Abstract
Background:
Gabapentin is effective for treating different types of headache including post-dural puncture headache (PDPH), also used for prophylaxis against migraine. We studied the effect of pre-operative administration of gabapentin on the characteristics of PDPH in parturients undergoing cesarean section (CS) under spinal anesthesia.
Materials and Methods:
Women undergoing elective cesarean section under spinal anesthesia were randomized to receive preoperative gabapentin 600 mg or placebo. Spinal anesthesia was achieved with 12.5 mg hyperbaric bupivacaine plus 25 μg fentanyl. Babies were followed up by Apgar scores, umbilical artery blood gases, breastfeeding difficulties, and need for NICU admission. The mothers were followed up for any side-effects of gabapentin for 24 h. Patients with PDPH were re-admitted and onset and duration of the headache were reported and severity was assessed using a visual analog scale (VAS) for 4 days from diagnosis. Paracetamol with caffeine and diclofenac were given for treatment, and the doses were adjusted according to VAS; also number of doses given for each group was recorded.
Results:
Eighty eight patients were randomized, and 2 were excluded. The incidence of headache and co-existing symptoms were similar in both groups. The onset of headache was significantly delayed in gabapentin group (P < 0.05). Also, severity and duration of headache were significantly less in gabapentin group (P < 0.05). The incidence of sedation was more in gabapentin group 11 (26.19%) versus placebo group 3 (6.81%). Neonatal outcomes were statistically insignificant between both groups.
Conclusion:
Pre-operative administration of gabapentin has no effect on incidence of (PDPH) but delays its onset and reduces its severity and duration in parturients undergoing cesarean section with spinal anesthesia without significant adverse effects on the mother or the baby.
Keywords: Gabapentinz, PDPH, spinal anesthesia
INTRODUCTION
PDPH is the most common complication after lumbar puncture for spinal anesthesia. Pregnant females are at particular risk due to sex, age, and the widespread use of spinal anesthesia. Headache may be severe enough to affect the ability of the mother to care for her baby. Many pharmacological agents have been used for treatment including gabapentin.[1] Gabapentin is an anti-convulsant, widely used for the treatment of different types of headache including the PDPH. We studied the effect of pre-operative administration of gabapentin on the characteristics of PDPH in parturients undergoing cesarean section under spinal anesthesia aiming to prophylactically reduce the incidence, severity, and duration of this post-dural puncture complication that has a significant impact on patients’ post-operative well-being.
MATERIALS AND METHODS
This is a double-blind, randomized, placebo-controlled study. We included 88 patients American Society of Anesthesiologist I and II primi or multigravida women, pregnant in a single full-term fetus, and who were planned for elective cesarean section under spinal anesthesia. None of these participants received spinal anesthesia before. Informed consent was obtained from the participants. We excluded women with history of chronic headache or any type of chronic pain and those on regular analgesics or anti-epileptic medications. Women with contraindications to spinal anesthesia, to gabapentin, or to any other medication in this study were also excluded. In addition, in case of any abnormalities in the fetus, the patient was excluded.
Participating parturients were divided into two groups; group G received 600 mg of gabapentin in the form of 2 capsules 300 mg each, and group P who received 2 capsules of starch identical to the gabapentin capsule. The participants were randomly assigned to one of both groups using computer-generated random numbers. Every participant was assigned a sequential number for the study, and the 2 capsules of gabapentin or placebo were placed in a small sequentially- numbered medication boxes. An assistant, not involved in the study, prepared the boxes, and another assistant blinded to the content of the boxes administered the capsules to the woman with the corresponding study number with a small sip of water 2 hours before admission to the operating room. Inside the operating room, an 18G venous cannula was inserted, and 1000 ml of Ringer's lactate solution was given to every patient as a preload. Standard monitors were applied. After positioning of the patient in the sitting position and sterilization of the back, spinal anesthesia was given using a 25-G quincke spinal needle, and a dose of 12.5 mg hyperbaric bupivacaine 0.5% plus 25 μg fentanyl was injected. Any drop in the blood pressure of more than 20% below the baseline was treated with 500 ml of 6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride injection, and if there is no response, a dose of 5-10 mg of ephedrine was given I.V. and the patient was excluded because headache is one of the possible side-effects of ephedrine, which may interfere with the aim of our study.
