Abstract
Poorly controlled diabetes has long been known as a catabolic disorder with profound loss of muscle and fat body mass resulting from a simultaneous reduction in protein synthesis and enhanced protein degradation. By contrast, retinal structure is largely maintained during diabetes despite reduced Akt activity and increased rate of cell death. Therefore, we hypothesized that retinal protein turnover is regulated differently than in other insulin-sensitive tissues, such as skeletal muscle. Ins2Akita diabetic mice and streptozotocin-induced diabetic rats exhibited marked reductions in retinal protein synthesis matched by a concomitant reduction in retinal protein degradation associated with preserved retinal mass and protein content. The reduction in protein synthesis depended on both hyperglycemia and insulin deficiency, but protein degradation was only reversed by normalization of hyperglycemia. The reduction in protein synthesis was associated with diminished protein translation efficiency but, surprisingly, not with reduced activity of the mTORC1/S6K1/4E-BP1 pathway. Instead, diabetes induced a specific reduction of mTORC2 complex activity. These findings reveal distinctive responses of diabetes-induced retinal protein turnover compared with muscle and liver that may provide a new means to ameliorate diabetic retinopathy.
Introduction
Diabetic retinopathy remains a leading cause of vision impairment and blindness in spite of medications that reduce hyperglycemia, hypertension, and hyperlipidemia. The worldwide diabetes epidemic, which includes both type 1 and type 2 diabetes, necessitates a better understanding of the fundamental processes that lead to vision impairment (1). Diabetes causes profound morbidity through its impact on sensory and autonomic nerves in the extremities, gastrointestinal and urinary tracts, heart, and retina (2). Nevertheless, the pathophysiologic basis for the retina’s vulnerability to diabetes remains obscure. We previously suggested that fundamental requirements to maintain vision may compromise the retina’s adaptability to metabolic stress (3). For example, neurons in the inner retina are subject to high metabolic requirements to maintain electrical gradients across unmyelinated neuronal membranes and to conduct bidirectional macromolecular transport through retinal ganglion cell axons to the lateral geniculate nucleus of the thalamus. However, a paucity of blood vessels in the inner retina results in a low partial pressure of oxygen (∼25 mmHg), whereas the need to optimize light transmission necessitates a relative lack of mitochondria (4,5). In contrast, the outer retina is supplied with ample oxygen from the choroidal circulation, and photoreceptor inner segments have a relatively high density of mitochondria. However, an extremely high basal metabolic activity of photoreceptor cells is required for phototransduction to regenerate photoreceptor outer segments and to maintain the dark current.
Insulin-deficient diabetes is a catabolic disorder characterized by skeletal muscle breakdown due to reduced protein synthesis and enhanced protein degradation in insulin-sensitive tissues, such as skeletal muscle and liver (6,7). Reduction of systemic insulin levels in type 1 diabetic patients is also associated with alterations in plasma protein synthesis (8). Although it had been proposed that such mechanisms were consistent across insulin-sensitive tissues and characteristic of diabetes, studies of brain and kidney have demonstrated otherwise. Indeed, diabetes is characterized by early renal hypertrophy as a consequence of reduced cathepsin activity and associated protein degradation (9), whereas no loss of protein occurs in rat brains and in animals in which skeletal muscle and liver protein degradation are dramatically increased (6). For >40 years, it has been known that protein synthesis and RNA content are tightly correlated in skeletal muscle (10) and that diabetes decreases both RNA content and ribosomal efficiency in skeletal muscle and liver (7,11,12). Protein synthesis in skeletal muscle is regulated by the mechanistic target of rapamycin complex 1 (mTORC1), which acts downstream of the insulin receptor/PI3K/Akt pathway (13). The insulin receptor/PI3K/Akt pathway is highly active in the retina under normal conditions, and although insensitive to feeding and fasting, in contrast to other insulin-sensitive tissues, its activity is reduced by diabetes (14,15). We also demonstrated that both systemic glycemic control and local insulin signaling are important for reversal of type 1 diabetes–associated retinal function defects (16). These collective observations led us to hypothesize that diabetes alters retinal metabolism, specifically protein synthesis and degradation, through alteration of glycemia and insulin signaling both locally and systemically in an mTOR-dependent manner.
We showed that both restoring ocular insulin receptor signaling and normalizing glycemia with the sodium-linked glucose transporter inhibitor phloridzin protected against retinal cell death in diabetic rats (16), thus revealing a novel interaction between growth factor deprivation and hyperglycemia affecting retinal neurodegeneration in type 1 diabetes. In the current study, we specifically assessed the role and regulation of the insulin receptor/Akt/mTOR pathway on retinal biosynthetic reactions essential for vision (17,18) and how they are affected under diabetic conditions. Retinal protein synthesis and degradation were quantified using in vivo and ex vivo methods in two experimental models of insulin-deficient diabetes. The specific impact of diabetes on mTORC1 and mTORC2 complexes was assessed. Collectively, the data reveal a marked diabetes-induced reduction of anabolic activity in the retina associated with a similar reduction in the mTORC2/Akt1/3 pathway, whereas the mTORC1/S6K1/4E-BP1 pathway remained unchanged. Of note, these alterations were reversed by systemic correction of hyperglycemia as well as by local stimulation of the insulin receptor signaling pathway. To our knowledge, this study is one of the first to investigate the regulation of catabolic mechanisms in the diabetic retina and thus provides new insights at the intersection of neurobiology and metabolism to understand the vulnerability of the retina. These findings may give rise to new strategies to control retinopathy in the face of diabetes by optimizing ocular insulin receptor action and nutrient concentrations.
Research Design and Methods
Animals, Diabetes, and Therapies
Spontaneously diabetic C57BL/6J Ins2Akita heterozygote male mice and littermate controls and Sprague-Dawley rats that received an intraperitoneal injection of streptozotocin (STZ) 65 mg/kg, as described previously (19), were used for Ins2Akita mouse and STZ rat experiments, respectively. Diabetic phenotype and genotype were confirmed by blood glucose levels >13.9 mmol/L (250 mg/dL). All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology Resolution on the Care and Use of Laboratory Animals. Continuous insulin therapy was administered during the second half of the duration of diabetes by implanting a subcutaneous insulin pellet to deliver ∼2 units bovine insulin per day for the duration of the experiment (LinShin Canada) (19). A second implant was given after 4 weeks for longer studies. Insulin-independent glycemic control was achieved by phloridzin therapy as previously described (16). Local insulin treatment was achieved by subconjunctival insulin administration (15 μL of a 100 nmol/L bovine crystalline insulin solution) on 4 consecutive days. This dose was chosen on the basis of preliminary studies showing activation of the retinal insulin receptor signaling pathway without affecting blood glucose levels (16). Phloridzin was administered subcutaneously twice daily, 12 h apart, for 4 days at a dose that normalized blood glucose levels in an insulin-independent manner (Table 1). The 4-, 8-, and 12-week diabetes duration studies were chosen because they lead to increased neuronal cell death, microvascular leakage, astrocyte defects, microglial cell activation, and impaired insulin receptor signaling (19–23).
Table 1.
