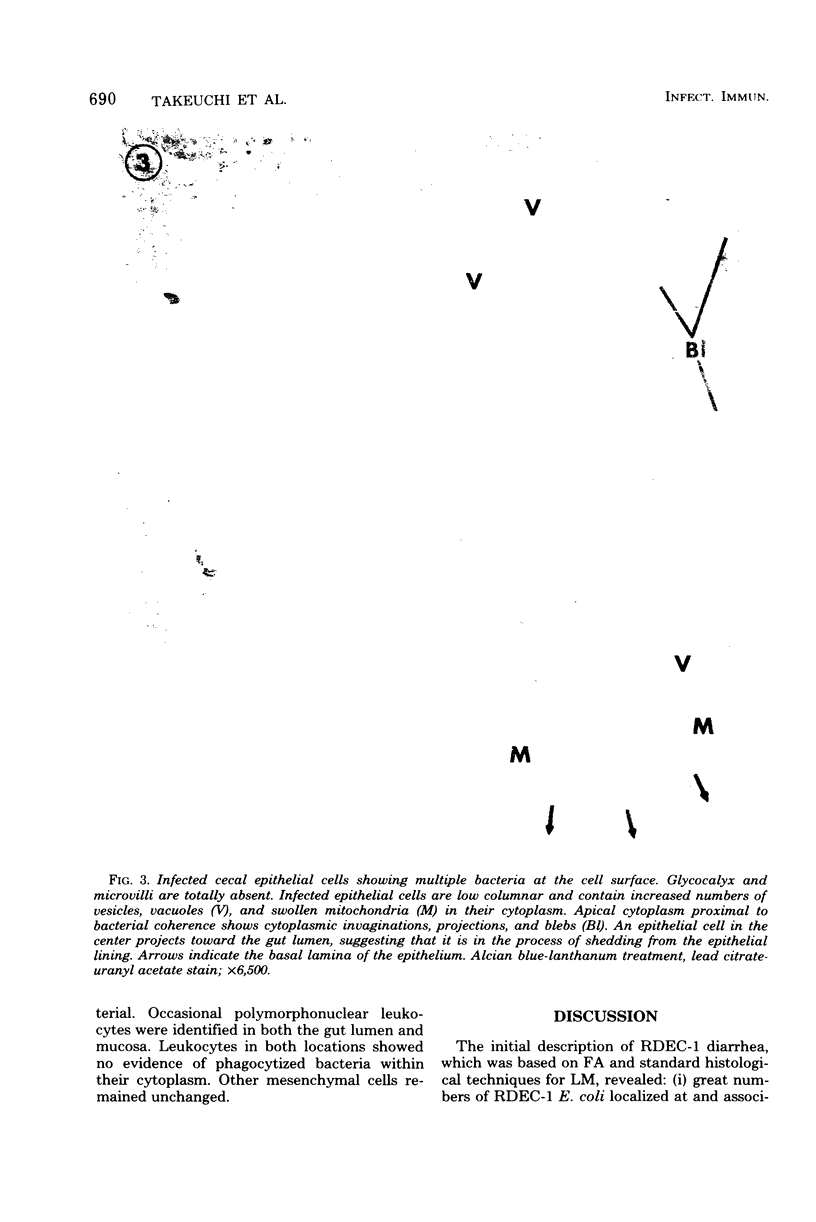
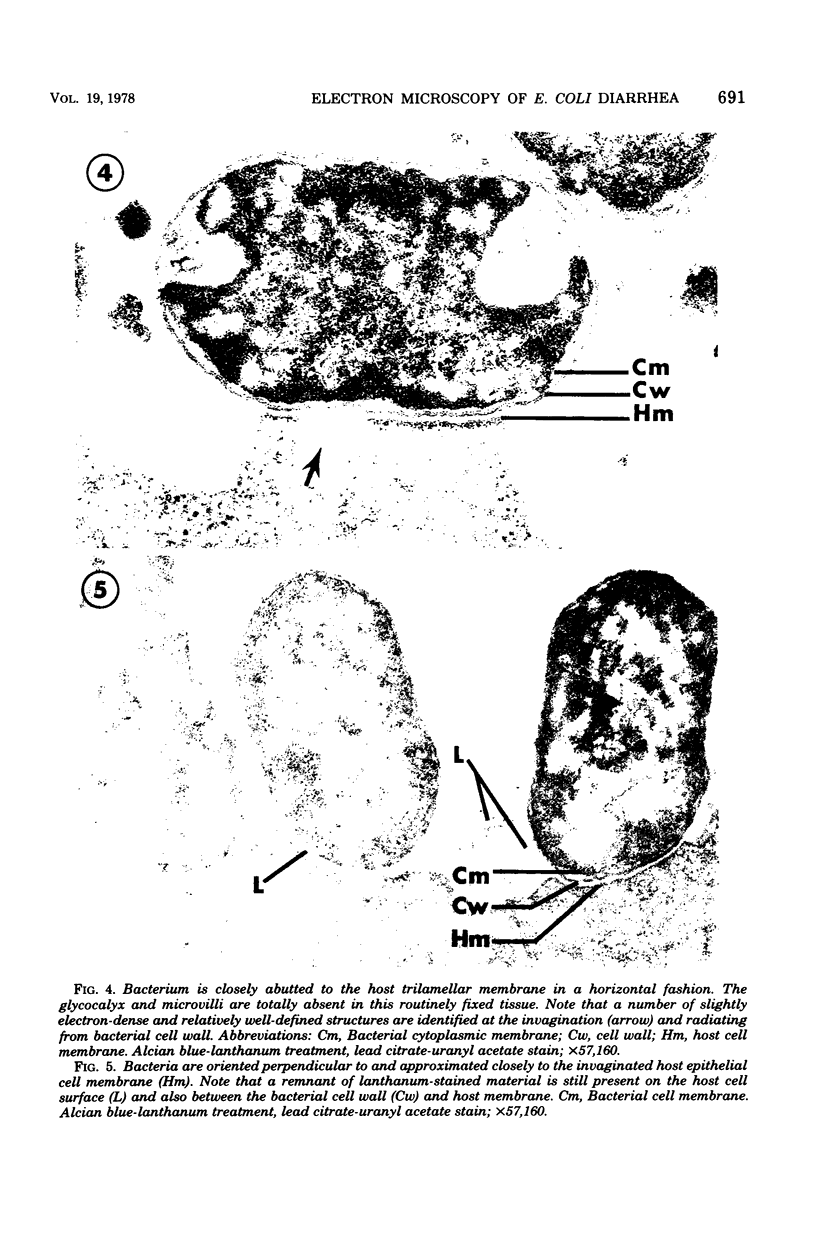
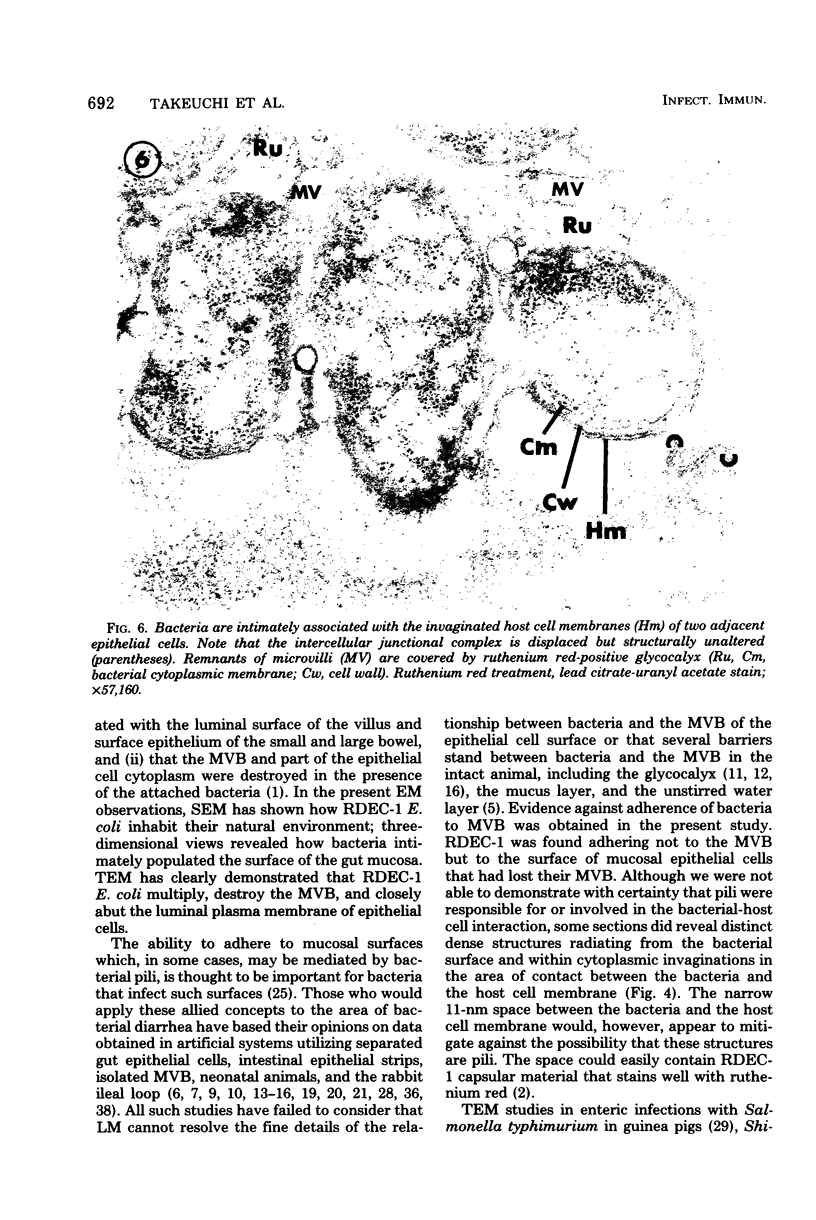
Abstract
RDEC-1 is a piliated strain of Escherichia coli that was isolated from and produces diarrhea in rabbits without invading the mucosa or synthesizing one of the classical enterotoxins. Previous histological and fluorescent-antibody studies of RDEC-1 diarrhea revealed an acute inflammatory response and large numbers of RDEC-1 associated with (adhering to) the mucosal surface of the ileum, cecum, and colon. The purpose of the present investigation was to further elucidate the histopathology by scanning (SEM) and transmission (TEM) electron microscopy. SEM revealed aggregates of bacteria on the surface of the gut; their distribution was patchy in the ileum and diffuse in the cecum and colon. Bacteria were in contact with each other and appeared to be closely associated with the epithelial surface. TEM showed that the brush border region of the epithelial cells was found to be in varying stages of degeneration, and the bacteria could not be seen adhering to the mucosal cells unless the brush border was absent. Bacteria were in close contact only with epithelial cells that had lost their brush border. The space between the bacteria and the epithelial cells was 11 nm, and it appeared to be filled, in most cases, with densely stained material. This E. coli rarely penetrated epithelial cells, but when it did; it was found in the supranuclear region and never reached the lamina propria. From previous and present studies, it seems probable that RDEC-1 produces diarrhea in rabbits by a mechanism that may be cytotoxic and differs from the classic mechanisms by which E. coli produces diarrhea.