After delivery of the baby, oxytocin infusion started, a sample was taken from the umbilical artery for blood gases, and the baby was assessed by Apgar score at 1 and 5 minutes post-delivery by the attending neonatologist. We also recorded the birth weight and the need for neonatal intensive care unit (NICU) admission. The babies were followed up for 24 h post-operatively by a neonatologist - not involved in the study - for difficulties in breast feeding. During the post-operative period, all women received the same fluid regimen and the same course of analgesics in the form of intramuscular diclofenac 50 mg alternating with intravenous paracetamol every 12 hours, and any breakthrough pain was treated with intramuscular 50 mg of meperidine. Antacids were given to all patients during this course of medications. Any possible adverse events of gabapentin as sedation, ataxia, tremors, dizziness, nausea, and vomiting were recorded for 24 hours post-operatively. In case of sedation, the level of which was assessed using Ramsay sedation score[2] [Appendix 1].
Appendix 1.
Ramsay sedation score

The patients were discharged from the hospital according to the policies followed in the obstetric department after 24 h with stable hemodynamics, adequate bowel motion, and full return of sensory and motor power after spinal anesthesia. All the participants were instructed about the possibility of headache and were encouraged to notify about its occurrence.
Post-dural headache has been defined by certain criteria as the headache occurring after a dural puncture and one that has a significant effect on the patients’ post-operative well-being i.e. headache, that is not only postural, but also continues for more than 24 hours at any level of intensity or so severe at any time that the patient is unable to maintain an upright position. In case of confirmed PDPH, the patient is re-admitted and the severity was assessed. Patients were asked directly to record the severity of their headache in an upright position on a visual analog scale (VAS) on days 1, 2, 3, and 4. The pain scale consisted of a 10 cm horizontal line marked from 0 (denoting no pain) to 10 (denoting worst possible imaginable pain). All participants who had a confirmed PDPH received the same protocol of bed rest, fluids, laxatives, antacids, and analgesics. For VAS scores 5 or above, 2 caplets of 500 mg paracetamol +65 mg of caffeine (panadol extra®) and 1 tablet of 50 mg of diclofenac potassium (cataflam®) were given every 8 hours. If the score drops to 3 or 4, only 1 paracetamol caplet with the diclofenac was given every 8 hours. Once the VAS score is 1 or 2, the previous doses are given every 12 h and stopped once the score drops to zero. The onset and duration of the headache and any other co-existing symptoms as nausea and vomiting, neck stiffness, and diplopia or photophobia were determined by asking the patient and were recorded. Emesis was scored as 0 = no emetic symptoms, 1 = nausea, or 2 = vomiting. The number of doses for analgesics given was also recorded.
Statistical methods
Results are expressed as means ± standard deviation (SD), median (range) or number (percent). Comparison between numerical data was performed using student t-test. Comparison between categorical data was performed using the chi squared test. Data were considered significant if P values were <0.05. Statistical analysis was performed with the aid of the MEDCALC computer program (version 12 windows)
RESULTS
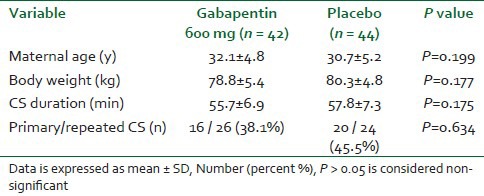
The study was conducted from May to November 2012; 88 women participated in the study, and 2 women were excluded from analysis. Demographic variables were similar in both groups [Table 1].
Table 1.