Systemic metabolic parameters
| Weight (g) |
Blood glucose (mg/dL) |
||||
|---|---|---|---|---|---|
| Diabetes duration | Experimental condition | n | Final | Before treatment | Final |
| STZ rats | |||||
| 4 weeks | CONTROL | 8 | 400 ± 9 | 127 ± 14 | 113 ± 8 |
| DIABETIC | 8 | 275 ± 14 | 501 ± 31 | 463 ± 18 | |
| DIABETIC + LOC INS | 8 | 282 ± 13 | 490 ± 38 | 465 ± 13 | |
| DIABETIC + PHL | 8 | 282 ± 13 | 492 ± 27 | 237 ± 45 | |
| DIABETIC + PHL + LOC INS | 8 | 259 ± 6 | 555 ± 19 | 155 ± 8 | |
| DIABETIC + SYS INS | 7 | 326 ± 8 | 448 ± 13 | 222 ± 13 | |
| 8 weeks | CONTROL | 8 | 475 ± 13 | 154 ± 19 | 140 ± 18 |
| DIABETIC | 8 | 260 ± 9 | 478 ± 30 | 512 ± 29 | |
| DIABETIC + LOC INS | 7 | 314 ± 22 | 535 ± 34 | 468 ± 25 | |
| DIABETIC + PHL | 9 | 291 ± 21 | 536 ± 27 | 174 ± 18 | |
| DIABETIC + PHL + LOC INS | 10 | 271 ± 13 | 486 ± 33 | 220 ± 19 | |
| DIABETIC + SYS INS | 16 | 365 ± 10 | 526 ± 13 | 174 ± 29 | |
| 12 weeks | CONTROL | 8 | 564 ± 19 | 116 ± 5 | 105 ± 2 |
| DIABETIC | 8 | 302 ± 17 | 453 ± 34 | 425 ± 25 | |
| DIABETIC + LOC INS | 9 | 305 ± 24 | 477 ± 27 | 418 ± 30 | |
| DIABETIC + PHL | 6 | 294 ± 20 | 461 ± 25 | 236 ± 22 | |
| DIABETIC + PHL + LOC INS | 8 | 320 ± 22 | 412 ± 28 | 178 ± 19 | |
| DIABETIC + SYS INS | 9 | 460 ± 9 | 456 ± 26 | 209 ± 29 | |
| Ins2Akita mice | |||||
| 4 weeks | CONTROL | 10 | 21.2 ± 0.3 | N/A | 180 ± 8 |
| DIABETIC | 10 | 21.6 ± 0.7 | N/A | 209 ± 32 | |
| 8 weeks | CONTROL | 9 | 29.4 ± 0.8 | N/A | 165 ± 12 |
| DIABETIC | 11 | 26.0 ± 0.5 | N/A | 450 ± 37 | |
| 12 weeks | CONTROL | 9 | 29.6 ± 0.9 | N/A | 177 ± 6 |
| DIABETIC | 10 | 25.8 ± 0.6 | N/A | 545 ± 16 | |
Data are mean ± SEM. LOC INS, local insulin; N/A, not applicable; PHL, phloridzin; SYS INS, systemic insulin.
Analysis of Protein Synthesis
Rats were anesthetized with ketamine 4 mg/kg and xylazine 0.4 mg/kg and remained unconscious during the procedure. A flooding dose of L-[2,3,4,5,6-3H]phenylalanine 100 μCi/mL in unlabeled phenylalanine 150 mmol/L (l mL/100 g body weight) was injected through the femoral vein immediately after anesthesia as described previously (24). Arterial blood was taken at the time of kill as was excision of the retina, gastrocnemius muscle, and liver. Radiolabeled phenylalanine incorporated into trichloroacetic acid–precipitable protein was immediately analyzed using dabsylation of the amino acid and radioactivity measurement. Plasma-specific radioactivity in the phenylalanine peak was measured by liquid scintillation counting, with appropriate correction for quench. Protein determinations were made by the biuret method. Rates of protein synthesis were calculated by the method of Garlick et al. (25).
Protein Degradation Rates Analysis Pulse-Chase Labeling
Rats were killed by decapitation following anesthesia, eyes were enucleated, and retinas were collected. Retinas were incubated in DMEM lacking L-methionine for 30 min at 37°C in a 95% O2, 5% CO2 incubator. After methionine depletion, retinas were transferred into DMEM containing L-[35S]methionine 1 mCi/mmol for 30 min. For the pulse-chase portion, radioactive incorporation was followed by two washes in DMEM and further incubated in fresh DMEM supplemented with 500 μmol/L cold L-methionine for 30 and 60 min. After the final incubation, retinas were removed and immediately frozen in liquid nitrogen to prevent further incorporation of label and protein degradation. The retinas were then processed as described for protein synthesis measurements.
Polysome Profile Analysis and Quantitative Real-Time PCR
After rapid isolation, four retinas were combined in 600 μL polysome buffer (50 mmol/L HEPES, 250 mmol/L KCl, 5 mmol/L MgCl2, 250 mmol/L sucrose, 1% Triton X-100, 1.3% deoxycholate, 100 μg/mL cycloheximide, 100 units/mL SUPERase•In RNase inhibitor) and homogenized with a dounce. The lysate was subjected to centrifugation at 3,000g for 15 min at 4°C. The supernatant was then layered onto a 20%–47% linear sucrose gradient (50 mmol/L HEPES, 250 mmol/L KCl, 5 mmol/L MgCl2) and centrifuged at 143,000g for 3 h at 4°C. Following centrifugation, the bottom of the centrifuge tube was punctured with a needle, and the sucrose density gradient was displaced upward (2 mL/min) through a spectrophotometer using Fluorinert (Isco, Lincoln, NE). Optical density at 254 nm was continuously recorded (chart speed = 150 cm/h). Two fractions representing the subpolysomal and polysomal portions of each gradient were collected directly into an equal volume of TRIzol reagent. RNA was extracted from each fraction according to the manufacturer’s protocol and resuspended in RNA Storage Solution. Relative abundance of mRNA of previously described targets of diabetes were assessed in subpolysomal and polysomal fractions using the 7900HT Sequence Detection System (Applied Biosystems). Ubiquitin was used as the endogenous control because ubiquitin levels were determined to be unchanged in an absolute quantification experiment.
Western Blotting, Activity Assay, and Immunoprecipitation
Retinas were homogenized by sonication in mTOR buffer (from K-LISA mTOR assay). Retinal lysates were used for immunoblot analysis as previously described (26) using the following antibodies: phospho-PKC-α from Millipore (Billerica, MA) and PKC-α, raptor, rictor, mTOR, phospho-mTOR (Ser2481), 4E-BP1, phospho-4E-BP1 (Thr37/46), S6 ribosomal protein, phospho-S6 (Ser235/236), and phospho-S6 (Ser240/244) from Cell Signaling (Boston, MA). Results were normalized by reprobing the same membranes using an antibody against β-actin.
For mTOR activity analysis, retinas were homogenized as stated before subsequent analysis of total mTOR activity using the K-LISA mTOR (Recombinant) Activity Assay (EMD Millipore) according to the manufacturer’s instructions. The assay uses a p70S6K-GST fusion protein that contains amino acids 322–425 of p70S6K as substrate. This portion of the p70S6K protein is phosphorylated by mTOR in both TORC1 and TORC2 complexes (27).
For immunoprecipitation, 500 μg of total retinal lysate (prepared as for immunoblotting) were incubated for 2 h at 4°C with a selected mouse monoclonal antibody targeting rictor or raptor (Santa Cruz). After three washes, protein G sepharose beads were added and incubated for 1 h at 4°C. The immune complex was then collected by centrifugation at 1,500g and washed three times with ice-cold buffer. Immunoblots were then performed to probe for pS2481-mTOR, total mTOR, raptor, and/or rictor.
Statistical Analysis
ANOVA models with heterogeneous variances, adjusted for the replication of the experiment, were fit to the data to assess differences among control, diabetic, and treated animals. The mean ± SEM and statistically significant differences are reported. Analyses were performed using nonrepeated-measures ANOVA followed by the Student-Newman-Keuls test for multiple comparisons or t test for a single comparison.
