureus by hepatic reticuloendothelial cells. An ultrastructural study. Lab Invest. 1969 Nov;21(5):406–414. [PubMed] [Google Scholar]
- Iwata K., Eda T., Kimura S., Aoyama I. [Studies on the extracellular enzymes of staphylococci. 1. Identification of pathogenic staphylococci by the use of deoxyribonuclease]. Nihon Saikingaku Zasshi. 1967 Oct;22(10):577–582. doi: 10.3412/jsb.22.577. [DOI] [PubMed] [Google Scholar]
- Iwata K., Eda T. [Studies on a unique strain of Staphylococcus showing high virulence for mice after intraperitoneal inoculation. 1. Its morphological and biological properties]. Nihon Saikingaku Zasshi. 1968 Mar;23(3):165–171. [PubMed] [Google Scholar]
- Iwata K., Eda T. [Studies on the unique staphylococcal strains exhibiting high virulence for mice by intraperitoneal inoculation. 2. Virulence of the strains]. Nihon Saikingaku Zasshi. 1968 May;23(5):348–354. doi: 10.3412/jsb.23.348. [DOI] [PubMed] [Google Scholar]
- Iwata K., Eda T. [Studies on unique staphylococcal strains exhibiting high virulence for mice by intraperitoneal inoculation. 3. Experimental analysis of its virulence]. Nihon Saikingaku Zasshi. 1968 Jun;23(6):392–400. doi: 10.3412/jsb.23.392. [DOI] [PubMed] [Google Scholar]
- KARLSBAD G., KESSEL R. W., DE PETRIS S., MONACO L. ELECTRON MICROSCOPE OBSERVATIONS OF BRUCELLA ABORTUS GROWN WITHIN MONOCYTES IN VITRO. J Gen Microbiol. 1964 Jun;35:383–390. doi: 10.1099/00221287-35-3-383. [DOI] [PubMed] [Google Scholar]
- Kapral F. A. The phagocytosis and intracellular fate of staphylococci. Ann N Y Acad Sci. 1965 Jul 23;128(1):285–300. doi: 10.1111/j.1749-6632.1965.tb11645.x. [DOI] [PubMed] [Google Scholar]
- Koenig M. G., Melly M. A. The importance of surface antigens in staphylococcal virulence. Ann N Y Acad Sci. 1965 Jul 23;128(1):231–250. doi: 10.1111/j.1749-6632.1965.tb11641.x. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. The phagocytosis and inactivation of staphylococci by macrophages of normal rabbits. J Exp Med. 1960 Jul 1;112:35–53. doi: 10.1084/jem.112.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R. J., MACKANESS G. B. ELECTRON MICROSCOPICAL OBSERVATIONS ON THE PERITONEAL MACROPHAGES OF NORMAL MICE AND MICE IMMUNISED WITH LISTERIA MONOCYTOGENES. II. STRUCTURE OF MACROPHAGES FROM IMMUNE MICE AND EARLY CYTOPLASMIC RESPONSE TO THE PRESENCE OF INGESTED BACTERIA. Br J Exp Pathol. 1963 Dec;44:608–611. [PMC free article] [PubMed] [Google Scholar]
- PEARSON G. R., FREEMAN B. A., HINES W. D. THIN-SECTION ELECTRON MICROGRAPHS OF MONOCYTES INFECTED WITH BRUCELLA SUIS. J Bacteriol. 1963 Nov;86:1123–1125. doi: 10.1128/jb.86.5.1123-1125.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D. E., MELLY M. A. Further observations on the behavior of staphylococci within human leukocytes. J Exp Med. 1960 Apr 1;111:533–558. doi: 10.1084/jem.111.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D. E., TOMPSETT R. The survival of staphylococci within human leukocytes. J Exp Med. 1952 Feb;95(2):209–230. doi: 10.1084/jem.95.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAYEGANI M. G., KAPRAL F. A. The eventual intracellular destruction of staphylococci by mononuclear cells. J Gen Microbiol. 1962 Dec;29:637–644. doi: 10.1099/00221287-29-4-637. [DOI] [PubMed] [Google Scholar]
- SUTER E. Interaction between phagocytes and pathogenic microorganisms. Bacteriol Rev. 1956 Jun;20(2):94–132. doi: 10.1128/br.20.2.94-132.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., RAMSEIAR H. CELLULAR REACTIONS IN INFECTION. Adv Immunol. 1964;27:117–173. doi: 10.1016/s0065-2776(08)60707-5. [DOI] [PubMed] [Google Scholar]