Abstract
Background:
The aim of this study was to compare the intravenous (IV) and caudal routes of administration of sufentanil for children undergoing orchidopexy and also to evaluate the effects on addition of caudal adrenaline and neostigmine.
Materials and Methods:
Sixty patients scheduled for orchidopexy were divided into the following groups: 1) Group IVSu received IV 0.5 μg/kg sufentanil and caudal saline; 2) Group CSu received caudal 0.5 μg/kg sufentanil and IV saline; 3) Group CSuAdr received caudal sufentanil plus adrenaline 5 μg/ml (1:200,000) and IV saline; 4) Group CSuNeo received caudal sufentanil plus neostigmine, and IV saline; and 5) Group CSuNeoAdr received caudal sufentanil plus neostigmine plus adrenaline, and IV saline. Heart rate and mean blood pressure >15% was treated with increasing isoflurane concentration. Consumption of isoflurane, side effects, quality of sleep, time to first administration of analgesic, and number of doses of 24-h rescue analgesic were recorded.
Results:
Groups were demographically similar. Isoflurane consumption showed the following association: Group IVSu = Group CSuNeo = Group CSuNeoAdr < Group CSu = Group CSuAdr (P < 0.02). VAS for sedation on reversal of anesthesia showed the following association: Group CSuNeo = Group CSuNeoAdr < Group CSu = Group CSuAdr = Group IVSu (P < 0.005). Time to the first administration of dipyrone showed the following association: Group IVSu = Group CSu = Group CSuAdr (3-4 h) < Group CSuNeo = Group CSuNeoAdr (10-11 h) (P < 0.05). Number of doses of rescue analgesic showed the following association: Group IVSu = Group CSu = Group CSuAdr > Group CSuNeo = Group CSuNeoAdr (P < 0.005). Incidence of adverse effects was similar among groups.
Conclusion:
Caudal sufentanil alone was no better than when administered in the IV route, and would just be justified by the association of neostigmine, but not adrenaline. Neostigmine association resulted in better perioperative analgesia.
Keywords: Caudal adrenaline, caudal neostigmine, caudal sufentanil, intravenous sufentanil, orchidopexy
INTRODUCTION
Caudal block is one of the most commonly used anesthetic techniques in subumbilical and genitourinary procedures, and caudal sufentanil has been advocated to be a useful adjuvant for perioperative analgesia, as traditional administration of caudal local anesthetic alone was inadequate on blocking peritoneal response during spermatic cord traction.[1]
Because of its greater lipid solubility, the onset of analgesia was quicker than with morphine or fentanyl, but the duration of sufentanil administered as a single caudal injection was shorter.[2] Furthermore, some investigators indicated that in adults, the effects of the more lipid-soluble opioid sufentanil were similar either after epidural or intravenous (IV) route of administration,[3] while it presented excessive sedation after IV administration.[4,5] In addition, it was suggested that the epidural administration of lipophilic opioids might progress with lower incidence of nausea compared to the IV route,[6] although it is controversial.[5]
The aim of this study was to evaluate the analgesic efficacy of either IV or caudal sufentanil, and to find whether the addition of either caudal adrenaline or neostigmine to caudal sufentanil would provide better perioperative profile for children undergoing orchidopexy.
MATERIALS AND METHODS
The study protocol was approved by the Ethical Committee of the institution (protocol registration in the Brazilian Ethical Research) and written parental or guardian informed consent was obtained. Using a double-blind prospective design, 60 boys of ASA physical status I or II scheduled for unilateral orchidopexy during combined caudal — general anesthesia were computer randomized to one of five groups (n = 12 in each group) [Table 1], and prospectively studied using a double-blinded, randomized, controlled design to examine analgesia and adverse effects. The preservative-free test drugs used were: Saline, sufentanil 0.5 mg/kg, neostigmine 2 mg/kg, and adrenaline 5 mg/ml (1:200,000) [Table 1].
Table 1.
