Abstract
Background:
Considering the important role of pituitary gland in regulating various endocrine axes and its unique anatomical location, various postoperative complications can be anticipated resulting from surgery on pituitary tumors. We examined and categorized the immediate postoperative complications according to various tumor pathologies.
Materials and Methods:
We carried out a prospective study in 152 consecutive patients and noted various postoperative complications during neurosurgical intensive care unit stay (within 48 hrs of hospital stay) in patients undergoing transsphenoidal removal of pituitary tumors.
Results:
In our series, various groups showed different postoperative complications out of which, cerebrospinal fluid leak was the commonest followed by diabetes insipidus, postoperative nausea and vomiting, and hematoma at operation site.
Conclusion:
Various immediate postoperative complications can be anticipated in transsphenoidal pituitary surgery even though, it is considered to be relatively safe.
Keywords: Complications, pituitary tumors, postoperative, transsphenoidal surgery
INTRODUCTION
Postoperative complications are of major concern in patient with intracranial lesion because it leads to a significant increase in morbidity and mortality.[1] Although tumors of the pituitary gland represent approximately 10% of diagnosed brain neoplasm, the transsphenoidal resection of pituitary tumors may account for as much as 20% of all intracranial operations performed for primary brain tumors.[2] Considering the important role of pituitary gland in regulating various endocrine axes and its anatomical location, various postoperative complications can be anticipated resulting from surgery on pituitary tumors. As compared to craniotomy, transsphenoidal surgery offers the advantage of low morbidity and mortality, preservation of normal pituitary function, lower incidence of permanent diabetes insipidus, lesser trauma to the frontal lobes and optic chiasm, less blood loss and no external scar.[3]
Having in mind all these reason, there exists a distinct possibility that the rate of complications might be influenced by the tumor pathology. No prospective study has been conducted to date to unravel these aspects of pituitary tumor resection.[4,5] We, therefore, planned a prospective study with primary aim to note various postoperative complications during intensive care unit (ICU) stay (within 48 hrs of hospital stay) in patients undergoing transsphenoidal removal of pituitary tumors.
MATERIALS AND METHODS
After receiving approval from the institutional ethics committee (T-08/31.07.2009) and written informed consent, all patients of either sex, undergoing transsphenoidal surgery through sub-labial route for pituitary tumor excision, were enrolled for period of one and a half year (August 2009 to January 2011). Surgeons with more than 6 years of experience in transsphenoidal surgery were involved in the study. Pregnant patients were excluded from this study.
All patients were premedicated with glycopyrrolate 0.2 mg intramuscularly, 30 mins before shifting to operating room. Demographic data (age, weight and gender), type of pituitary tumor, and hormone profile were noted. In the operation theatre standard monitors were attached. General anesthesia was induced with fentanyl 2 μg/kg and propofol 2 mg/kg or thiopentone 3-5 mg /kg. Trachea was intubated with rocuronium 1 mg/kg. Depending upon the preoperative status of cortisol level, patients were supplemented with 100-mg dose of hydrocortisone intravenously at the time of induction. Anesthesia was maintained with either intravenous (propofol infusion) or inhalational (sevoflurane or isoflurane) agent per the choice of anesthesiologist in-charge. All patients received 60% nitrous oxide in oxygen. Major intraoperative complications such as bradycardia, hypertension, CSF leak, etc., were noted. After extubation, patients were shifted to neurosurgical ICU. Any complication occurring during 48 hours of stay in neurosurgical ICU were recorded, which we defined as ‘early postoperative complication’. Subsequently, patients were transferred to the ward and were followed until discharge from hospital. Tumors were grouped either macro-adenoma or micro-adenoma depending on diameter size on MRI imaging (macro-adenoma more than 10 mm and micro-adenoma less than 10 mm in any dimension). We categorized patients into five groups based on the histological type of tumor. These were growth hormone (GH) secreting adenoma, Cushing's disease, Prolactin-secreting adenoma, Non-functioning adenoma (NFPA) and pituitary apoplexy.
