Synopsis
Allogeneic hematopoietic cell transplantation (HCT) is an important therapeutic option for a variety of malignant and nonmalignant conditions. As the number of allogeneic HCT continues to increase, greater attention to improvements in supportive care, infectious prophylaxis, immunosuppressive medications and DNA-based tissue typing are continuously being made. However, graft versus host disease (GVHD) remains the most frequent and serious complication following allogeneic HCT and limits the broader application of this important therapy. Recent advances in the understanding of the pathogenesis of GVHD have led to new approaches to its management, including utilizing its role in preserving the graft versus leukemia effect following allogeneic transplant. Here we review the important elements in the complex immunological interactions involving cytokine networks, chemokine gradients and the direct mediators of cellular cytotoxicity that cause clinical GVHD, and discuss the risk factors and strategies for management of GVHD.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an important therapeutic option for a variety of malignant and nonmalignant conditions [1]. The indication for its use has expanded, especially among older patients, over the last several years through novel strategies utilizing donor leukocyte infusions, non-myeloablative conditioning and umbilical cord blood (UCB) transplantation [2]. As the number of allogeneic HCT continues to increase, with more than 20,000 allogeneic transplantations performed annually worldwide, greater attention to improvements in supportive care, infectious prophylaxis, immunosuppressive medications and DNA-based tissue typing are continuously being made. Despite advances, graft versus host disease (GVHD) remains the most frequent and serious complication following allogeneic HCT and limits the broader application of this important therapy [3]. GVHD can be considered an exaggerated manifestation of a normal inflammatory mechanism in which donor lymphocytes encounter foreign antigens in a milieu that fosters inflammation. In the context of hematological malignancies, a delicate balance exists between the harmful consequences of GVHD and the beneficial effects incurred when donor lymphocytes attack recipient malignant cells, a process referred to as the graft versus leukemia/tumor (GVL) effect. Given the increasing number of transplant recipients, there will be an increasing population of patients with GVHD. Recent advances in the understanding of the pathogenesis of GVHD have led to new approaches to its management, including utilizing its role in preserving the GVL effect following allogeneic transplant. Here we review the important elements in the complex immunological interactions involving cytokine networks, chemokine gradients and the direct mediators of cellular cytotoxicity that cause clinical GVHD, and discuss the risk factors and strategies for management of GVHD.
ACUTE GVHD
Epidemiology and Risk Factors
In 1966, Billingham formulated three requirements for the development of GVHD: the graft must contain immunologically competent cells; the recipient must express tissue antigens that are not present in the transplant donor; and the recipient must be incapable of mounting an effective response to eliminate the transplanted cells [4]. It is now known that T cells are the immunologically competent cells, and when tissues containing T cells (blood products, bone marrow, and solid organs) are transferred from one person to another who is unable to eliminate those cells, GVHD can develop [5, 6].
Allogeneic HCT is the most common setting for the development of GVHD where recipients receive immunoablative chemotherapy and/or radiation prior to hematopoietic cell infusion containing donor T cells. GVHD ultimately develops when donor T cells respond to recipient tissue antigens secondary to mismatches between major and/or minor histocompatibility antigens between the donor and recipient. The major histocompatibility complex (MHC) contains the genes that encode tissue antigens. In humans, the MHC region lies on the short arm of chromosome 6 and is called the HLA (human leukocyte antigen) region [7]. Class I HLA (A, B, and C) proteins are expressed on almost all nucleated cells of the body at varying densities. Class II (DR, DQ, and DP) proteins are primarily expressed on hematopoietic cells (B cells, dendritic cells, monocytes, and activated T cells), but their expression can be induced on many other cell types following inflammation or injury. High-resolution DNA typing of HLA genes with polymerase chain reaction (PCR)-based techniques have now largely replaced earlier methods. The incidence of GVHD is directly related to HLA disparity [8, 9] and with more HLA-mismatches, the likelihood of developing GVHD increases [10, 11]. Recent data from the National Marrow Donor Program (NMDP) suggests that high-resolution matching for HLA-A, -B, -C, and –DRBI (8/8 match) maximizes posttransplant survival [12, 13].
Despite HLA identity between a patient and donor, the incidence of acute GVHD ranges from 26% to 32% in recipients of sibling donor grafts, and 42% to 52% in recipients of unrelated donor grafts (CIBMTR Progress Report January – December 2008). The incidence is likely related to genetic differences that lie outside the HLA loci, or “minor” histcompatibility antigens (HA), which are immunogenic peptides derived from polymorphic proteins presented on the cell surface by MHC molecules [14]. Some minor HAs, such as HY and HA-3, are expressed on all tissues and are targets for both GVHD and GVL, while other minor HAs, such as HA-1 and HA-2, are expressed abundantly on hematopoietic cells (including leukemic cells) and may induce a greater GVL effect with less GVHD [14, 15]. However, the precise elucidation of the majority of human minor antigens remains to be accomplished [14, 16].
The impact of donor and recipient polymorphisms in cytokine genes with critical roles in the classical ‘cytokine storm’ of GVHD has been examined as a risk factor for GVHD [17]. A variety of polymorphic genes, including tumor necrosis factor (TNF)-α, interleukin 10 (IL-10), and interferon-γ (IFN γ) variants, have been associated with GVHD, although not always [18–20]. At present, there is no unequivocal evidence that polymorphic genes for cytokines or other proteins involved in innate immunity [21–24] sufficiently influence GVHD and transplant outcome to change clinical practice. Nonetheless, future strategies to identify the best possible transplant donor will likely incorporate both HLA and non-HLA genetic factors.