Full text
PDF

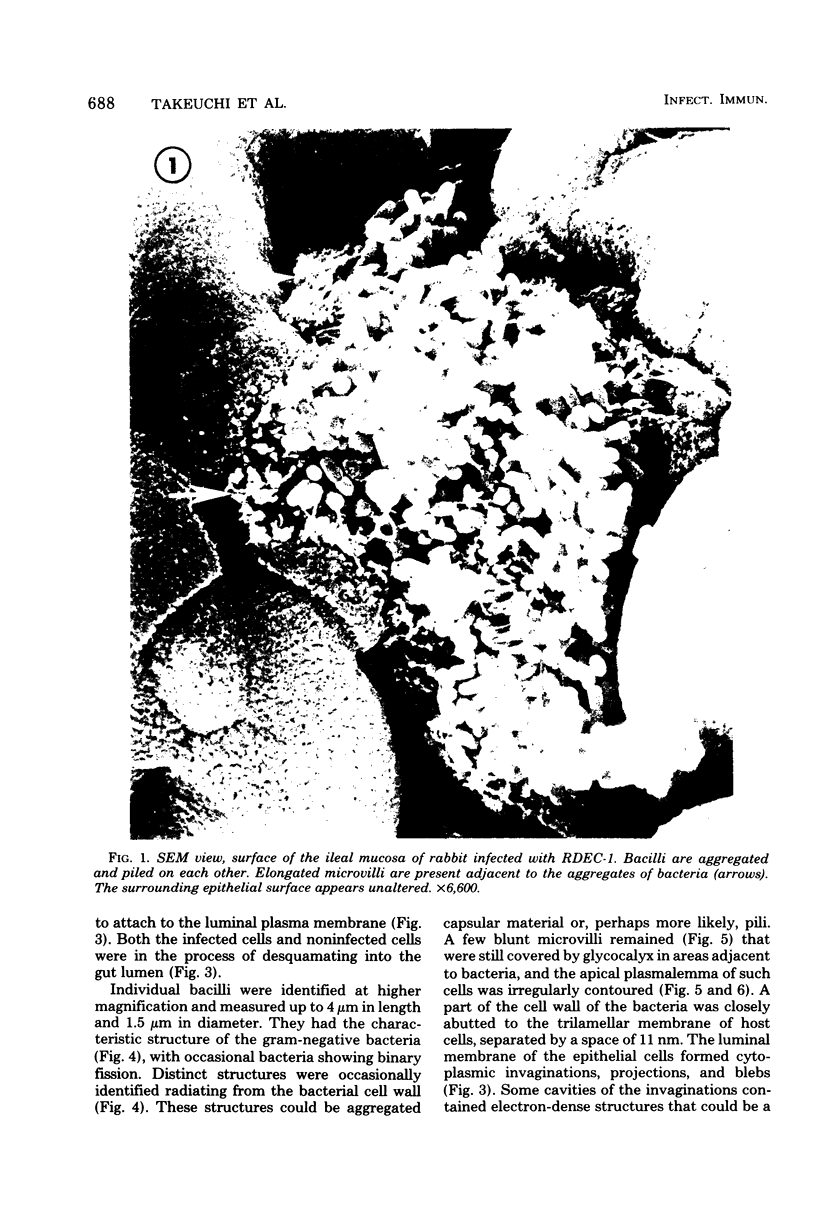



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantey J. R., Blake R. K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977 Mar;135(3):454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., O'Hanley P. D., Blake R. K. A rabbit model of diarrhea due to invasive Escherichia coli. J Infect Dis. 1977 Nov;136(5):640–648. doi: 10.1093/infdis/136.5.640. [DOI] [PubMed] [Google Scholar]
- Davis C. P., Savage D. C. Habitat, succession, attachment, and morphology of segmented, filamentous microbes indigenous to the murine gastrointestinal tract. Infect Immun. 1974 Oct;10(4):948–956. doi: 10.1128/iai.10.4.948-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker M. M., Yeivin R., Sacks T. G. Pathogenesis of Escherichia coli enteritis in the ligated rabbit gut. Isr J Med Sci. 1967 May-Jun;3(3):445–452. [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Ito S. Structure and function of the glycocalyx. Fed Proc. 1969 Jan-Feb;28(1):12–25. [PubMed] [Google Scholar]
- Ito S. The enteric surface coat on cat intestinal microvilli. J Cell Biol. 1965 Dec;27(3):475–491. doi: 10.1083/jcb.27.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E. D. Progesterone level and response to oxytocin at term. Lancet. 1968 Sep 7;2(7567):570–570. doi: 10.1016/s0140-6736(68)92430-6. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. O., Dekker R. A., Bluemink J. G. Ligand-mediated osmium binding: its application in coating biological specimens for scanning electron microscopy. J Ultrastruct Res. 1973 Nov;45(3):254–258. doi: 10.1016/s0022-5320(73)80051-6. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- McNeish A. S., Turner P., Fleming J., Evans N. Mucosal adherence of human enteropathogenic Escherichia coli. Lancet. 1975 Nov 15;2(7942):946–948. doi: 10.1016/s0140-6736(75)90360-8. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Nagy B., Isaacson R. E., Orskov I. Occurrence of K99 antigen on Escherichia coli isolated from pigs and colonization of pig ileum by K99+ enterotoxigenic E. coli from calves and pigs. Infect Immun. 1977 Feb;15(2):614–620. doi: 10.1128/iai.15.2.614-620.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Nakamura A., Sakazaki R. Pathogenic properties of "enteropathogenic" Escherichia coli from diarrheal children and adults. Jpn J Med Sci Biol. 1968 Oct;21(5):333–349. [PubMed] [Google Scholar]
- Rambourg A. Morphological and histochemical aspects of glycoproteins at the surface of animal cells. Int Rev Cytol. 1971;31:57–114. doi: 10.1016/s0074-7696(08)60057-1. [DOI] [PubMed] [Google Scholar]