Demographic variables and duration of CS
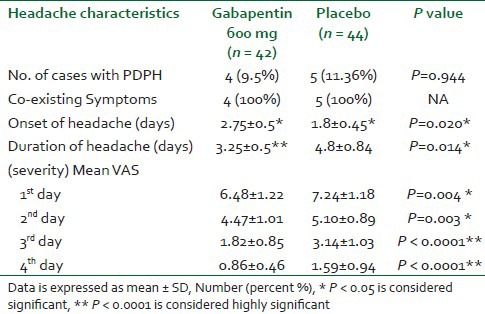
The incidence of headache and co-existing symptoms were similar in both groups. The onset of headache was significantly delayed in gabapentin group (P < 0.05) [Table 2]. The severity (Mean VAS) and duration of headache were significantly less in gabapentin group all over the 4 days (P < 0.05) [Table 2].
Table 2.
Headache characteristics (incidence, onset, duration, and Mean VAS scores while sitting)
Number of paracetamol and diclofenac tablets were significantly less in gabapentin group (13.25 ± 0.96) compared to placebo group (20.8 ± 2.68) [Table 3]. The incidence of sedation was more in gabapentin group 11 (26.19%) versus 3 (6.81%) in placebo group [Table 4]. The incidence of other side-effects was the same in both groups [Table 4]. There were no statistically significant differences between the two groups as regard the neonatal outcomes [Table 5].
Table 3.
Number of paracetamol and diclofenac tablets given

Table 4.
Incidence of maternal adverse reactions in the first 24 h post-operatively
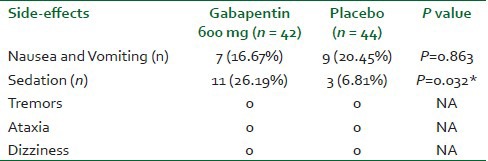
Table 5.
Neonatal outcomes

DISCUSSION
PDPH is a complication of regional anesthesia, more often seen in parturients, because of their risk factors of young age and female gender. In spinal anesthesia, the PDPH is mainly dependent on the size and type of needle. Before starting treatment, confirmation of diagnosis is essential because 5-16% of headaches after dural puncture are not PDPH.[3]
The characteristic manifestation of PDPH is its postural component; it increases with sitting or standing and improves with supine position. The pain occurs in the temporal, frontal, or occipital regions bilaterally and can be associated with backache, nausea, neck stiffness, cranial nerve signs, and localized muscle spasms; a history of dural puncture and postural component of the headache are important in diagnosis.[4] The most accepted mechanism for induction of headache is leakage of cerebrospinal fluid through dural hole resulting in cerebral vasodilatation, increased arterio-venous pressure gradient, dural traction and compression of cranial contents from loss of the cranial fluid cushion.[5] It is important to differentiate PDPH from other causes as tension headache, migraine headache, drug withdrawal, preeclampsia, meningitis, and subarachnoid hemorrhage.[3]
Current treatment for PDPH involves complete bed rest, hydration, analgesics, oral or intravenous caffeine, sumatriptan, ACTH, corticosteroids, gabapentin, and epidural patch.[6] The present study showed that the pre-operative administration of 600 mg of gabapentin significantly delays the onset and reduces the severity and duration of the PDPH. The decreased severity and duration of the headache in the gabapentin group was reflected on the analgesic doses needed for treatment, which was significantly lower in the same group. However, there was no difference in the incidence of the headache in both groups.
Gabapentin is an anti-epileptic used as monotherapy or adjunctive therapy in the treatment of partial seizures with or without secondary generalization. Although gabapentin is an analogue of gamma-aminobutyric acid (GABA), it is neither a GABA agonist nor antagonist and its mechanism of action is unknown. Gabapentin is also used in the treatment of neuropathic pain, migraine prophylaxis, and restless legs syndrome.[7,8]
The effect of gabapentin on already established post-spinal headache has been previously reported. Erol DD[1] investigated the tolerability and efficacy of administration of gabapentin in PDPH in 20 patients who developed PDPH after spinal anesthesia. Patients were randomized to receive either gabapentin (group 1) or placebo (group 2). VAS scores were significantly lower in group 1 than in group 2 for 4 days, and he concluded that the administration of oral gabapentin in patients with PDPH after spinal anesthesia is an effective and safe treatment. Also, two cases of PDPH, who failed to respond to traditional analgesics, after treatment with gabapentin 400 mg 3 times daily, the headache was relieved remarkably in 24 h.[9]
Gabapentin was used before by Moore et al.[10] in a similar group of pregnant females undergoing cesarean section in a study conducted to assess the efficacy of different doses of gabapentin on post-cesarean section pain. They concluded that a single dose of gabapentin 600 mg given 1 h before cesarean section significantly improves pain scores in the first 48 h post-partum and increases patient satisfaction.
