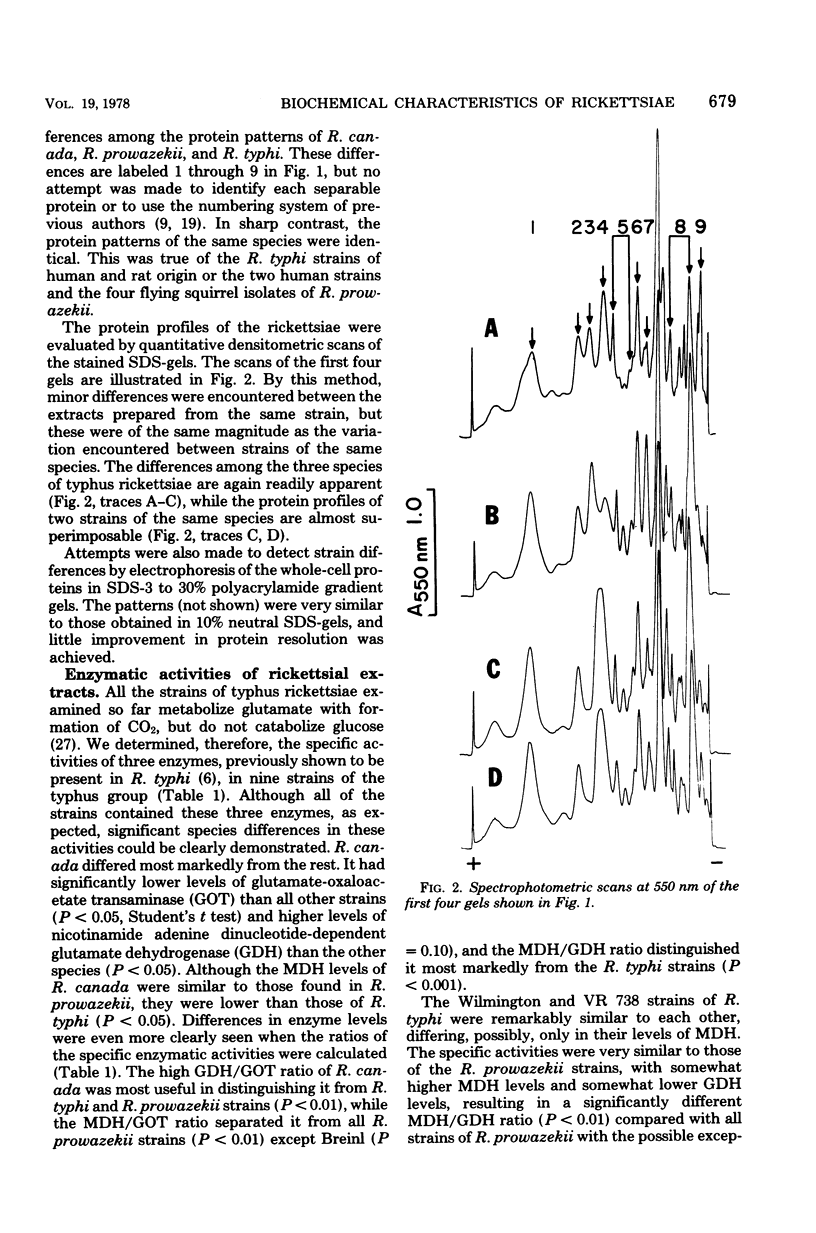
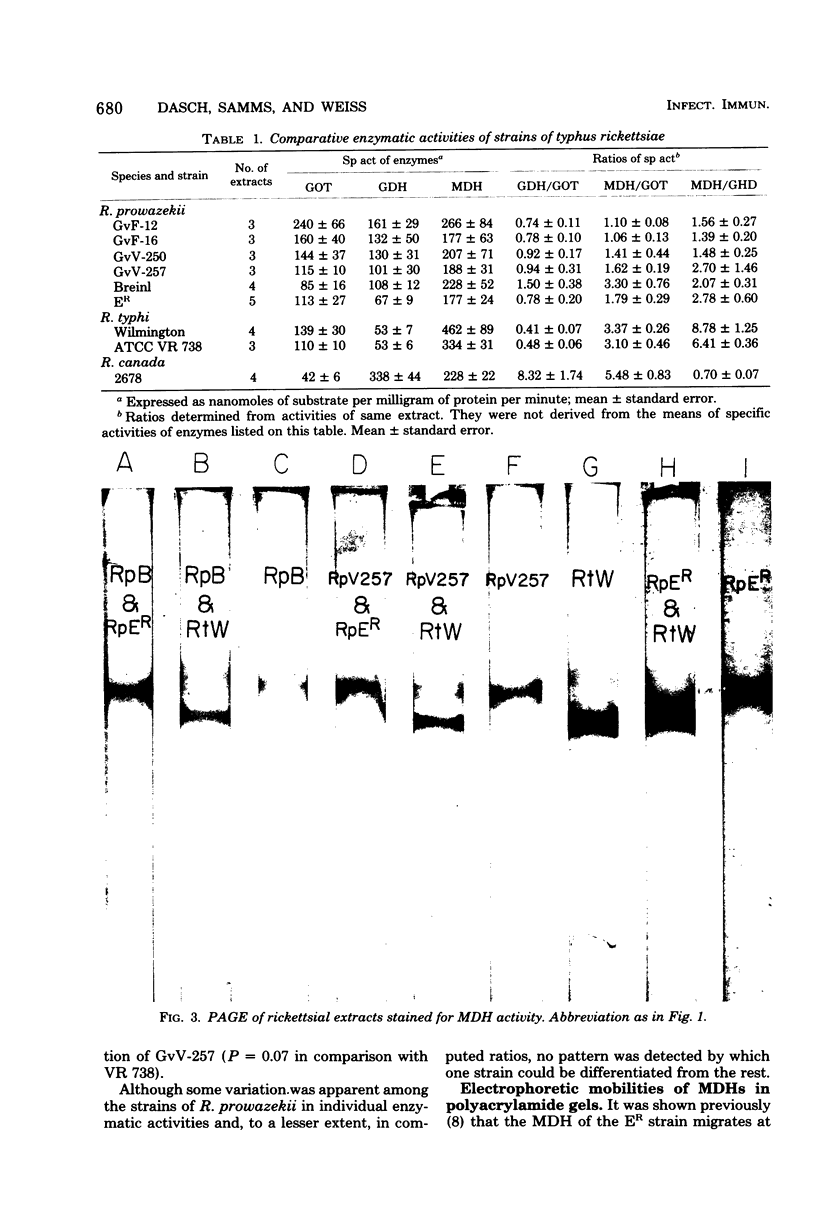
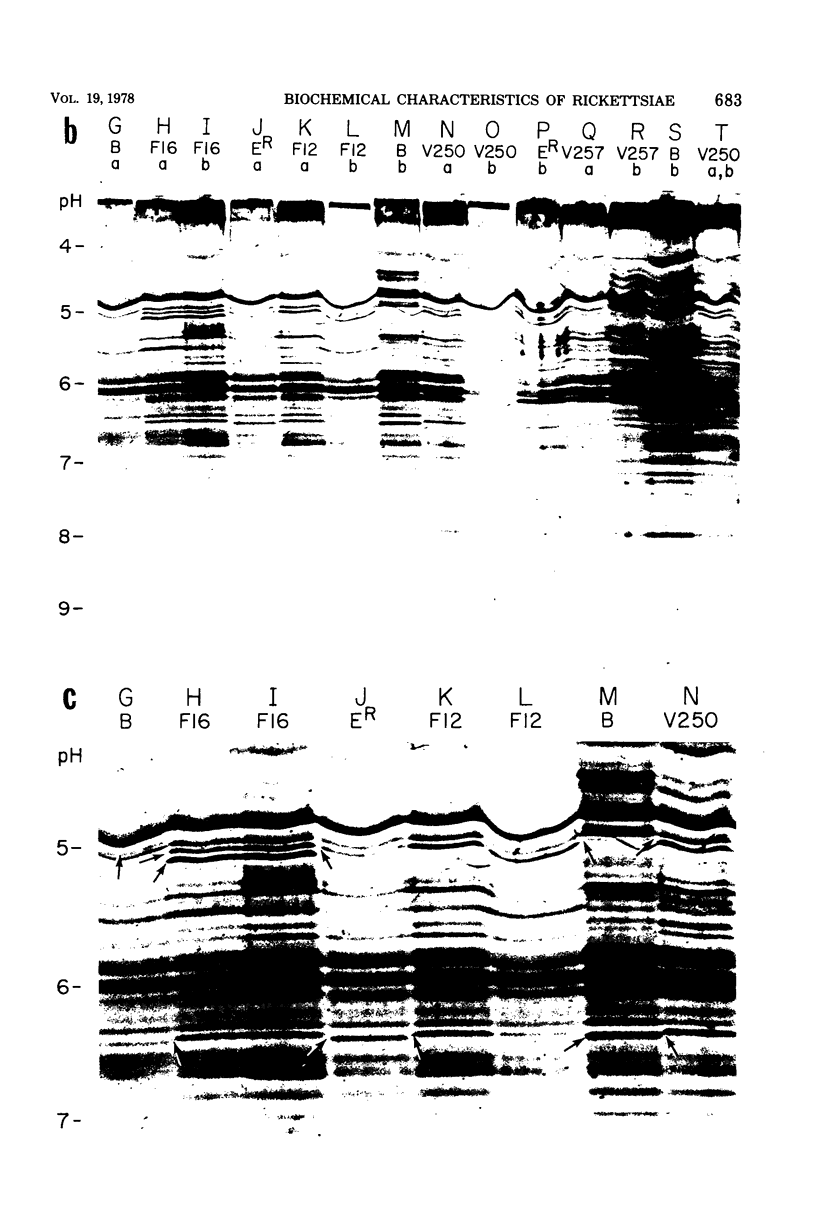
Abstract
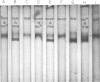
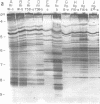
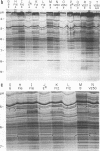
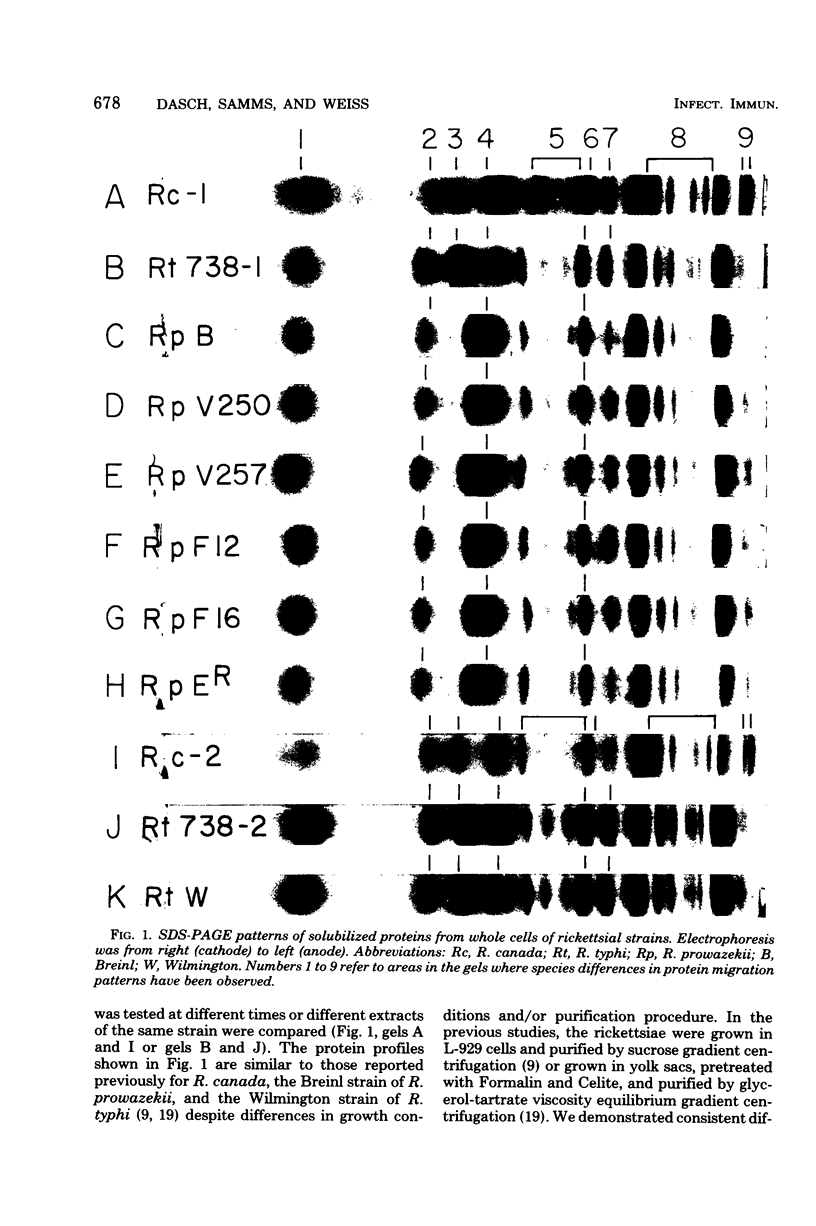
Six strains of Rickettsia prowazekii, two derived from human infections and four isolated from flying squirrels, two strains of R. typhi, and the single available strain of R. canada, were characterized by several biochemical procedures. The electrophoretic patterns on polyacrylamide gels of rickettsial proteins solubilized by sodium dodecyl sulfate revealed several species differences, but strains of the same species appeared to have identical patterns. Cytoplasmic fractions of the rickettsiae were examined for enzymatic activities and for polyacrylamide gel isoelectric focusing patterns. Some species differences were encountered in the activities or ratios of activities of glutamate-oxaloacetate transaminase, glutamate dehydrogenase, and malate dehydrogenase. When polyacrylamide gels were stained for malate dehydrogenase after electrophoresis, a single band became apparent with single extracts or mixtures of two strains of R. prowazekii, but two bands were seen with mixtures of a strain of R. prowazekii and one of R. typhi. The isoelectric focusing patterns of the soluble proteins revealed numerous species differences, especially between R. canada and the other two species, and a few differences among the strains of R. prowazekii. The