We looked for following postoperative complications:
Prolonged ventilation (more than 12 hours) — Patients who were extubated at the conclusion of surgery but required reintubation in ICU due to any reason were included in this category.
Postoperative nausea/vomiting (PONV) — Nausea and vomiting were recorded as single complication.
CSF leak — Evident CSF leakage from nose or in the posterior pharynx as complained by patient. Whenever CSF leak was observed during surgery, sella turcica as well as sphenoid sinus were packed with fat graft taken from thigh by the surgeon.
Meningitis — Signs of fever, deterioration of consciousness, positive CSF analysis with signs of meningismus
Diabetes insipidus (DI) — Defined as urine output greater than 300 mL/hour for more than 3 hours with a urine specific gravity of less than 1.002
Dyselectrolytemia — Serum Sodium > 150 or < 135, Serum Potassium > 5.5 or < 3.5 mmol/L
Deterioration/Loss of vision: This includes peripheral field cut or central diminution or complete loss of vision.
New cranial nerve deficit or palsy
Postoperative intracranial bleeding/hematoma at operation site grouped as single complication
Hydrocephalus: This includes gross distension of ventricles evident on postoperative CTscan.
STATISTIC ANALYSIS
Statistical analysis was done using software SPSS-15. Data are expressed as either mean (standard deviation) or median (range) and number (percentage).
RESULTS
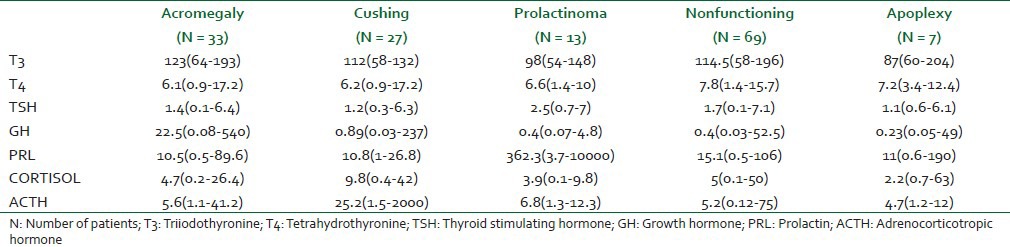
A total of 152 patients underwent sub-labial transsphenoidal surgery. In three patients, tumors were diagnosed as meningioma, thus, were excluded. Therefore, 149 patients were included for the final analysis. Demographic characteristics, duration of surgery and length of hospital stay are shown in Table 1. Median age of patient was 38 (12-79) year. There was preponderance of males in all categories of tumors except Cushing's disease which had female predominance. Baseline hormone profiles are also shown in Table 2. In our study, the most common pituitary tumor was found to be nonfunctioning (46%) followed by GH secreting adenoma (22%). Apoplexy group was constituted by minimal number (5%) of patients [Table 1].
Table 1.
Demographic profile [Mean (SD)], tumor characteristics, intraoperative variables and length of hospital stay in patients undergoing transsphenoidal surgery for pituitary adenoma
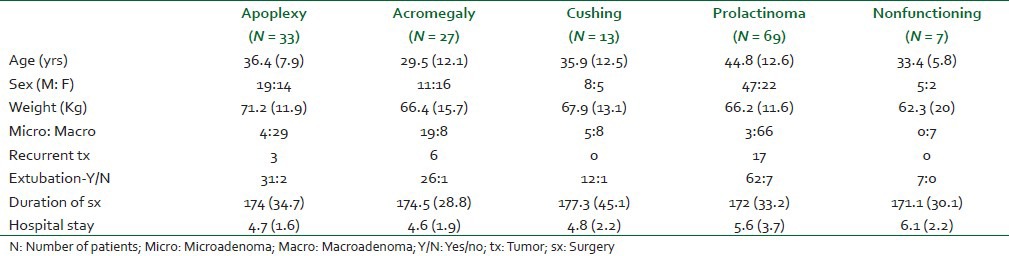
Table 2.