Results
Diabetes Inhibits Retinal Protein Synthesis Rates in Mice and Rats, and the Effect Is Reversed by Systemic Insulin Treatment
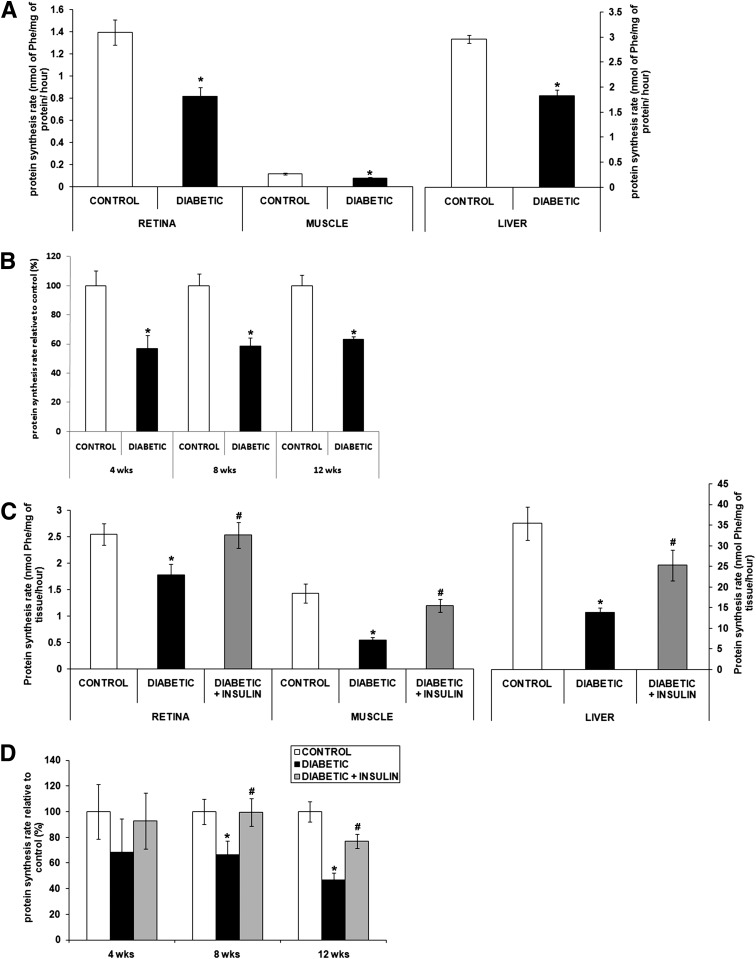
Insulin deficiency in type 1 diabetes diminishes protein synthesis rates in insulin-responsive tissues, including muscle and liver, but the effect on protein synthesis in the retina is not known. Therefore, we compared protein synthesis rates in liver, skeletal muscle, and retina under normal and diabetic conditions using the Ins2Akita mouse and STZ rat models of type 1 diabetes (Fig. 1). The flooding dose of phenylalanine method was used because it is well standardized and facilitates comparison of protein synthesis rates in multiple tissues of the same animal and in response to systemic manipulations. Details of the systemic metabolic parameters are provided in Table 1. Within 15 min after administration of a flooding dose of phenylalanine, the specific radioactivity of phenylalanine was equilibrated among the blood, liver, skeletal muscle, and retina precursor pools in control (0.134 ± 0.019 disintegrations per min [DPM]/μmol phenylalanine), diabetic (0.133 ± 0.016 DPM/μmol phenylalanine), and insulin-treated diabetic animals (0.134 ± 0.011 DPM/μmol of phenylalanine). These data indicate equivalent tissue uptake of the tracer independent of disease or treatment condition. Consistent with a higher basal Akt activity in normal retina than in gastrocnemius muscle (14), retinal tissue exhibited a basal rate of protein synthesis that was greater than gastrocnemius muscle. Also consistent with prior studies (7), liver and skeletal muscle protein synthesis rates were reduced by 38% and 33%, respectively, in diabetic Ins2Akita mice (Fig. 1A). The same method showed that these mice had a similar 44% reduction of retinal protein synthesis at 4 weeks after diabetes onset that persisted over the next 8 weeks (Fig. 1B).
Figure 1.
Diabetes disrupts retinal protein synthesis in diabetic rats and mice. The in vivo flooding dose of phenylalanine method was used to measure protein synthesis rate in retina, muscle, and liver from Ins2Akita diabetic mice and littermate controls (A and B) and from STZ-induced diabetic rats treated or not with systemic insulin and age-matched controls (C and D). Graphic representation of the decreased protein synthesis rate in the Ins2Akita diabetic mice (A) and its reversal by systemic insulin administration measured in retina, gastrocnemius muscle, and liver after 8 weeks of diabetes in STZ-induced rats (C). Graphic representation of the time course study of the retinal protein synthesis rate in Ins2Akita diabetic mice (B) and STZ-diabetic rats (D) relative to control (n = 8/group). *Significantly different from control (P < 0.05). #Significantly different from diabetic (P < 0.05). Phe, phenylalanine.
The same method was also used to study protein synthesis in STZ-induced diabetic rats. In this model, 8 weeks of diabetes reduced the protein synthesis rate in skeletal muscle and liver by 61% and 63%, respectively, and that in the retina by 33% (Fig. 1C). Retinal protein synthesis rates declined by 52% after 12 weeks of diabetes (Fig. 1D). These results demonstrate conserved impairment of retinal protein synthesis in two models of insulin-deficient diabetes. To determine whether systemic insulin therapy could restore retinal protein synthesis, diabetic rats were implanted with slow-release insulin implants as previously described (19). In keeping with previous results (7), this treatment restored protein synthesis rates in both liver and muscle of STZ-diabetic rats (Fig. 1C). Insulin therapy also completely reversed the defect in retinal protein synthesis after 8 weeks of diabetes (Fig. 1C) but only partially after 12 weeks (Fig. 1D). These results demonstrate that diabetes has a negative impact on protein synthesis similar to that in muscle, liver, and retina and that insulin replacement is able to correct the deficit in all three tissues.
Diabetes Diminishes Retinal Protein Degradation Without Affecting Total Content of RNA or Ribosomal RNA or Polysomal Aggregation
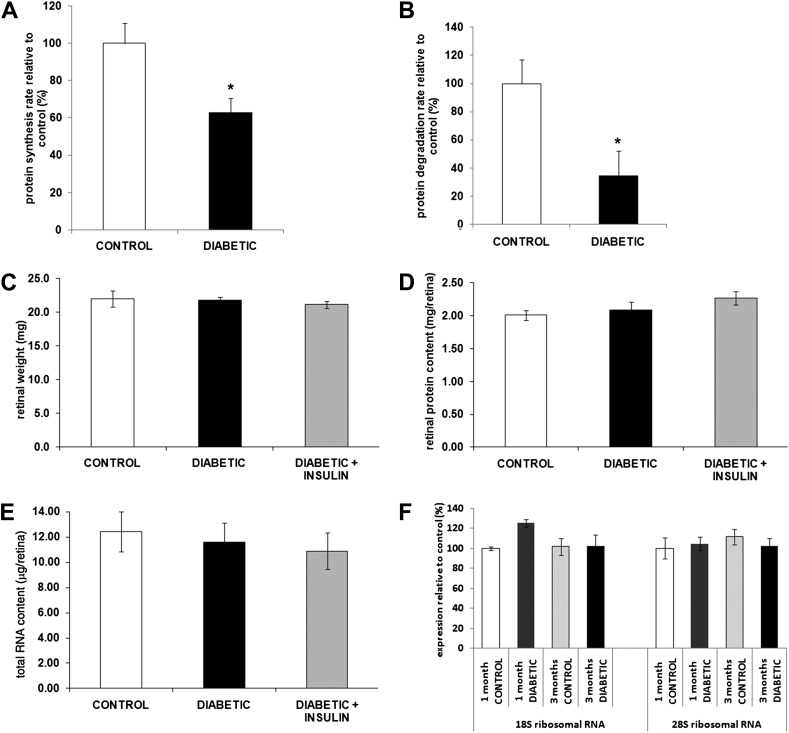
Diabetes elevates protein degradation and reduces protein synthesis in skeletal muscle, leading to the net loss of lean body mass and protein content (28). Unlike muscle, neuronal tissue is not depleted during catabolic states; that is, brain and nerve function is preserved until later stages of starvation. In untreated diabetic rodents, retinal ganglion cell and inner plexiform layers undergo minor, but progressive decreases in total retinal thickness (19). However, the one-third reduction in the rate of retinal protein synthesis does not correlate with a comparable net loss of retinal protein mass, which could reflect concomitant changes in protein degradation. To test this, retinal protein synthesis and degradation rates were estimated in ex vivo retinas from normal and diabetic rats by following radiolabeled methionine incorporation and radiolabel loss in pulse-chase measurements, respectively. Methionine incorporation into retinal protein showed that the rate of protein synthesis was reduced 39% in 12-week diabetic rats relative to retina from normal control rats (Fig. 2A), which is similar to what was observed with the flooding dose of phenylalanine method in vivo (Fig. 1D). Of note, pulse-chase analysis indicated that protein degradation was markedly reduced by 66% in retina from diabetic mice (Fig. 2B). Thus, the deficit in the protein synthetic rate was compensated by a reduction of comparable magnitude in protein degradation. As expected, analysis of retinal total mass and protein content in normal and 12-week diabetic rats and diabetic rats treated with insulin showed no significant differences (Fig. 2C and D).