patterns of the two human strains, Breinl and ER, differed in at least one location, and both differed from the flying squirrel strains in the displacement of one band. One of the flying squirrel strains, GvF-16, contained a protein band not seen in the other five strains. Despite these minor differences, a striking similarity was revealed by all the biochemical tests performed between the R. prowazekii strains of human and flying squirrel origin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Balayera N. M., Nikdskaya V. N. Enhanced virulence of the vaccine strain E of Rickettsia prowazeke on passaging in white mice and guinea pigs. Acta Virol. 1972 Jun;16(1):80–82. [PubMed] [Google Scholar]
- Baptist J. N., Shaw C. R., Mandel M. Zone electrophoresis of enzymes in bacterial taxonomy. J Bacteriol. 1969 Jul;99(1):180–188. doi: 10.1128/jb.99.1.180-188.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeman F. M., Masiello S. A., Williams M. S., Elisberg B. L. Epidemic typhus rickettsiae isolated from flying squirrels. Nature. 1975 Jun 12;255(5509):545–547. doi: 10.1038/255545a0. [DOI] [PubMed] [Google Scholar]
- Coolbaugh J. C., Progar J. J., Weiss E. Enzymatic activities of cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Jul;14(1):298–305. doi: 10.1128/iai.14.1.298-305.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J., Meddins B. M. Polyacrylamide gel electrophoresis of mycoplasma proteins in sodium dodecyl sulphate. J Gen Microbiol. 1973 May;76(1):239–242. doi: 10.1099/00221287-76-1-239. [DOI] [PubMed] [Google Scholar]