Groups
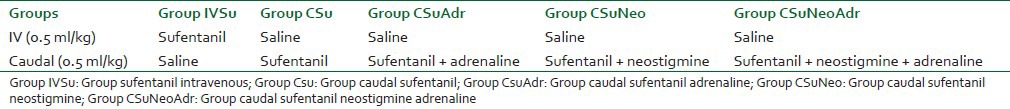
All patients were premedicated with IV midazolam 0.05 mg/kg in the holding room plus IV 10 mg/kg atropine. Anesthesia was induced in the surgical room with IV etomidate (0.3-0.4 mg/kg) and orotracheal intubation was facilitated by atracurium 0.5 mg/kg and lidocaine spray. A third of the initial IV dose of atracurium was repeated every 30 min until 30 min before the end of the surgical procedure. After intubation, patients were placed in the lateral position and the caudal injection of the test drug was performed by an experienced anesthesiologist. The caudal and the IV test drugs were diluted in saline to a final volume of 0.5 ml/kg and were administered simultaneously and at the same rate (3 ml/min) by two anesthesiologists who were blinded to the study protocol. General anesthesia was maintained with isoflurane in the normal semi-open circle system with 50% nitrous oxide/50% oxygen mixture, under a delivered fresh gas flow of 3 l. The targeted end-tidal isoflurane concentration was adjusted to keep the blood pressure and heart rate within 15% range of baseline values. The inhalation anesthetic agents were discontinued at the completion of skin closure, and the total volume spent during the procedure was immediately noted. Routine intraoperative monitoring consisted of noninvasive blood pressure measured at 3-min interval, continuous electrocardiography, pulse oximetry, body temperature, and capnography. The muscle relaxation was pharmacologically reversed with atropine 25 mg/kg IV and neostigmine 50 mg/kg IV. The degree of sedation was scored by an anesthesiologist, who was blinded to the study protocol, after extubation, using the concept of the 10-cm visual analog scale (10-cm VAS), with the lowest score given for “not sedated at all” and the highest score for “as sedated as possible.”
Patients were moved to the recovery area after extubation. In the recovery room, patients were individually taken care of by a nurse unaware of the anesthetic technique employed, and the mother or guardian was asked to be with the child, as it is our routine. The mother was encouraged to handle the child. Dipyrone (10 mg/kg) was administered IV at any sign of crying or discomfort, at an interval of minimum 4 h, at the discretion of the trained nurse, and the pain impression was always scored by the same anesthesiologist using the 10-cm VAS, with the lowest score given for “no sign of pain” and the highest score for “excruciating pain,” based on the corresponding facial pain numerical scales. The time to first administration of the analgesic since anesthetic induction was recorded in minutes. The number of times that rescue analgesic dipyrone (oral and/or IV) was administered during the first 24 h after anesthesia induction was recorded. The quality of sleep was rated using the VAS (0-10 cm), with the lowest score given for “did not sleep at all, uncomfortable” and the highest score for “slept all night, comfortable” by the mother or guardian who slept next to the child. Any adverse effects were recorded, focusing on nausea and vomiting. Nausea was scored using the 10-cm VAS, with the lowest score given for “no nausea at all” and the highest score for “the worst nausea possible.” Any adverse effect, including the occurrence of vomiting, was noted.
The power of the study was based on preliminary data. We hypothesized that caudal sufentanil would result in analgesia that would be increased by 100% after the addition of epidural neostigmine. Taking into consideration α = 5% and β = 80%, this would require a minimum of eight patients per group. Groups were compared for demographic data, duration of surgery, and time to administration of first rescue analgesics by one-way analysis of variance (ANOVA) and Chi-square test. Incidence of adverse events and adjuvant drug use were compared among groups by Chi-square corrected for multiple testing. Blood pressure, heart rate, the time to the first administration dipyrone, isoflurane spent, number of analgesic administration during the first 24 h, and VAS scores were compared among groups by two-way ANOVA for repeated measures. P < 0.05 was considered significant. Post-hoc tests (Tukey and Benferroni analysis) were applied to correct P values. Data were expressed as mean ± SD except for the number of rescue analgesics [median (25-75%)].
RESULTS
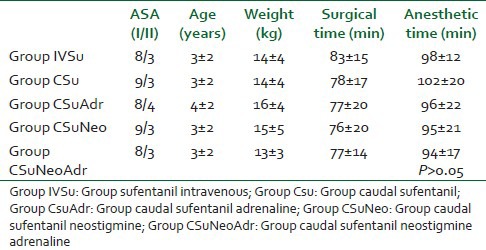
The groups were demographically the same in relation to ASA physical status, age, weight, surgical time, and anesthetic time (which included the surgical time plus the time to perform the caudal block plus the time for extubation) [Table 2]. One patient from Group IVSu and one from Group CSuNeoAdr were excluded due to incomplete data collection. The mean blood pressure and pulse rate at fixed intervals were similar among groups during all perioperative procedures (data not shown, P > 0.05).
Table 2.