A possible mechanism for gabapentin-mediated analgesia is the modulation of glutamate receptors (NMDA and AMPA/kainite). Gabapentin seems to decrease both NMDA and non-NMDA-mediated glutamate currents in the superficial lamina of the spinal cord of the rats[11] and also inhibits nociceptive responses to intrathecal NMDA and AMPA in vivo.[12]
Suarez et al.[13] suggested that sodium entry through pre-synaptic NMDA-K channels facilitates axon excitability and the interaction of gabapentin with this mechanism might contribute to its analgesic benefits.
Another suggested mechanism for gabapentin is that it binds to the voltage-dependent calcium channels.[14] Dirks et al.[15] determined that a single dose of gabapentin reduced post-operative morphine use and pain during movement in the first 4 hours after surgery.
Turan et al.[16] showed that gabapentin decreased post-operative analgesic consumption and pain scores in patients undergoing rhinoplasty and endoscopic sinus surgery.
However, Short et al.[17] conducted a study on women undergoing elective cesarean section and were randomized to 3 groups to receive 300 or 600 mg gabapentin or placebo, 1 h before surgery. The study showed that the pre-operative dose didn’t improve post-operative pain management or maternal satisfaction.
Sedation is a common side-effect of gabapentin. In the present study, the number of parturients in the gabapentin group who reported sedation was 11 versus 3 in the group who didn’t receive gabapentin. However, according to the Ramsey sedation score that we used, all the parturients had a score below 3, which didn’t interfere with their caring for the babies and didn’t delay their hospital discharge. Also, other side-effects of gabapentin were not reported in any of the two groups.
It is known that gabapentin crosses the placenta and is secreted in the breast milk.[18] That is why we examined the neonates for Apgar scores, umbilical artery pH and NICU admission and difficulties in breast feeding initiation. The results were similar among both groups.
The gabapentin pregnancy registry reviewed 39 women who had received gabapentin during pregnancy, of which 36 had received gabapentin throughout pregnancy and concluded that the exposure didn’t tend to any increased risk for adverse fetal or maternal events.[19]
As regard the effects of gabapentin on breastfeeding, many studies have been conducted on infants who were breastfed during maternal use of gabapentin for epilepsy with doses up to 2.1 gm and no adverse events on the newborn were noted.[18]
However, the dose of gabapentin chosen for our study was based on the studies that mentioned that a single oral dose of either 300 mg or 600 mg given to the mother before cesarean section appeared to have no effect on breastfeeding initiation or the baby well-being.[10,17] Does increasing the pre-operative dose of gabapentin will have more beneficial effects on the PDPH? And if yes, this will be on the expense of seeing some adverse effects on the mother and the baby? More studies need to be done to answer these questions.