Figure 2.
Diabetes reduces retinal protein synthesis and degradation independent of retinal weight, total protein, total RNA, and ribosomal RNA content. Retinas from 3-month diabetic STZ rats treated or not with systemic insulin and age-matched controls were harvested for assessment of the impact of diabetes on retinal protein synthesis (A), degradation rate (B), and composition (C–F). Diabetes reduced retinal protein synthesis (A) and retinal degradation (B) as measured by the pulse-chase method without affecting retinal wet weight (C), protein content (D), total RNA content (E), and ribosomal RNA content (F) (n ≥ 20/group). *Significantly different from control.
In skeletal muscle and liver, the diabetes-associated impairment in protein synthesis is accompanied by a reduction in total RNA and ribosomal RNA content (11,29). To determine whether this was the case for retinal tissue during diabetes, retinal total RNA and 18S and 28S ribosomal RNA contents were compared in normal and diabetic rats. No significant differences were observed in total RNA levels in normal and 12-week diabetic rats or 12-week diabetic rats treated with insulin (Fig. 2E). In addition, ribosomal RNA content in retinas of normal and diabetic rats was not significantly different after either 1 or 3 months of diabetes (Fig. 2F). Thus, in contrast to skeletal muscle and liver, in retina, the diabetes-induced reduction in protein synthesis was not a result of a commensurate fall in RNA levels.
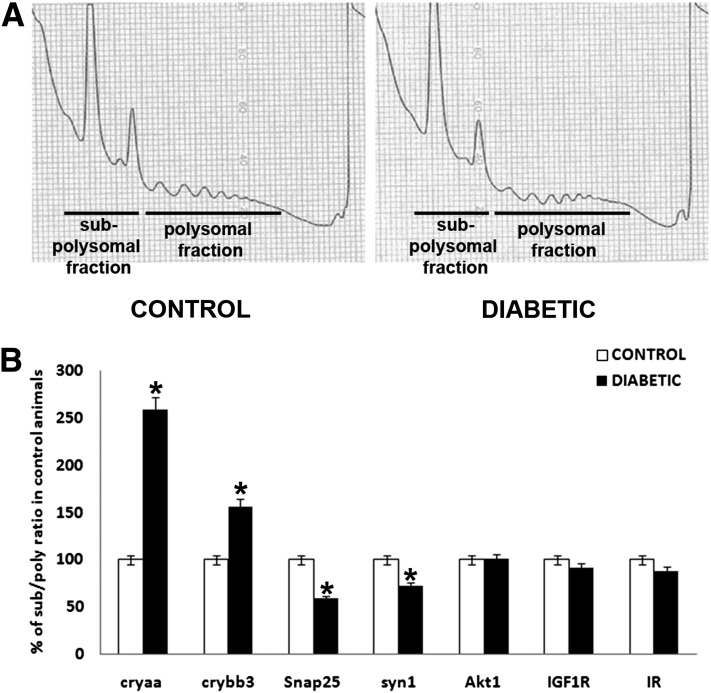
To further confirm the diabetes-induced reduction in translational efficiency, polysome profiles were obtained from the retina of normal and 12-week diabetic rats. Figure 3A shows representative images of the polysome profiles for both groups with no significant changes, suggesting that the overall reduction of protein synthesis was due to impairment in both peptide chain initiation and elongation.
Figure 3.
Diabetes reduces retinal protein synthesis through an impairment in both peptide chain initiation and elongation while affecting specific mRNA translation rates. The impact of diabetes on polysome profiles and RNA pools of the subpolysomal and polysomal fractions was also analyzed. Representative polysome profiles are presented and show that translation efficiency is due to impairment in both peptide chain initiation and elongation in the retina during diabetes (A). RNA isolated from both subpolysomal and polysomal fractions was analyzed by quantitative RT-PCR and demonstrated the decreased translation of α-A-crystallin (cryaa) and β-B3-crystallin (crybb3) mRNA and increased translation of synaptic genes Snap25 and synapsin 1 (syn1) during diabetes (B). *Significantly different from control.
Diabetes Decreases Synthesis of Specific Retinal Proteins, Including Synaptic Proteins
To determine whether diabetes differentially affected the synthesis of specific proteins, subpolysomal and polysomal fractions were recovered to determine the relative distribution of several mRNA species in retinas from normal and 12-week diabetic rats. Of note, analysis of mRNA content within the subpolysomal and polysomal fractions showed that diabetes altered the distribution of specific mRNAs differently. In particular, α-A-crystallin and β-B3-crystallin mRNAs were shifted into the polysome fraction and the Snap25 and synaptophysin 1 synaptic mRNAs were shifted into the subpolysomal fraction, whereas Akt, INSR, and Igf1r mRNAs remained unchanged (Fig. 3B). These data parallel increased α-A-crystallin and β-B3-crystallin protein contents (30) and decreased Snap25 and synaptophysin 1 protein contents (31) observed in diabetic rat retinas examined after 12 weeks of diabetes. Thus, it appears that although diabetes reduces global rates of protein synthesis in the retina and other insulin-sensitive tissues, the translation of all retinal mRNAs was not affected equally. Moreover, the translation of certain mRNAs was increased (i.e., synaptic proteins) rather than reduced in the retina of diabetic compared with control animals. The changes in synthesis of selective retinal proteins contrast with the general loss of protein reported in other insulin-sensitive tissues.