Baseline hormone profile [median (range)] in different pituitary adenoma groups
Eleven patients (7.4%) required postoperative ventilation due to various reasons like intraoperative bleeding (45%), residual effect of anesthetics (36%), seizure (9%) and hypothermia, temperature less than 34°C (9%) and they were extubated few hours later in neurosurgical ICU. They were not included in analysis of postoperative complications. Other intraoperative complications observed were, CSF leak, hypertension and bradycardia in 15%, 6% and 1% of patients, respectively. CSF leak was the commonest complication (40%) in all the groups while new onset of cranial nerve complication was observed in only one patient (0.7%) [Tables 3 and 4].
Table 3.
Immediate postoperative (with in 48 hrs) complications in patients undergoing transsphenoidal surgery for pituitary adenoma [number (percentage)
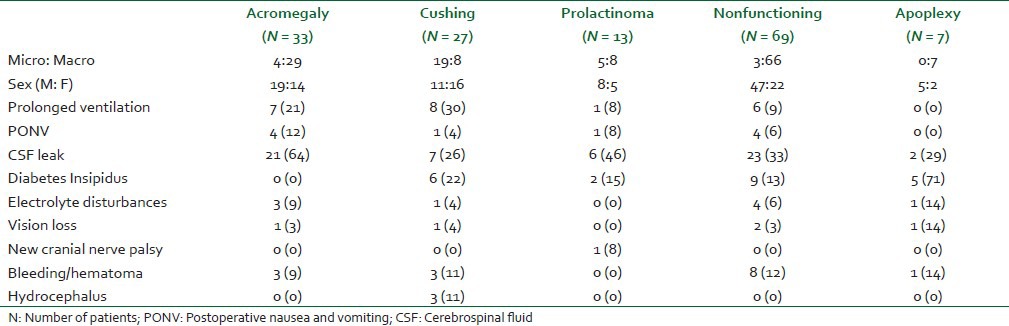
Table 4.
Overall complications in various groups of pituitary adenoma
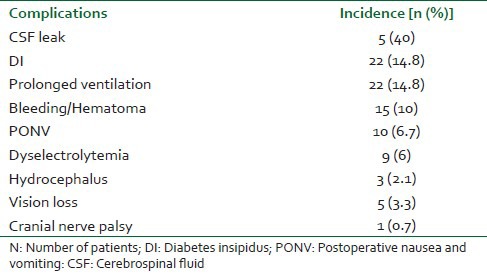
Fifteen patients (10%) required tracheal reintubation and ventilatory support in postoperative period because of deterioration in neurological condition [Table 4]. An emergent computed tomography (CT) scan showed either intracranial bleed or hematoma at operative site as the cause of deterioration in these patients. Emergent exploration was conducted for these patients. Postoperative complications within 48 hours are shown in Table 3. The duration of hospital stay was comparable among groups [Table 1].
DISCUSSION
The findings of present study conducted on patients undergoing transsphenoidal removal of pituitary adenoma revealed:
CSF leak was the commonest postoperative complication.
Other common complications noted were DI, prolonged postoperative ventilation, PONV and electrolyte disturbances.
The length of hospital stay was comparable for patients irrespective of nature of tumor pathology.