- Shea S. M. Lanthanum staining of the surface coat of cells. Its enhancement by the use of fixatives containing Alcian blue or cetylpyridinium chloride. J Cell Biol. 1971 Dec;51(3):611–620. doi: 10.1083/jcb.51.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between two apparently different enterotoxins produced by enteropathogenic strains of Escherichia coli of porcine origin. J Med Microbiol. 1970 Aug;3(3):387–401. doi: 10.1099/00222615-3-3-387. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. Studies on Escherichia coli enterotoxin. J Pathol Bacteriol. 1967 Apr;93(2):531–543. doi: 10.1002/path.1700930212. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR J., MALTBY M. P., PAYNE J. M. Factors influencing the response of ligated rabbit-gut segments to injected Escherichia coli. J Pathol Bacteriol. 1958 Oct;76(2):491–499. doi: 10.1002/path.1700760218. [DOI] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Formal S. B., Sprinz H. Exerimental acute colitis in the Rhesus monkey following peroral infection with Shigella flexneri. An electron microscope study. Am J Pathol. 1968 Mar;52(3):503–529. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Jervis H. R., Nakazawa H., Robinson D. M. Spiral-shaped organisms on the surface colonic epithelium of the monkey and man. Am J Clin Nutr. 1974 Nov;27(11):1287–1296. doi: 10.1093/ajcn/27.11.1287. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Jervis H. R., Sprinz H. The globule leucocyte in the intestinal mucosa of the cat: a histochemical, light and electron microscopic study. Anat Rec. 1969 May;164(1):79–99. doi: 10.1002/ar.1091640106. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H., LaBrec E. H., Formal S. B. Experimental bacillary dysentery. An electron microscopic study of the response of the intestinal mucosa to bacterial invasion. Am J Pathol. 1965 Dec;47(6):1011–1044. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Zeller J. A. Scanning electron microscopic observations on the surface of the normal and spirochete-infested colonic mucosa of the rhesus monkey. J Ultrastruct Res. 1972 Aug;40(3):313–324. doi: 10.1016/s0022-5320(72)90103-7. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Zeller J. A. Ultrastructural identification of spirochetes and flagellated microbes at the brush border of the large intestinal epithelium of the rhesus monkey. Infect Immun. 1972 Dec;6(6):1008–1018. doi: 10.1128/iai.6.6.1008-1018.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetterling J. M., Takeuchi A., Madden P. A. Ultrastructure of Cryptosporidium wrairi from the guinea pig. J Protozool. 1971 May;18(2):248–260. doi: 10.1111/j.1550-7408.1971.tb03316.x. [DOI] [PubMed] [Google Scholar]
- Wilson M. R., Hohmann A. W. Immunity to Escherichia coli in pigs: adhesion of enteropathogenic Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1974 Oct;10(4):776–782. doi: 10.1128/iai.10.4.776-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]