Systemic Glycemic Control and Ocular Insulin Receptor Signaling Influence Retinal Protein Synthesis and Degradation
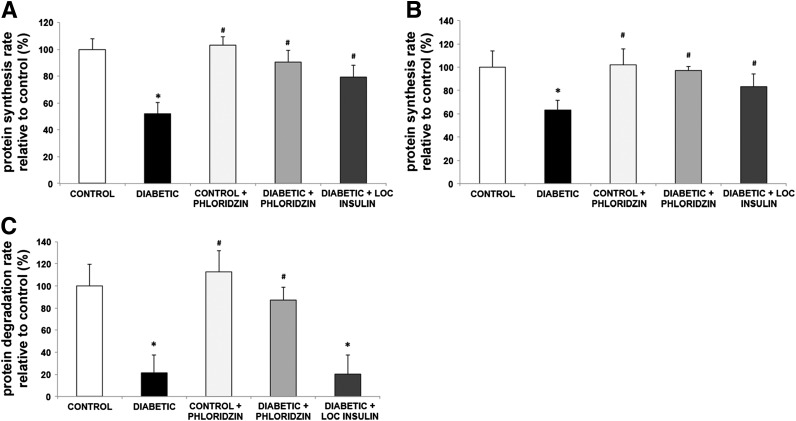
We showed previously that both reduced ocular insulin receptor signaling and systemic hyperglycemia play critical roles in the regulation of retinal cell death (16). Diabetes affects retinal protein synthesis and degradation, and systemic insulin administration partially reverses these metabolic changes, so we examined the respective contribution of local insulin signaling and systemic glucose normalization on these aspects of retinal metabolism. Sole activation of local insulin signaling by subconjunctival insulin administration or systemic glycemic normalization by phloridzin treatment partially reversed the diabetes-induced reduction in retinal protein synthesis measured in vivo with the flooding dose of phenylalanine method (Fig. 4A) and ex vivo with radiolabeled methionine incorporation (Fig. 4B). Of note, phloridzin treatment restored the protein degradation rate in the retinas of 12-week diabetic rats, whereas periocular insulin administration had no effect (Fig. 4C). These findings underscore the importance of excess nutrient concentrations in the retina in the context of insulin deficiency.
Figure 4.
Blood glucose normalization reverses both retinal protein synthesis and retinal protein degradation induced by diabetes, whereas local insulin only reverses the reduction in protein synthesis. Rats with 3 months of diabetes were treated by administration of phloridzin twice daily or insulin subconjunctivally once daily for the last 4 days. Protein synthesis rate was then measured using the flooding dose of phenylalanine method, and results show that both treatments partially reverse the protein synthesis decrease induced by diabetes (A). Similarly, retinas from diabetic and age-matched control rats treated with either phloridzin twice daily or insulin subconjunctivally once daily for the last 4 days were harvested and incubated ex vivo with radiolabeled methionine to measure protein synthesis rate (B) followed by pulse-chase method to measure protein degradation rate (C). Phloridzin treatment for 4 days reversed both protein synthesis and protein degradation rates, whereas ocular insulin only reversed the protein synthesis defects induced by diabetes (n ≥ 8/group). *Significantly different from control (P < 0.05). #Significantly different from diabetic (P < 0.05). LOC, local.
Diabetes-Induced Reduction in Retinal Protein Synthesis Is Associated With Diminished mTORC2 Activity
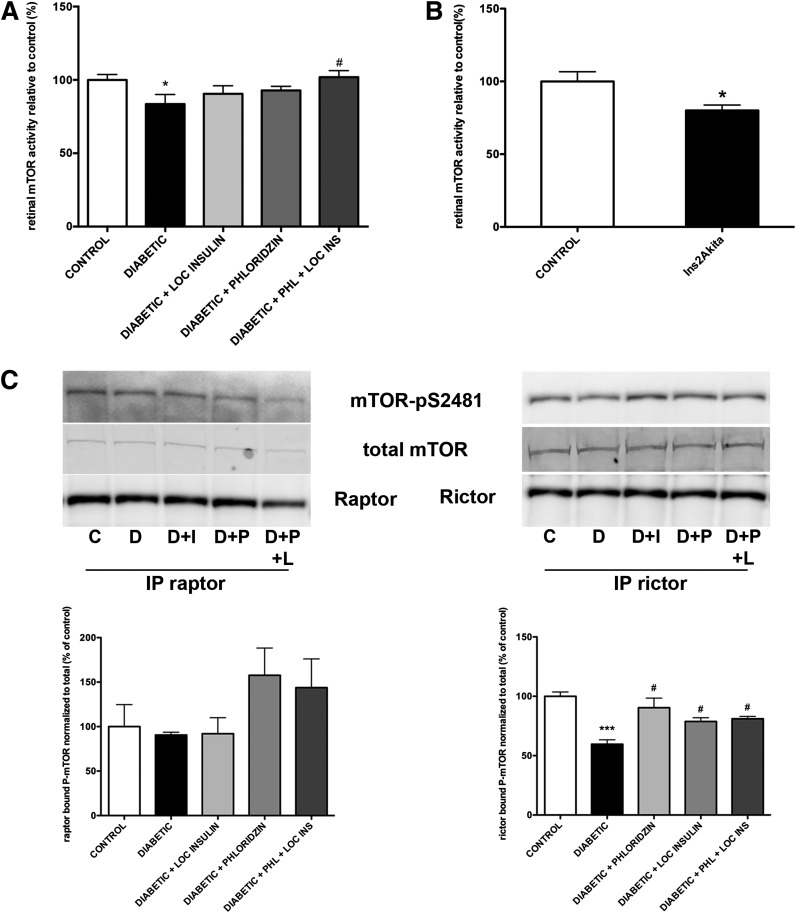
The Akt/mTORC1 pathway controls protein translation in insulin-sensitive tissues, including skeletal muscle and liver. Diabetes reduces liver and skeletal muscle protein synthesis through reduction of Akt2 activity and subsequent reduction of mTORC1 activity, as demonstrated by reduced phosphorylation of p70S6K, 4E-BP1, and S6 ribosomal protein (32). In the retina, diabetes reduces Akt1, Akt3, and p70S6K activity (15). Thus, we hypothesized that the diabetes-induced reduction in retinal protein synthesis reflects diminished mTORC1 complex activity. By using an assay that does not discriminate between mTORC1 and mTORC2, we found that diabetes significantly reduced retinal total mTOR activity by 16% in diabetic rats (Fig. 5A) and 20% in Ins2Akita mice (Fig. 5B). Phloridzin and periocular insulin treatment both partially restored retinal mTOR activity, whereas combined treatments completely restored it.
Figure 5.
Diabetes reduces total retinal mTOR activity and mTORC2 associated mTOR phosphorylation. Rats with 3 months of diabetes were treated with either phloridzin twice daily or insulin subconjunctivally once daily for the last 4 days. Retinal mTOR activity was then measured, which demonstrated that mTOR activity was significantly reduced in the retina during diabetes and was reversed by the combined treatment (A). Retinal mTOR activity was also measured in 3-month diabetic Ins2Akita mice and demonstrated a similar reduction (B) (n ≥ 8/group). *Significantly different from control (P < 0.05). #Significantly different from diabetic (P < 0.05). Specific activity of the mTORC1 and mTORC2 complexes was assessed by immunoprecipitation of rictor and raptor followed by assessment of the bound mTOR activity using an antibody against pS2481 (C). Diabetes did not reduce raptor-bound mTOR phosphorylation, but it significantly reduced rictor-bound mTOR phosphorylation. This reduction was reversed by both phloridzin and local insulin treatment. C, control; D, diabetic; D+I, diabetic + insulin; D+P, diabetic + phloridzin; D+P+L, diabetic + phloridzin + local insulin; IP, immunoprecipitation. ***Significantly different from control (P < 0.001); #Significantly different from diabetic (P < 0.05).