Common complications
CSF leak
It was the most common complication noted in each group in our study (40%). It was noted in all types of pituitary tumors with highest incidence in acromegalics (64%), followed by 46% in prolactinomas and 33% in NFPA. Postoperative CSF leak has some relationship with the tumor type and size. This complication has been most commonly reported with FSH adenoma and Cushing's disease in the retrospective study of 592 patients.[4] Surgical revision, tumor consistency, and tumor margins were independently associated with intra-operative leaks, while the tumor size, consistency, and margins were risk factors of postoperative leaks.[4] However, Shiney et al.[5] in their retrospective review found no such relationship of postoperative CSF leak with tumor size. They also concluded that non-adenomatous disease and presence of an intraoperative leak were independent predictors of a postoperative leak.[5]
Acromegalics had highest rate of CSF rhinorhea in our study. Most tumors in this group were found to be macro adenoma (90%) and so size-related causal relationship may be a possible explanation for more CSF leak in acromegalics patients. This possibility explains more CSF leak in other groups (prolactinomas, nonfunctioning and apoplexy) too. On the other hand most of tumors in Cushing (70%) were micro-adenoma and still it developed CSF leak in 26% of patients. Probably, high cortisol levels leads to thinning of texture of subarachnoid, thereby, increasing its vulnerability to intraoperative breach easily. Other reason may be that most of these patients were operated by neurosurgeon with lesser experience.
Diabetes insipidus
Diabetes insipidus was found to be the next common complication (14.8%) in our series; however, it was transient in nature. If not treated DI results in life threatening fluid and electrolyte imbalance. In our study this complication was frequently observed in patients with Apoplexy (71%) followed by Cushing's (22%) and Prolactinoma (15%) patients, but none in acromegalics. Randeva and colleagues[6] reported 16% incidence of transient diabetes insipidus in patients of pituitary apoplexy during their hospital stay. The cause of DI after transsphenoidal surgery may be due to compression or destruction of the posterior pituitary gland, interruption of the blood supply to the gland or edema to the pituitary stalk. Pituitary apoplexy is more often limited to the anterior lobe of the pituitary; however, in some instances it might involve the posterior lobe. A high incidence of DI (71%) in our series in this group of patients may be because of late presentation of these patients to our specialized center owing to lack of health awareness and absence of adequate health services in many parts of our country. It is likely that by the time these patients present to our center, some damage of posterior pituitary has already occurred. From our observation it is apparent that this complication does not bear any size causal relationship. This has been amply demonstrated by absence of this complication in acromegalics a (majority of this group presented with macro-adenoma) and 22% of incidence in Cushing's (majority of which had micro-adenoma). Pesky et al.[7] reported transient and permanent DI in 9.0% and 1.4% patients, respectively. They further observed significantly higher incidence of transient DI in secretary adenoma compared to NFPA (16.6% vs. 3.4%). However, our observations suggest that occurrence of this complication is independent of tumor pathology. According to Nemergut et al.[8] patients more likely to develop postoperative transient DI are those with secretary macro-adenoma. They also reported a temporal relationship between intraoperative leak and postoperative DI, both transient and permanent.[8] We found no relationship between CSF leak, tumor size or tumor pathology. In acromegalics, in spite of macro-adenoma in majority of patients and high incidence of CSF leak, there was no incidence of DI. Similarly despite presence of macro adenoma in large population of NFPA group incidence of DI was only 13%. A possible reason may be that probably it is easy to resect macro-adenomas thereby, sparing trauma to posterior pituitary in most patients.
Prolonged ventilation
Tracheal reintubation and prolonged ventilation in patients result in pulmonary complications. Major indication of reintubation in neurosurgical patients is neurological deterioration due to any reason. No study has highlighted this aspect of transsphenoidal surgery. Intracranial bleed or hematoma at operative site was the reason of neurological worsening in our series which warranted mechanical ventilatory support. Its rate was highest in ACTH secreting (30%), GH secreting (21%) and NFPA tumors (9%).