Demographic data
The isoflurane consumption (ml/kg/h) was similar among the patients from Group IVSu, Group CSuNeo, and Group CSuNeoAdr (P > 0.05). Their intraoperative anesthetic consumption was lesser when compared to patients who received caudal sufentanil alone or in combination with adrenaline (Group CSu = Group CSuAdr) (P < 0.02) [Table 3]. The VAS for sedation at the reversal time was similar among Group IVSu, Group CSu, and Group CSuAdr (P > 0.05). However, patients who received caudal neostigmine had lower VAS sedation and were classified as more awake at the reversal time (P < 0.005) [Table 3].
Table 3.
Perioperative analgesic data
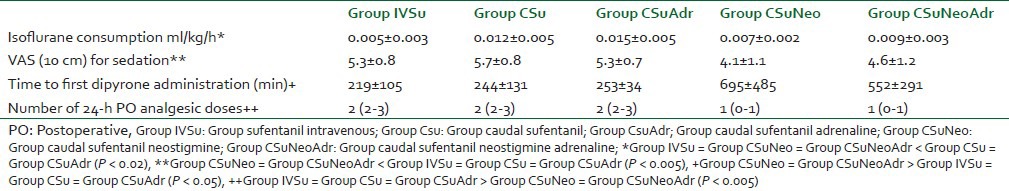
The time to the first administration of postoperative analgesics was similar among patients from Group IVSu, Group CSu, and Group CSuAdr (3-4 h, P > 0.05) [Table 3]. The time to first administration of rescue analgesics for patients who received caudal sufentanil plus neostigmine (Group CSuNeo and Group CSuNeoAdr) was similar, but higher when compared to the other groups (10-11 h, P < 0.05) [Table 3].
The number of postoperative doses of dipyrone in 24 h, administered at the discretion of a trained nurse, was similar among the patients in Group IVSu, Group CSu, and Group CSuAdr (P > 0.05) [Table 3]. However, patients who received caudal sufentanil and caudal neostigmine (Group CSuNeo and Group CSuNeoAdr) needed lesser doses in 24 h evaluation (P < 0.005) [Table 3]. The facial pain VAS at analgesic administration was similar among all groups (5-6 cm, data not shown, P > 0.05).
The incidence of postoperative nausea or vomiting was similar among groups. The VAS scores for nausea were: Group IVSu (1.9 ± 2.9), Group CSu (1.2 ± 2.2), Group CSuAdr (1.5 ± 2.7), Group CSuNeo (1.2 ± 2), Group SCSuNeoAdr (1.8 ± 2.6) (P > 0.05). Four children in Group IVSu had decrease in the cardiac frequency higher than 20% and were treated with titrated IV atropine (P > 0.05). No other intraoperative adverse effects were observed. Postoperatively, two patients from Group IVSu, one from Group CSuNeo, two from Group CSuAdr, one from Group CSu, and one from Group CSuNeoAdr had vomited once, but did not require pharmacological treatment (P > 0.05). All groups of patients had a comparable appetite and quality of night rest during the first 24 h following the operation (P > 0.05). There was no need for additional analgesics other than dipyrone.
DISCUSSION
We confirmed the findings of previous workers that the postoperative analgesic effect of the more lipid-soluble opioid sufentanil was similar when administered either epidurally or IV[3,4,5] in children, while it presented pronounced sedation when administered IV.[4,5] In order to evaluate the perioperative analgesic effect of sufentanil, this study was designed to compare two different routes of administration, i.e. IV and caudal. Once IV administration of sufentanil (0.145 ng/ml plasma concentration) was demonstrated to reduce isoflurane minimum alveolar concentration to 50%,[7] isoflurane intraoperative consumption was used as a parameter to assess the analgesic potency of both IV and epidural routes. The fact that the intraoperative isoflurane consumption was higher in the groups that received sufentanil caudal alone or sufentanil with added adrenaline, compared with patients who had IV sufentanil, reflects rather a sedative effect of IV sufentanil resulting in less isoflurane consumption and a higher VAS for sedation at the reversal time of the anesthesia, probably secondary to its own sedative effect.