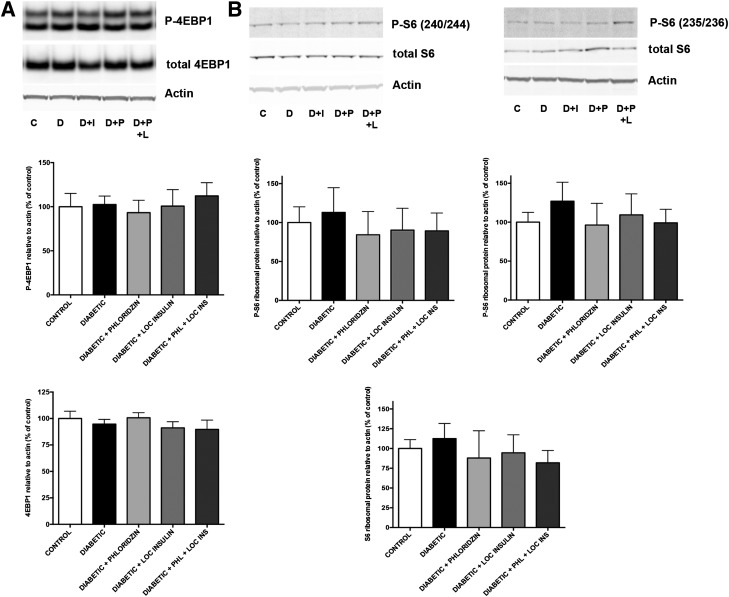
We next tested the specific impact of diabetes on the activity of the two distinct mTOR complexes mTORC1 and mTORC2. Contrary to other insulin-sensitive tissues, diabetes did not reduce serine 2481 phosphorylation of mTOR bound to raptor, suggesting an absence of effect on mTORC1 activity (Fig. 5C). Of note, diabetes reduced by 40% the phosphorylation of mTOR bound to rictor, suggesting a reduction in mTORC2 activity (Fig. 5C). Consistent with the protein synthesis restoration, phloridzin and local insulin administration restored rictor-bound mTOR phosphorylation. To further establish the specificity of the impact of diabetes on mTORC1 and mTORC2 activities, we analyzed the levels of expression and phosphorylation of downstream effectors of each pathway. Indicators of mTORC1 activity, 4E-BP1 and its phosphorylation on threonines 37 and 46 (Fig. 6A) as well as S6 ribosomal protein and its phosphorylation on serines 240/244 and serines 235/236 (Fig. 6B), were unaffected by diabetes. Similarly, no changes in S6K1 level of expression or phosphorylation on threonine 389 were detected in retinas from diabetic animals (data not shown). The combination of these results strongly supports the absence of expected perturbation of the retinal mTORC1 complex during diabetes (Fig. 5C and D). In skeletal muscle, mTOR also modulates protein synthesis through regulation of translation initiation factor levels and phosphorylation, including eIF2B (33). Thus, we tested the impact of diabetes on eIF2B levels and phosphorylation, and consistent with the lack of effect of diabetes on S6K1 and 4E-BP1, no changes in either eIF2B expression or phosphorylation levels were observed in retinal lysates from Ins2Akita diabetic mice compared with littermates controls (data not shown).
Figure 6.
Diabetes does not affect the expression and phosphorylation of downstream targets of mTORC1. Rats with 3 months of diabetes were treated with either phloridzin twice daily or insulin subconjunctivally once daily for the last 4 days. Analysis of the levels of expression of 4E-BP1 and its phosphorylation on threonines 37 and 46 (A) and S6 ribosomal protein and its phosphorylation on serines 240/244 and serines 235/236 (B), downstream targets of TORC1, showed that neither was reduced by diabetes (n ≥ 5/group). C, control; D, diabetic; D+I, diabetic + insulin; D+P, diabetic + phloridzin; D+P+L, diabetic + phloridzin + local insulin.
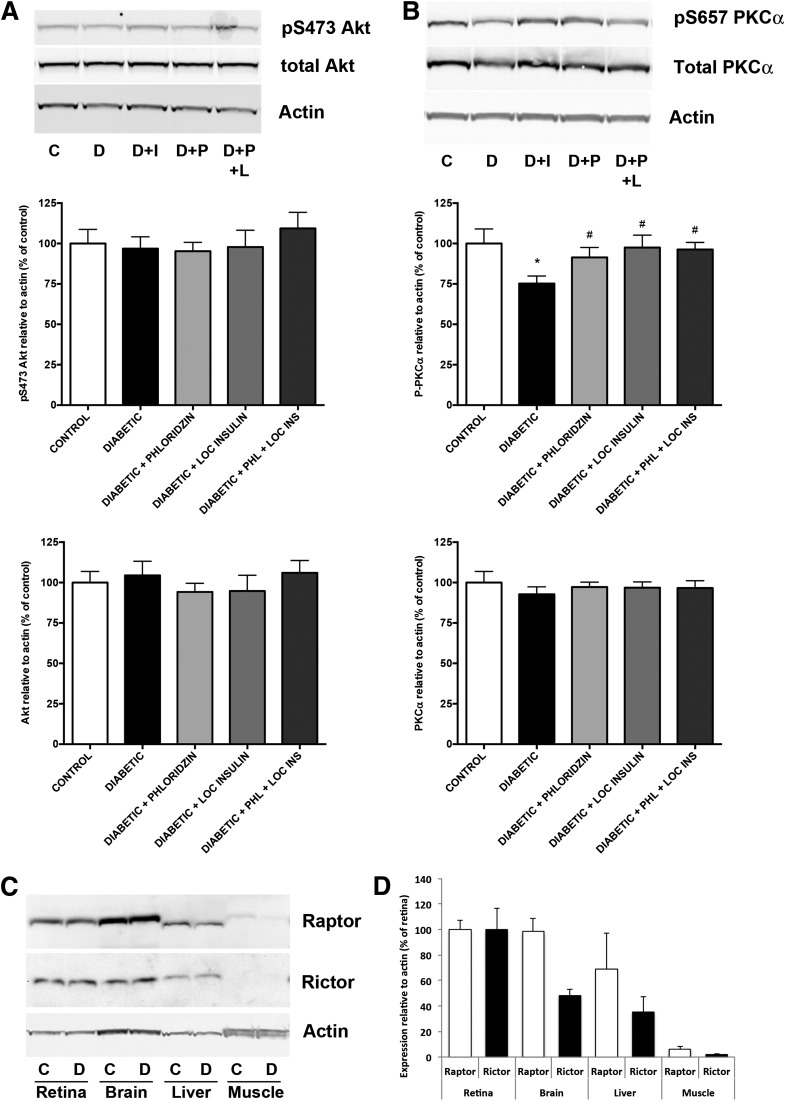
Although the role of mTORC2 in the regulation of protein synthesis is not as clearly defined as for mTORC1, conditional deletion of rictor leads to small cerebellar Purkinje neurons independent of mTORC1 (34), and both mTORC1 and mTORC2 affect the dendritic arbor morphology in hippocampal neurons (35). As we previously reported (15), Akt1 serine 473 phosphorylation was not reduced by diabetes in the retina (Fig. 7A), suggesting that either a kinase other than PDK1 or mTORC2 may be involved in the regulation of this site in the retina or that alteration of phosphatase activity might prevent its dephosphorylation and compensate for decreased mTORC2 activity. We also analyzed the expression and phosphorylation of PKC-α, a known downstream target of mTORC2, and showed that PKC-α phosphorylation on serine 657 was reduced by 25% in diabetic retinal samples (Fig. 7B) consistent with reduced mTORC2 complex activity. The differential regulation of the mTOR/Akt pathway in the retina might reflect the differential ratio of raptor and rictor expressed in the retina. Indeed, the basal level of expression of rictor is higher in retina than in liver and muscle. Comparatively, the levels of expression of raptor are more consistent, at least across the retina, brain, and liver (Fig. 7C and D). Taken together, this reflects a different, if not more prominent, role of mTORC2 in the retina. Collectively, these data reveal the S6K1/4E-BP1 pathway-independent alteration of retinal protein synthesis by diabetes, and the associated specific reduction in mTORC2 signaling.
Figure 7.
Diabetes affects a downstream target of mTORC2, the predominant TOR-associated complex in the retina. Rats with 3 months of diabetes were treated with either phloridzin twice daily or insulin subconjunctivally once daily for the last 4 days. Analysis of the level of serine 473 phosphorylation of Akt (A) and PKC-α (B), downstream targets of TORC2, showed that PKC-α was reduced by diabetes, and this effect is reversed by both local insulin and systemic phloridzin treatment (n ≥ 5/group). *Significantly different from control (P < 0.001). #Significantly different from diabetic (P < 0.05). C, control; D, diabetic; D+I, diabetic + insulin; D+P, diabetic + phloridzin; D+P+L, diabetic + phloridzin + local insulin. Further analysis of TORC complexes showed that the retina has a comparable level of expression to raptor but a higher level of expression of rictor compared with other insulin-sensitive tissues, including brain, liver, and skeletal muscle. A representative picture (C) is shown as well as a graphical representation of the quantification (D) (n = 4/group).