- Dasch G. A., Weiss E. Characterization of the Madrid E strain of Rickettsia prowazekii purified by renografin density gradient centrifugation. Infect Immun. 1977 Jan;15(1):280–286. doi: 10.1128/iai.15.1.280-286.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisemann C. S., Osterman J. V. Proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1976 Jul;14(1):155–162. doi: 10.1128/iai.14.1.155-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito J. J., Obijeski J. F. High resolution polyacrylamide gradient gel electrophoresis. Prep Biochem. 1976;6(6):431–442. doi: 10.1080/00327487608069128. [DOI] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. I. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973 Oct;8(4):519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten E. Glutamate dehydrogenases in genus Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Feb;81(1):49–58. doi: 10.1111/j.1699-0463.1973.tb02186.x. [DOI] [PubMed] [Google Scholar]
- Ignatovich V. F. Enhancement of the antigenic activity and virulence of the vaccine strain E of Rickettsia prow azeki by passages in cell culture. Acta Virol. 1975 Nov;19(6):481–485. [PubMed] [Google Scholar]
- Kazár J., Brezina R., Urvölgyi J. Studies on the E strain of Rickettsia prowazeki. Bull World Health Organ. 1973;49(3):257–265. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKiel J. A., Bell E. J., Lackman D. B. Rickettsia canada: a new member of the typhus group of rickettsiae isolated from Haemaphysalis leporispalustris ticks in Canada. Can J Microbiol. 1967 May;13(5):503–510. doi: 10.1139/m67-065. [DOI] [PubMed] [Google Scholar]