Regarding perioperative analgesia, the association of neostigmine to caudal sufentanil resulted in intraoperative analgesia exemplified by the overall lower isoflurane consumption, and postoperative analgesia exemplified by 10-11 h of analgesia, compared to 3-4 h observed in the groups that did not receive caudal neostigmine. In the present study, the dose of caudal neostigmine was 2 μg/kg, as it was previously demonstrated to be effective dose in adults[8] and children.[9,10] Epidural neostigmine has been shown to potentiate opioids. In fact, in our present study, caudal sufentanil alone was no better than when administered in the IV route, and the caudal route would just be justified by the association of neostigmine, but not adrenaline in this population. Epidural combination of neostigmine (6-7 μg/kg) with sufentanil 10 μg provided similar duration of analgesia as epidural sufentanil 20 μg, and allowed analgesia devoid of side effects in the first stage of labor.[11] Other authors have described 1 h of postoperative analgesia after caudal 0.5 μg/kg sufentanil combined with levobupivacaine[12] and no significant differences with regard to the surgical stress response in children, demonstrating no advantage in adding 0.5 μg/kg sufentanil to bupivacaine over bupivacaine alone in the caudal block.[13]
The prolonged analgesic action of central neostigmine demonstrated in the present study reflected the cholinergic involvement in nociception. An electron microscopy analysis demonstrated that cholinergic boutons are presynaptic to dorsal horn neurons as well as to the terminals of sensory primary afferents, suggesting that they are likely to modulate incoming somatosensory information.[14] The authors suggested that this newly identified dorsal horn cholinergic system in monkeys was the source of the ACh involved in the analgesic effects of epidural neostigmine.[14] The association of the highly lipid-soluble opioids sufentanil, which has a quicker onset of action, with neostigmine, resulted in at least summation of the analgesic effects. The quicker onset of action and shorter duration of sufentanil would be compensated by the slower onset of action but longer duration of neostigmine, resulting in a clinically useful association, which is devoid of important side effects.
Another point to be considered was the addition of adrenaline to caudal sufentanil. Although the literature suggests an improvement in analgesia when epidural adrenaline is combined to opioid plus local anesthetic,[15,16] we observed that adrenaline did not potentiate the analgesic effect of sufentanil alone or in combination with neostigmine, which would imply that the effects observed by others[15,16] were in fact analgesic enhancement of the combination of epidural local anesthetic and adrenaline. Similar to the α1-agonist adrenaline, no significant differences were found among the groups in either pain scores or requirement of additional doses of analgesics after the association of sufentanil and the α2-agonist clonidine with bupivacaine for caudal anesthesia after hypospadias repair in children.[17]
In reality, data from the literature suggest that epidural local anesthetics would potentiate both opioid[18,19] and adrenaline.[15,16] Therefore, although local anesthetic represents the gold standard for caudal block, we were interested to evaluate if the liposoluble opioid sufentanil administered by epidural route was beneficial. Since epidural sufentanil was described to potentiate local anesthetics[18] probably in a synergistic way,[19] the incorporation of caudal local anesthetics in the protocol could interfere with the final results of each individual drug evaluated.
With regard to the adverse effects, lower incidence of nausea has been described following epidural lipophilic opioid administration in adults.[6] In the present study, the scores of incidence of vomiting and nausea were similar among groups, which would reflect either that indeed epidural sufentanil and neostigmine did not potentiate each other its emetic effect, or fault of power for this adverse effect. Nevertheless, higher epidural neostigmine doses have been previously demonstrated to not cause emesis[8,20,21] even when combined with epidural opioids.[22,23] In the present study, isoflurane administration was adjusted according to the cardiovascular changes, and one finding was that four children in Group IVSu had a decrease in cardiac frequency higher than 20% and were treated with titrated IV atropine, although it was not statistically significant when compared to the other groups. Systemically administered sufentanil was described to result in persistent bradycardia for at least 24 h in dogs anesthetized with sevoflurane.[24] Similarly, intrathecal high-dose neostigmine (200 mg) was previously described to result in analgesia, with peculiar adverse effects including bradycardia not responding to intravenous atropine.[25] Subsequently, bradycardia reversible by atropine was described following smaller intrathecal doses such as 25-100 mg.[26,27] Nevertheless, such vagotonic effects were described only after intrathecal administration, but not after epidural administration, even when high epidural dose of 30 μg/kg was administered in pediatric patients undergoing genitourinary surgery.[28] Therefore, one would not expect any vagotonic action after epidural neostigmine, and therefore, the profound anesthesia was not a direct action on cardiovascular system, but rather analgesic contemplation of caudal neostigmine in the intraoperative setting, observed by the lower isoflurane consumption in all patients receiving caudal neostigmine as the adjuvant.
To conclude, in pediatric orchidopexy, IV sufentanil was similar to caudal sufentanil alone with regard to analgesia, whilst the IV route was accompanied with desirable intraoperative sedation. While adrenaline did not potentiate caudal sufentanil, only neostigmine added to caudal sufentanil offered clinical benefit over either caudal or IV sufentanil alone, with regard to perioperative analgesia.