CONCLUSION
A single pre-operative dose of 600 mg of gabapentin has no effect on the incidence of the post-dural puncture headache but delays its onset and reduces its severity and duration in parturients undergoing cesarean section with spinal anesthesia without significant adverse effects on the mother or the baby.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Erol DD. The effect of oral gabapentin on postdural puncture headache. Acute Pain. 2006;8:169–73. [Google Scholar]
- 2.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morewood GH. A rational approach to the cause, prevention and treatment of postdural puncture headache. Can Med Assoc J. 1993;149:1087–93. [PMC free article] [PubMed] [Google Scholar]
- 4.Choi A, Laurito CE, Cunningham FE. Pharmacologic management of postdural puncture headache. Ann Pharmacother. 1996;30:831–9. doi: 10.1177/106002809603000722. [DOI] [PubMed] [Google Scholar]
- 5.Turnbull DK, Shepherd DB. Postdural puncture headache: Pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718–29. doi: 10.1093/bja/aeg231. [DOI] [PubMed] [Google Scholar]
- 6.Scavone BM, Wong CA, Sullivan JT, Yaghmour E, Sherwani SS, McCarthy RJ. The Efficacy of a prophylactic epidural blood patch in preventing post dural puncture headache in parturients after inadvertent dural puncture. Anesthesiology. 2004;101:1422–7. doi: 10.1097/00000542-200412000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Sweetman SC. London: Pharmaceutical Press; [Last accessed on 2013 May 17]. Martindale: The complete drug reference. Available from: http://www.medicinescomplete.com/ [Google Scholar]
- 8.Mulleners WM, Chronicle EP. Anticonvulsants in migraine prophylaxis: A Cochrane review. Cephalalgia. 2008;28:585–97. doi: 10.1111/j.1468-2982.2008.01571.x. [DOI] [PubMed] [Google Scholar]
- 9.Lin YT, Sheen MJ, Huang ST, Horng HC, Cherng CH, Wong CS, et al. Gabapentin relieves post-dural puncture headache – a report of two cases. Acta Anaesthesiol Taiwan. 2007;45:47–51. [PubMed] [Google Scholar]
- 10.Moore A, Costello J, Wieczorek P, Shah V, Taddio A, Carvalho JC. Gabapentin improves post-cesarean delivery pain management: A randomized, placebo-controlled trial. Anesth Analg. 2011;112:167–73. doi: 10.1213/ANE.0b013e3181fdf5ee. [DOI] [PubMed] [Google Scholar]
- 11.Shimoyama M, Shimoyama N, Hori Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain. 2000;85:405–14. doi: 10.1016/S0304-3959(99)00283-3. [DOI] [PubMed] [Google Scholar]
- 12.Yoon MH, Choi JI, Jeong SW. Spinal gabapentin and antinociception: Mechanisms of action. J Korean Med Sci. 2003;18:255–61. doi: 10.3346/jkms.2003.18.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suárez LM, Suárez F, Del Olmo N, Ruiz M, González-Escalada JR, Solís JM. Presynaptic NMDA auto-receptors facilitate axon excitability: A new molecular target for the anticonvulsant gabapentin. Eur J Neurosci. 2005;21:197–209. doi: 10.1111/j.1460-9568.2004.03832.x. [DOI] [PubMed] [Google Scholar]
- 14.Maneuf YP, Gonzalez MI, Sutton KS, Chung FZ, Pinnock RD, Lee K. Cellular and molecular action of the putative GABA-mimetic gabapentin. Cell Mol Life Sci. 2003;60:742–50. doi: 10.1007/s00018-003-2108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dirks J, Fredensborg BB, Christensen D, Fomsgaard JS, Flyger H, Dahl JB. A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology. 2002;97:560–4. doi: 10.1097/00000542-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Turan A, Memiş D, Karamanlioğlu B, Yağiz R, Pamukçu Z, Yavuz E. The analgesic effects of gabapentin in monitored anesthesia care for ear-nose-throat surgery. Anesth Analg. 2004;99:375–8. doi: 10.1213/01.ANE.0000136646.11737.7B. [DOI] [PubMed] [Google Scholar]
- 17.Short J, Kristi Downey K, Bernstein P, Shahand V, Carvalho JC. A single preoperative dose of gabapentin does not improve post cesarean delivery pain management: A randomized, double-blind, placebo-controlled dose-finding trial. Anesth Analg. 2012;115:1336–42. doi: 10.1213/ANE.0b013e31826ac3b9. [DOI] [PubMed] [Google Scholar]
- 18.Ohman I, Vitols S, Tomson T. Pharmacokinetics of gabapentin during delivery, in the neonatal period, and lactation: Does a fetal accumulation occur during pregnancy? Epilepsia. 2005;6:1621–4. doi: 10.1111/j.1528-1167.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 19.Mountoris G. Gabapentin exposure in human pregnancy: Results from the gabapentin pregnancy registry. Epilepsy Behav. 2003;4:310–7. doi: 10.1016/s1525-5050(03)00110-0. [DOI] [PubMed] [Google Scholar]