Discussion
The goal of the current study was to investigate the metabolic basis of the early stages of diabetic retinopathy based on the following observations: 1) the retina has a high metabolic activity despite relative hypoxia in the inner retina, and 2) diabetes rapidly reduces high basal insulin receptor and Akt activity and increases the rate of neuronal cell death. Specifically, we used in vivo and ex vivo approaches to test the hypothesis that diabetes impairs protein synthesis of the postmitotic adult retina specifically through alteration of the insulin receptor/Akt/mTOR axis. The data reveal that 1) diabetes reduces retinal protein synthesis (i.e., translational efficiency) and protein degradation in a targeted manner, without loss of ribosomal RNA content (i.e., translational capacity); 2) diabetes specifically alters the Akt/mTORC2 pathway without affecting the mTORC1/S6K1/4E-BP1 pathway, which regulates protein synthesis in skeletal muscle; and 3) periocular administration of insulin and systemic reduction of plasma glucose with phloridzin restores biosynthetic activity, but only the latter restores the protein degradation levels. Therefore, this study shows the unique pattern of alteration of the Akt/mTOR pathway in the retina under diabetic conditions and its association with essential anabolic activity.
Earlier studies demonstrated that diabetes rapidly alters retinal protein synthesis in alloxan-treated rabbits (36,37). These studies demonstrated a potential commonality between the mechanisms leading to the various complications of diabetes; however, the current study demonstrates significant differences in the mechanisms involved. Protein synthesis is affected in skeletal muscle, liver, and retina, but retinal protein content and total mass do not change over time, unlike in the other two insulin-sensitive tissues. The reason for this difference may be related to the role and function of these tissues. First, skeletal muscle and liver are energy reservoirs that accommodate variable nutrient requirements for organs that do not store energy substrates (i.e., brain, retina). This aspect of their function is reflected by their capacity to undergo wide mass variations, including progressive loss of mass during uncontrolled diabetes. Second, muscle and liver are acutely sensitive to insulin action, whereas the retina is not affected by fluctuating plasma insulin levels associated with feeding and fasting (14). This difference might be due to retinal function requiring both high metabolic activity and tissue stability for proper visual function. Common signaling pathways regulate both cell survival and biosynthesis (15,38), so loss of protein synthesis is part of a multifaceted disruption of anabolism that results in cell death when adaptive mechanisms fail. This process likely begins in persons with diabetes when the retina appears normal but subtle signs of retinal sensory impairment, such as reduced electroretinographic responses and visual field sensitivity, become manifest (39,40). On a pathophysiologic level, diabetes leads to loss of axons (41) and cotton wool spots that indicate reduced retinal axonal transport (42).
The present data suggest that reversal of diabetes-induced protein synthesis deficits by periocular insulin and phloridzin treatment are independent of the amino acid imbalance occurring in diabetes and consistent with the absence of effect on mTORC1 activity, which is regulated by amino acid availability (43). The importance of understanding the regulation of retinal biosynthetic pathways extends to a variety of blinding disorders, including diabetic retinopathy and retinitis pigmentosa (rod–cone degeneration), conditions for which satisfactory treatments are lacking (1,44). Protein synthesis in muscle or liver is differentially affected by insulin, with clear tissue- and protein-specific effects (45). In retinitis pigmentosa, mutations in visual cycle genes lead to blindness, but stimulation of mTOR signaling with insulin can extend photoreceptor viability (46). The present data suggest that in the retina, perturbations of mTORC2 rather than mTORC1 could be related to the reduction in protein synthesis under diabetic conditions. Studies using novel mTOR inhibitors in renal cell carcinoma and mouse embryonic fibroblast cells have suggested a potential role for mTORC2 in protein synthesis regulation (47,48); however, in both studies, the inhibitors used did not allow complete discrimination between the two mTOR complexes, whereas in the present study, only mTORC2 activity was altered in parallel with reduced protein synthesis while the S6K1/4E-BP1 pathway remained unaffected. Of note, specific deletion of rictor in the central nervous system leads to a subtle decrease in cell size, an effect previously seen in Akt3 knockout mice that correlates with the present data and could be attributed to a decrease in protein translation (34,49).
This work clearly illustrates both the similarities and specificities of the mechanisms of diabetic retinopathy regarding the systemic metabolic perturbations and complications associated with diabetes. Although diabetes alters the metabolism of every organ, the mechanisms shown here by which it affects retinal function are tissue specific. Additional work is needed to more clearly define cell-specific alterations, but the current study provides new insights into the regulation of protein homeostasis required for adaptation to injury (50). Prolonged impairment of protein synthesis and degradation may limit the ability of the retina to adapt to injury and result in retinal degeneration, and maintenance of retinal anabolic pathways may help to sustain retinal function.
Article Information
Acknowledgments. The authors thank Diane Fingar of the Cellular and Developmental Biology department of the University of Michigan for helpful guidance on the analysis of mTORC1 and mTORC2.
Funding. This project and its authors were supported by National Institutes of Health grants EY-020895 (to P.E.F.), DK-13499 and DK-15658 (to L.S.J. and S.R.K.), EY-20582 (to S.F.A. and T.W.G.), and DK-094292 (to T.W.G.); the A. Alfred Taubman Medical Research Institute (to T.W.G.); a Research to Prevent Blindness Physician-Scientist Award (to T.W.G.); and American Diabetes Association grant 1-04-RA-31 (to T.W.G.). This work used core services supported by National Institutes of Health grant DK-097153 to the University of Michigan as well as the Core Center for Vision Research funded by EY-007003 from the National Eye Institute.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.E.F. contributed to the data collection and analysis and writing of the manuscript. M.K.L. contributed to the data collection and editing of the manuscript. S.P. contributed to the data analysis and editing of the manuscript. L.S.J., S.R.K., S.F.A., and T.W.G. contributed to obtaining the funding, the data analysis, and editing of the manuscript. T.W.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med 2012;366:1227–1239 [DOI] [PubMed] [Google Scholar]
- 2.Tesfaye S, Boulton AJ, Dyck PJ, et al. Toronto Diabetic Neuropathy Expert Group Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonetti DA, Barber AJ, Bronson SK, et al. JDRF Diabetic Retinopathy Center Group Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 2006;55:2401–2411 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed J, Braun RD, Dunn R, Jr, Linsenmeier RA. Oxygen distribution in the macaque retina. Invest Ophthalmol Vis Sci 1993;34:516–521 [PubMed] [Google Scholar]
- 5.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 2003;121:547–557 [DOI] [PubMed] [Google Scholar]
- 6.Dice JF, Walker CD, Byrne B, Cardiel A. General characteristics of protein degradation in diabetes and starvation. Proc Natl Acad Sci U S A 1978;75:2093–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pain VM, Garlick PJ. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem 1974;249:4510–4514 [PubMed] [Google Scholar]