Postoperative nausea and vomiting
PONV is not a life-threatening complication but results in considerable distress to the patients. Its incidence postcraniotomy may be as high as 50%.[9,10,11] However, incidence of PONV following transsphenoidal surgery is quite low. Flynn and colleagues[11] reported 7.5% incidence of PONV in SLTS with 10% incidence in acromegalics. In our study too, its overall rate was 6.7% with 12% in acromegalics while in other groups of patients it ranged from 0 to 8%. Lower incidence of PONV in them compared to craniotomy, could be due to lesser invasive nature of the procedure resulting minimal disturbance of the CTZ. Furthermore, the smaller incision and lesser disruption of surrounding structures may lead to lesser inflammation and pain with consequent lesser PONV. Flynn et al.[11] further linked PONV with some risk factors like intraoperative CSF leak, fat grafting and lumbar drain placement. Manipulation of CSF pressure may be directly responsible for the higher incidence of postoperative emesis.[12] In our study also, PONV incidence was more in presence of CSF leak, in acromegalics, prolactinomas, and non-functioning tumor groups but it was less in Cushing and apoplexy patients. We believe that perhaps the size of the tumor predisposes to PONV. Majority of patients with acromegaly, prolactinoma and nonfunctioning group had large tumor size (macro-adenoma) which probably required greater manipulation, thereby resulting in higher PONV. On the other hand, most of the Cushing's presented with micro-adenomas which could be resected without much disruption of surrounding structures. However, in apoplexy group though all the patients had macro-adenoma and high incidence (29%) of CSF leak, there was no PONV. We do not have any plausible explanation, however, because of emergent presentation, these patients were aggressively managed with steroids (Dexamethasone) resulting in some protection from PONV.
Electrolyte disturbances
Electrolyte disturbances are mainly seen in the form of serum sodium imbalances. Hypernatremia is a manifestation of DI and hyponatremia is because of syndrome of inappropriate anti diuretic hormone secretion (SIADH), hypocortisolism and rarely because of cerebral salt wasting syndrome.[13] While hypernatremia manifests (due to DI) during early postoperative period, hyponatremia usually presents after few days in the postoperative period.[14] Hypokalemia too can occur but its incidence is very low. An unrecognized or improperly treated Na+ imbalance may result in catastrophe. Kristof et al.[15] showed that water and electrolyte (Na+) disturbances occurred in 75% of their patients following transsphenoidal surgery. Sane and colleagues[14] reported 35% incidence of hyponatremia subsequent to transsphenoidal surgery. Compared to these studies, we noticed 6% of hypernatremia which was due to transient DI. Incidence of hyponatremia (14%) was also very low in our series. With acute fall in serum sodium, neurologic symptoms resulting from cerebral edema can be seen at serum sodium level below120 meq/l. There exists a distinct possibility that asymptomatic mild hyponatremia (between 135 meq/l and 120 meq/l) which developed slowly, due to SIADH, escaped our attention. Hypernatremia (Serum sodium more than 150 meq/L) in our study was seen in all groups except prolactinomas. The latter group of patients despite having macro-adenoma in large majority did not develop high Na+ at all. We cannot find proper explanation for this. Since a rising serum Na+ following pituitary tumor resection is a manifestation of DI, probably DI in this group of patients was managed early with pharmacological agents, thereby, restoring and maintaining normal Na+ levels. Other reason for absence of hypernatremia in this group of patients may have resulted form surgical management of this group of patients by more experienced neurosurgeon.
RARE COMPLICATIONS
Some groups showed rare but serious complications like new cranial nerve deficit, intraventricular hematoma, visual deterioration and hydrocephalus. Postoperative hematoma was frequently encountered in patients with pituitary apoplexy (14%) and this group also showed higher number of patients with postoperative visual deterioration (14%).
Postoperative hematoma at operation site
Hematoma formation at the operation site following any intracranial procedure is the most devastating complication which usually develops within 24 hours and results in serious morbidity or even death, if not managed urgently. Its principal cause is inadequate hemostasis achieved during surgery. Its incidence following craniotomy has been reported to be 2.2%.[16] In the present study 15 patients (10%) developed this complication. Hematoma formation was almost equally distributed among all except prolactinoma group in which no patient developed this complication. Our observation suggests that this complication is unrelated to the size tumor or duration of the surgery. Similarly secretary or non-secretary nature of the tumor has no bearing on its occurrence. We could not figure out reasons for lack of this complication in prolactinoma patients. Could it be that high prolactin level provides some protection against hematoma formation by influencing the coagulation system or this simply reflects that probably these patients were operated on by more experienced neurosurgeon? This requires further exploration.