Footnotes
Source of Support: The work was carried out under support of the Clinic for Pain Management of the Teaching Hospital of the School of Medicine of Ribeirão Preto, University of São Paulo, Brazil
Conflict of Interest: None declared
REFERENCES
- 1.Wang T, Xiang Q, Liu F, Wang G, Liu Y, Zhong L. Effects of caudal sufentanil supplemented with levobupivacaine on blocking spermatic cord traction response in pediatric orchidopexy. J Anesth. 2013;27:650–6. doi: 10.1007/s00540-013-1613-9. [DOI] [PubMed] [Google Scholar]
- 2.Rostaing S, Bonnet F, Levron JC, Vodinh J, Pluwska F. Pharmacokinetics of epidural clonidine on analgesia and pharmacokinetics of epidural fentanyl in postoperative patients. Anesthesiology. 1991;75:42–5. doi: 10.1097/00000542-199109000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SE, Tan S, White PF. Sufentanil analgesia following cesarean section: Epidural versus intravenous administration. Anesthesiology. 1988;68:129–33. doi: 10.1097/00000542-198801000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Geller E, Chrubasik J, Graf R, Chrubasik S, Schult-Monting J. A randomized double-blind comparison of epidural sufentanil versus intravenous sufentanil or epidural fentanyl analgesia after major abdominal surgery. Anesth Analg. 1993;76:1243–50. doi: 10.1213/00000539-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Miguel R, Barlow I, Morrell M, Scharf J, Sanusi D, Fu E. A prospective, randomized, double-blind comparison of epidural and intravenous sufentanil infusions. Anesthesiology. 1994;81:346–52. doi: 10.1097/00000542-199408000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Grant RP, Dolman JF, Harper JA, White SA, Parsons DG, Evans KG, et al. Patient controlled lumbar epidural fentanyl compared with patient controlled intravenous fentanyl for post-thoracotomy pain. Can J Anesth. 1992;39:214–9. doi: 10.1007/BF03008779. [DOI] [PubMed] [Google Scholar]
- 7.Krane EJ, Jacobson LE, Lynn AM, Parrot C, Tyler DC. Caudal morphine for postoperative analgesia in children: A comparison with caudal bupivacaine and intravenous morphine. Anesth Analg. 1987;66:647–53. [PubMed] [Google Scholar]
- 8.Lauretti GR, de Oliveira R, Reis MP, Juliâo MC, Pereira NL. Study of three different doses of epidural neostigmine coadministered with lidocaine for postoperative analgesia. Anesthesiology. 1999;90:1534–8. doi: 10.1097/00000542-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Mahajan R, Grover VK, Chari P. Caudal neostigmine with bupivacaine produces a dose-independent analgesic effect in children. Can J Anaesth. 2004;51:702–6. doi: 10.1007/BF03018429. [DOI] [PubMed] [Google Scholar]
- 10.Karaaslan K, Gulcu N, Ozturk H, Sarpkaya A, Colak C, Kocoglu H. Two different doses of caudal neostigmine co-administered with levobupivacaine produces analgesia in children. Paediatr Anaesth. 2009;19:487–93. doi: 10.1111/j.1460-9592.2009.02969.x. [DOI] [PubMed] [Google Scholar]
- 11.Roelants F, Lavand’homme PM. Epidural neostigmine combined with sufentanil provides balanced and selective analgesia in early labor. Anesthesiology. 2004;101:439–44. doi: 10.1097/00000542-200408000-00025. [DOI] [PubMed] [Google Scholar]
- 12.Erol A, Tavlan A, Tuncer S, Topal A, Yurtcu M, Reisli R, et al. Caudal anesthesia for minor subumbilical pediatric surgery: A comparison of levobupivacaine alone and levobupivacaine plus sufentanil. J Clin Anesth. 2008;20:442–6. doi: 10.1016/j.jclinane.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Erol A, Tuncer S, Tavlan A, Reisli R, Aysolmaz G, Otelcioglu S. Addition of sufentanil to bupivacaine in caudal block effect on stress responses in children. Pediatr Int. 2007;49:928–32. doi: 10.1111/j.1442-200X.2007.02479.x. [DOI] [PubMed] [Google Scholar]