- 8.Jaleel A, Klaus KA, Morse DM, et al. Differential effects of insulin deprivation and systemic insulin treatment on plasma protein synthesis in type 1 diabetic people. Am J Physiol Endocrinol Metab 2009;297:E889–E897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olbricht CJ, Geissinger B. Renal hypertrophy in streptozotocin diabetic rats: role of proteolytic lysosomal enzymes. Kidney Int 1992;41:966–972 [DOI] [PubMed] [Google Scholar]
- 10.Millward DJ, Garlick PJ, James WP, Nnanyelugo DO, Ryatt JS. Relationship between protein synthesis and RNA content in skeletal muscle. Nature 1973;241:204–205 [DOI] [PubMed] [Google Scholar]
- 11.Jefferson LS, Liao WS, Peavy DE, Miller TB, Appel MC, Taylor JM. Diabetes-induced alterations in liver protein synthesis. Changes in the relative abundance of mRNAs for albumin and other plasma proteins. J Biol Chem 1983;258:1369–1375 [PubMed] [Google Scholar]
- 12.McNurlan MA, Garlick PJ. Protein synthesis in liver and small intestine in protein deprivation and diabetes. Am J Physiol 1981;241:E238–E245 [DOI] [PubMed] [Google Scholar]
- 13.Rapley J, Oshiro N, Ortiz-Vega S, Avruch J. The mechanism of insulin-stimulated 4E-BP protein binding to mammalian target of rapamycin (mTOR) complex 1 and its contribution to mTOR complex 1 signaling. J Biol Chem 2011;286:38043–38053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiter CE, Sandirasegarane L, Wolpert EB, et al. Characterization of insulin signaling in rat retina in vivo and ex vivo. Am J Physiol Endocrinol Metab 2003;285:E763–E774 [DOI] [PubMed] [Google Scholar]
- 15.Reiter CE, Wu X, Sandirasegarane L, et al. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes 2006;55:1148–1156 [DOI] [PubMed] [Google Scholar]
- 16.Fort PE, Losiewicz MK, Reiter CE, et al. Differential roles of hyperglycemia and hypoinsulinemia in diabetes induced retinal cell death: evidence for retinal insulin resistance. PLoS One 2011;6:e26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faridi J, Fawcett J, Wang L, Roth RA. Akt promotes increased mammalian cell size by stimulating protein synthesis and inhibiting protein degradation. Am J Physiol Endocrinol Metab 2003;285:E964–E972 [DOI] [PubMed] [Google Scholar]
- 18.Lee YR, Park J, Yu HN, Kim JS, Youn HJ, Jung SH. Up-regulation of PI3K/Akt signaling by 17beta-estradiol through activation of estrogen receptor-alpha, but not estrogen receptor-beta, and stimulates cell growth in breast cancer cells. Biochem Biophys Res Commun 2005;336:1221–1226 [DOI] [PubMed] [Google Scholar]
- 19.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 1998;102:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW, Penn State Retina Research Group Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes 1998;47:1953–1959 [DOI] [PubMed] [Google Scholar]
- 21.Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci 2004;45:3330–3336 [DOI] [PubMed] [Google Scholar]
- 22.Park SH, Park JW, Park SJ, et al. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia 2003;46:1260–1268 [DOI] [PubMed] [Google Scholar]
- 23.Reiter CE, Gardner TW. Functions of insulin and insulin receptor signaling in retina: possible implications for diabetic retinopathy. Prog Retin Eye Res 2003;22:545–562 [DOI] [PubMed] [Google Scholar]
- 24.Farrell PA, Fedele MJ, Vary TC, Kimball SR, Lang CH, Jefferson LS. Regulation of protein synthesis after acute resistance exercise in diabetic rats. Am J Physiol 1999;276:E721–E727 [DOI] [PubMed] [Google Scholar]
- 25.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J 1980;192:719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Losiewicz MK, Fort PE. Diabetes impairs the neuroprotective properties of retinal alpha-crystallins. Invest Ophthalmol Vis Sci 2011;52:5034–5042 [DOI] [PubMed] [Google Scholar]
- 27.Ali SM, Sabatini DM. Structure of S6 kinase 1 determines whether raptor-mTOR or rictor-mTOR phosphorylates its hydrophobic motif site. J Biol Chem 2005;280:19445–19448. [DOI] [PubMed]
- 28.Rodríguez T, Busquets S, Alvarez B, et al. Protein turnover in skeletal muscle of the diabetic rat: activation of ubiquitin-dependent proteolysis. Int J Mol Med 1998;1:971–977 [DOI] [PubMed] [Google Scholar]
- 29.Kent JD, Kimball SR, Jefferson LS. Effect of diabetes and insulin treatment of diabetic rats on total RNA, poly(A)+ RNA, and mRNA in skeletal muscle. Am J Physiol 1991;260:C409–C416 [DOI] [PubMed] [Google Scholar]
- 30.Fort PE, Freeman WM, Losiewicz MK, Singh RS, Gardner TW. The retinal proteome in experimental diabetic retinopathy: up-regulation of crystallins and reversal by systemic and periocular insulin. Mol Cell Proteomics 2009;8:767–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanGuilder HD, Brucklacher RM, Patel K, Ellis RW, Freeman WM, Barber AJ. Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur J Neurosci 2008;28:1–11 [DOI] [PubMed] [Google Scholar]
- 32.Anthony JC, Reiter AK, Anthony TG, et al. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes 2002;51:928–936 [DOI] [PubMed] [Google Scholar]
- 33.Kubica N, Crispino JL, Gallagher JW, Kimball SR, Jefferson LS. Activation of the mammalian target of rapamycin complex 1 is both necessary and sufficient to stimulate eukaryotic initiation factor 2Bvarepsilon mRNA translation and protein synthesis. Int J Biochem Cell Biol 2008;40:2522–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carson RP, Fu C, Winzenburger P, Ess KC. Deletion of rictor in neural progenitor cells reveals contributions of mTORC2 signaling to tuberous sclerosis complex. Hum Mol Genet 2013;22:140–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urbanska M, Gozdz A, Swiech LJ, Jaworski J. Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons. J Biol Chem 2012;287:30240–30256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chihara E. Impairment of protein synthesis in the retinal tissue in diabetic rabbits: secondary reduction of fast axonal transport. J Neurochem 1981;37:247–250 [DOI] [PubMed] [Google Scholar]
- 37.Chihara E, Sakugawa M, Entani S. Reduced protein synthesis in diabetic retina and secondary reduction of slow axonal transport. Brain Res 1982;250:363–366 [DOI] [PubMed] [Google Scholar]
- 38.Nakamura M, Barber AJ, Antonetti DA, et al. Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J Biol Chem 2001;276:43748–43755 [DOI] [PubMed] [Google Scholar]
- 39.Bearse MA, Jr, Adams AJ, Han Y, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res 2006;25:425–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson GR, Scott IU, Quillen DA, Walter LE, Gardner TW. Inner retinal visual dysfunction is a sensitive marker of non-proliferative diabetic retinopathy. Br J Ophthalmol 2012;96:699–703 [DOI] [PubMed] [Google Scholar]
- 41.Chihara E, Matsuoka T, Ogura Y, Matsumura M. Retinal nerve fiber layer defect as an early manifestation of diabetic retinopathy. Ophthalmology 1993;100:1147–1151 [DOI] [PubMed] [Google Scholar]
- 42.McLeod D. Why cotton wool spots should not be regarded as retinal nerve fibre layer infarcts. Br J Ophthalmol 2005;89:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 1998;273:14484–14494 [DOI] [PubMed] [Google Scholar]
- 44.Stitt AW, Lois N, Medina RJ, Adamson P, Curtis TM. Advances in our understanding of diabetic retinopathy. Clin Sci (Lond) 2013;125:1–17 [DOI] [PubMed] [Google Scholar]
- 45.Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes 2001;50:2652–2658 [DOI] [PubMed] [Google Scholar]
- 46.Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci 2009;12:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldman ME, Apsel B, Uotila A, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 2009;7:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nayak BK, Feliers D, Sudarshan S, et al. Stabilization of HIF-2α through redox regulation of mTORC2 activation and initiation of mRNA translation. Oncogene 2013;32:3147–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Easton RM, Cho H, Roovers K, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol 2005;25:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verma P, Chierzi S, Codd A, et al. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci 2005;25:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]