Vision loss
Owing to close proximity of optic chiasm to pituitary there is potentially a high risk of damaging it with resultant loss of vision. Other reasons for vision loss are: Postoperative hematoma or devascularization of optic apparatus, fracture of orbit, cerebral vasospasm and prolapsed of optic chiasm into empty sella.[17] Following pituitary adenoma resection its incidence varies 0.5% to 2.4%.[18] In our series this complication was seen in 5 (3.3%) patients and most common cause was development of hematoma requiring emergency exploration to restore vision. The highest incidence seen in apoplexy may be because these patients presented with vision deterioration in the emergency. Absence of this complication in prolactinoma patients again highlight the fact tumor size does not determine this complication and probably patients in this group were operated by more experienced neurosurgeon.
Hydrocephalus
Hydrocephalus in pituitary adenoma occurs as a result of obstruction of third ventricle by suprasellar extension of the tumor. It may develop following pituitary tumor resection because of intraventricular bleeding and later due to meningitis. We observed this complication in 2.3% of our patients. This complication was restricted to Cushing's groups only. We have no explanation for this. Absence of this complication in other group of patients emphasizes the fact that neither size nor duration of surgery is responsible for its occurrence.
LIMITATIONS
The surgery performed in our patients involved neurosurgeons with variable experience; however all surgeons have more than 6 years of experience. But we have here different learning curves included into this study. This may attribute the incidence of various complications in various groups. The multivariate analysis could not be performed due to small sample size in each group. Long-term follow-ups and outcome of these patients were not carried out.[19,20]
CONCLUSION
Transsphenoidal surgery is considered a safe procedure but it does carry the risks of various postoperative complications. In our series, various groups showed different postoperative complications in which CSF leak was the commonest followed by DI, PONV and hematoma at operation site. Tumor size or pathology may be the contributory factor for these findings.
FOOT NOTE
This study was carried out at All India Institute of Medical Sciences, New Delhi, India, as part of research thesis during super specialization training (DM) in the same institute by corresponding author.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Manninen PH, Raman SK, Boyle K, el-Beheiry H. Early postoperative complications following neurosurgical procedures. Can J Anesth. 1999;46:7–14. doi: 10.1007/BF03012507. [DOI] [PubMed] [Google Scholar]
- 2.Jane JA, Jr, Sulton LD, Laws ER., Jr Surgery for primary brain tumors in US academic training centers: Results from the Residency Review Committee for neurological surgery. J Neurosurg. 2005;103:789–93. doi: 10.3171/jns.2005.103.5.0789. [DOI] [PubMed] [Google Scholar]
- 3.Lecleraq TA, Griooli F. Avoidance of diabetes insipidus in transsphenoidal hypohysectomy: A modified technique of selective hypophysecotomy. J Neurosurg. 1983;58:682–84. doi: 10.3171/jns.1983.58.5.0682. [DOI] [PubMed] [Google Scholar]