- 14.Pawlowski SA, Gaillard S, Ghorayeb I, Ribeiro-da-Silva A, Schlichter R, Cordero-Erausquin M. A novel population of cholinergic neurons in the macaque spinal dorsal horn of potential clinical relevance for pain therapy. J Neurosci. 2013;33:3727–37. doi: 10.1523/JNEUROSCI.3954-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adami C, Veres-Nyéki K, Spadavecchia C, Rytz U, Bergadano A. Evaluation of peri-operative epidural analgesia with ropivacaine, ropivacaine and sufentanil, and ropivacaine, sufentanil and epinephrine in isoflurane anesthetized dogs undergoing tibial plateau levelling osteotomy. Vet J. 2012;194:229–34. doi: 10.1016/j.tvjl.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Uvarov DN, Antipin EE, Zemtsovskiĭ MIa, Borisov DB, Nedashkovskiĭ EV. Effect of adrenaline on the quality of postoperative epidural analgesia. Anesteziol Reanimatol. 2011;3:66–9. [PubMed] [Google Scholar]
- 17.De Mey JC, Strobbet J, Poelaert J, Hoebeke P, Mortier E. The influence of sufentanil and/or clonidine on the duration of analgesia after a caudal block for hypospadias repair surgery in children. Eur J Anaesthesiol. 2000;17:379–82. doi: 10.1046/j.1365-2346.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- 18.Buyse I, Stockman W, Columb M, Vandermeersch E, Van de Velde M. Effect of sufentanil on minimum local analgesic concentrations of epidural bupivacaine, ropivacaine and levobupivacaine in nullipara in early labour. Int J Obstet Anesth. 2007;16:22–8. doi: 10.1016/j.ijoa.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Vercauteren M, Meert TF. Isobolographic analysis of the interaction between epidural sufentanil and bupivacaine in rats. Pharmacol Biochem Behav. 1997;58:237–42. doi: 10.1016/s0091-3057(97)00011-7. [DOI] [PubMed] [Google Scholar]
- 20.Lauretti GR, de Oliveira R, Perez MV, Paccola CA. Postoperative analgesia by intraarticular and epidural neostigmine following knee surgery. J Clin Anesth. 2000;12:444–8. doi: 10.1016/s0952-8180(00)00189-6. [DOI] [PubMed] [Google Scholar]
- 21.Ross VH, Pan PH, Owen MD, Seid MH, Harris L, Clyne B, et al. Neostigmine decreases bupivacaine use by patient-controlled epidural analgesia during labor: A randomized controlled study. Anesth Analg. 2009;109:524–31. doi: 10.1213/ane.0b013e31819518e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omais M, Lauretti GR, Paccola CA. Epidural morphine and neostigmine for postoperative analgesia after orthopedic surgery. Anesth Analg. 2002;95:1698–701. doi: 10.1097/00000539-200212000-00042. [DOI] [PubMed] [Google Scholar]
- 23.Tekin S, Topcu I, Ekici NZ, Caglar H, Erincler T. Comparison of analgesic activity of the addition to neostigmine and fentanyl to bupivacaine in postoperative epidural analgesia. Saudi Med J. 2006;27:1199–203. [PubMed] [Google Scholar]
- 24.Polis I, Moens Y, Hoeben D, Tshamala M, Hoybergs Y, Gasthuys F. Cardiopulmonary effects of sufentanil long acting on sevoflurane anesthesia in dogs. Vet Anaesth Analg. 2006;33:111–21. doi: 10.1111/j.1467-2995.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 25.Lauretti GR, Reis MP, Prado WA, Klamt JG. Dose response study of intrathecal morphine versus intrathecal neostigmine, their combination, or placebo for postoperative analgesia in patients undergoing anterior and posterior vaginoplasty. Anesth Analg. 1996;82:1182–7. doi: 10.1097/00000539-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Lauretti GR, Hood DD, Eisenach JC, Pfeifer BL. A multi-center study of intrathecal neostigmine for analgesia following vaginal hysterectomy. Anesthesiology. 1998;89:913–8. doi: 10.1097/00000542-199810000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Lauretti GR, Mattos AL, Reis MP, Prado WA. Intrathecal neostigmine for postoperative analgesia after orthopedic surgery. J Clin Anesth. 1997;9:473–7. doi: 10.1016/s0952-8180(97)00103-7. [DOI] [PubMed] [Google Scholar]
- 28.Batra YK, Arya VK, Mahajan R, Chari P. Dose response study of caudal neostigmine for postoperative analgesia in paediatric patients undergoing genitourinary surgery. Paediatr Anaesth. 2003;13:515–21. doi: 10.1046/j.1460-9592.2003.01066.x. [DOI] [PubMed] [Google Scholar]