- Sayed I. A., Hatten B. A. Isoelectric focusing of mycoplasma proteins. Appl Environ Microbiol. 1976 Oct;32(4):603–609. doi: 10.1128/aem.32.4.603-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyring G. W., Miller R. W., Purkayastha V. Improved method for characterization of bacterial dehydrogenases using acrylamide gel disc electrophoresis. Anal Biochem. 1970 Aug;36(2):511–520. doi: 10.1016/0003-2697(70)90387-8. [DOI] [PubMed] [Google Scholar]
- WEISS E. Some aspects of variation in rickettsial virulence. Ann N Y Acad Sci. 1960 Nov 21;88:1287–1297. doi: 10.1111/j.1749-6632.1960.tb20120.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peterson J. C. Enzymatic activities leading to pyrimidine nucleotide biosynthesis from cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Aug;14(2):439–448. doi: 10.1128/iai.14.2.439-448.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D. In vitro studies on rickettsia-host cell interactions: intracellular growth cycle of virulent and attenuated Rickettsia prowazeki in chicken embryo cells in slide chamber cultures. Infect Immun. 1975 Jun;11(6):1391–1404. doi: 10.1128/iai.11.6.1391-1401.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman D. R., Weiss E., Dasch G. A., Bozeman F. M. Biological properties of Rickettsia prowazekii strains isolated from flying squirrels. Infect Immun. 1977 Jun;16(3):853–860. doi: 10.1128/iai.16.3.853-860.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]