- 4.Han ZL, He DS, Mao ZG, Wang HJ. Cerebrospinal fluid rhinorrhea following trans-sphenoidal pituitary macroadenoma surgery: Experience from 592 patients. Clin Neurol Neurosurg. 2008;110:570–9. doi: 10.1016/j.clineuro.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Shiley SG, Limonadi F, Delashaw JB, Barnwell SL, Andersen PE, Hwang PH, et al. Incidence, etiology, and management of cerebrospinal fluid leaks following trans-sphenoidal surgery. Laryngoscope. 2003;113:1283–8. doi: 10.1097/00005537-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass JA. Classical pituitary apoplexy: Clinical features, management and outcome. Clin Endocrinol (Oxf) 1999;51:181–8. doi: 10.1046/j.1365-2265.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- 7.Persky MS, Brunner E, Copper PR, Cohen NL. Perioperative complication of transsphenoidal excision for pituitary adenomas. Skull Base Surgery. 1996;6:231–5. doi: 10.1055/s-2008-1058631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemergut EC, Zuo Z, Jane JA, Jr, Laws ER., Jr Predictors of diabetes insipidus after transsphenoidal surgery: A review of 881 patients. J Neurosurg. 2005;103:448–54. doi: 10.3171/jns.2005.103.3.0448. [DOI] [PubMed] [Google Scholar]
- 9.Fabling JM, Gan TJ, El-Moalem HE, Warner DS, Borel CO. A randomized, double-blind comparison of ondansetron versus placebo for prevention of nausea and vomiting after infratentorial craniotomy. J Neurosurg Anesthesiol. 2002;14:102–7. doi: 10.1097/00008506-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Kathirvel S, Dash HH, Bhatia A, Subramaniam B, Prakash A, Shenoy S. Effect of prophylactic ondansetron on postoperative nausea and vomiting after elective craniotomy. J Neurosurg Anesthesiol. 2001;13:207–12. doi: 10.1097/00008506-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Flynn BC, Nemergut EC. Postoperative nausea and vomiting and pain after transsphenoidal surgery: A Review of 877 patients. Anesth Analg. 2006;103:162–7. doi: 10.1213/01.ane.0000221185.08155.80. [DOI] [PubMed] [Google Scholar]
- 12.Samadani U, Huang JH, Baranov D, Zager EL, Grady MS. Intracranial hypotension after intraoperative lumbar cerebrospinal fluid drainage. Neurosurgery. 2003;52:148–51. doi: 10.1097/00006123-200301000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Barrow DL, Tindall GT. Loss of vision after transsphenoidal surgery. Neurosurgery. 1990;27:60–8. doi: 10.1097/00006123-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Sane T, Rantakan K, Poranen A, Tabtela R, Valimali M, Pelkonen R. Hyponatremia after transsphenoidal surgeon for pituitary tumors. J Clin Endocrinol Metab. 1994;79:1395–8. doi: 10.1210/jcem.79.5.7962334. [DOI] [PubMed] [Google Scholar]
- 15.Kristof RA, Rother M, Neuloh G, Klingmüller D. Incidence, clinical manifestations, and course of water and electrolyte metabolism disturbances following transsphenoidal pituitary adenoma surgery: A prospective observational study. J Neurosurg. 2009;11:555–62. doi: 10.3171/2008.9.JNS08191. [DOI] [PubMed] [Google Scholar]
- 16.Taylor WA, Thomas NW, Wellings JA, Bell BA. Timing of postoperative intracranial hematoma development and implications for the best use of neurosurgical intensive care. J Neurosurg. 1995;82:48–50. doi: 10.3171/jns.1995.82.1.0048. [DOI] [PubMed] [Google Scholar]
- 17.Atkin SL, Coady AM, White MC, Mathew B. Hyponatremia secondary to cerebral salt wasting syndrome following routine pituitary surgery. Eur J Endocrinol. 1996;135:245–7. doi: 10.1530/eje.0.1350245. [DOI] [PubMed] [Google Scholar]
- 18.Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: Review of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–36. doi: 10.1097/00006123-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Patil CG, Lad SP, Harsh GR, Laws ER, Jr, Boakye M. National trends, complications, and outcomes following transsphenoidal surgery for Cushing's disease from 1993 to 2002. Neurosurg Focus. 2007;23:E7. doi: 10.3171/foc.2007.23.3.9. [DOI] [PubMed] [Google Scholar]
- 20.Chang EF, Zada G, Kim S, Lamborn KR, Quinones-Hinojosa A, Tyrrell JB, et al. Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J Neurosurg. 2008;108:736–45. doi: 10.3171/JNS/2008/108/4/0736. [DOI] [PubMed] [Google Scholar]