In addition to genetic factors, other risk factors which have been associated with the development of GVHD include older donor and recipient age [25–28], multiparous female donor [28, 29], advanced malignant condition at transplantation [9, 29], donor type [28], and donor hematopoeitic cell source [30–32]. Over the last decade, there has been a shift in clinical practice from the use of intraoperative harvested bone marrow (BM) to G-CSF mobilized peripheral blood stem cells (PBSC) as the donor hematopoietic cell source. However, definitive data demonstrating long-term advantages of PBCS rather than BM are lacking. One meta-analysis found that both acute and chronic GVHD are more common following peripheral blood stem cell transplant (PBSCT) compared with bone marrow transplant (BMT) and indicated a trend towards decreased relapse rate following PBSCT [31]. The relative risk (RR) for acute GVHD after PBSCT was 1.16 (95% CI, 1.04 to 1.28) compared with BMT; the RR for chronic GVHD after PBSCT was 1.53 (95% CI, 1.25 to 2.05); and the RR for relapse after PBSCT was 0.81 (95% CI, 0.62 to 1.05). Thus, the survival benefit of PBSC versus BM remains in question. A large prospective, randomized, multicenter clinical trial of PBSC versus BM in unrelated donor transplantation conducted through the Bone Marrow Transplant Clinical Trials Network has recently finished accrual.
For those without a suitable HLA-matched donor, umbilical cord blood (UCB) has become an alternative to bone marrow or peripheral blood stem cells [33–36]. The incidence and severity of acute GVHD appears to be lower following UCB transplant than after HLA-matched marrow unrelated donor transplant, despite HLA disparities between the donor and recipient [37, 38]. In an effort to meet the minimum cell dose required to ensure reliable engraftment, the simultaneous transplantation of two partially HLA-matched UCB units has been studied [39]. A recent report comparing transplantation with two partially HLA-matched UCB units versus a single unit demonstrated an increased incidence and earlier presentation of acute GVHD associated with the double UCB graft [40].
Prevention
Prevention of acute GVHD is an integral component to the management of patients undergoing allogeneic HCT. The primary strategy employed is in the use of pharmacologic GVHD prophylaxis. The most widely used GVHD prophylaxis following full intensity conditioning includes a combination of a calcineurin inhibitor (e.g. cyclosporine, tacrolimus) with methotrexate (MTX). This standard regimen was initially described in 1986 [41] and since then several clinical trials have shown the superiority of this combination in reducing the incidence of GVHD and improving survival compared to either agent alone [42–45]. Calcineurin inhibitors, cyclosporine and tacrolimus, impede the function of the cytoplasmic enzyme, calcineurin, which is critical in the activation of T cells. The most common side effects include hypomagnesemia, hyperkalemia, hypertension, and nephrotoxicity [46]. Large randomized studies comparing tacrolimus-MTX to cyclosporine-MTX have demonstrated a reduced incidence of grade II-IV acute GVHD with tacrolimus, but no overall survival advantage [43, 46].
Recently, sirolimus, a widely used immunosuppressant in solid organ transplantation [47], has become attractive to use as a GVHD prophylactic agent because of the non-overlapping toxicities with calcineurin inhibitors and the different mechanism of action. Sirolimus binds uniquely to FK binding protein 12 (FKBP12) and then complexes with mTOR (mammalian Target of Rapamycin) [48]. Several studies have shown that the combination of sirolimus and tacrolimus has resulted in rapid engraftment, low incidence of acute GVHD, reduced transplant-related toxicity, and improved survival [49]. A prospective, randomized, multicenter trial is currently being conducted through the BMT CTN (protocol 0402) comparing sirolimus-tacrolimus versus tacrolimus-MTX following HLA-matched, related donor peripheral blood stem cell transplantation.
A commonly used GVHD prophylaxis following reduced-intensity conditioning includes a combination of a calcineurin inhibitor (e.g. cyclosporine, tacrolimus) with mycophenolate mofeitil (MMF) instead of MTX. MMF, the prodrug of mycophenolic acid, selectively inhibits inosine monophosphate dehydrogenase, an enzyme critical to the de novo synthesis of guanosine nucleotide, which is needed for proliferation of T cells. In a prospective randomized trial, patients who received MMF as part of GVHD prophylaxis experienced significantly less severe mucositis and more rapid neutrophil engraftment than those who received MTX [50]. Although the optimal prophylaxis regimen following reduced-intensity HCT is not well established, mycophenolate mofetil (MMF) has been shown to be safe in this context [51–55]. MMF is also often preferred to MTX in UCB transplants due to its advantageous toxicity profile with respect to neutropenia and mucositis.
Many centers have previously attempted to decrease the risk of GVHD by ex vivo T cell depletion. Despite significant reductions in the incidence and severity of GVHD, T cell depletion has not achieved wide acceptance because of high rates of graft rejection, life-threatening infections and leukemia relapse [56–58]. In vivo T cell depletion has also been widely studied utilizing alemtuzumab, a monoclonal antibody specific for CD52 antigen expressed abundantly on the surface of normal and malignant lymphocytes [59, 60], or anti-thymocyte globulin (ATG), a polyclonal antibody mixture of either horse or rabbit origin directed against multiple epitopes of human T cells [61]. These approaches are associated with significant reduction in acute GVHD, but at the cost of impaired immune reconstitution and increased risk of leukemia relapse. Thus, the focus of most prevention strategies remains pharmacological manipulation of T cells following transplant.
Pathophysiology
Acute GVHD is mediated by donor lymphocytes infused into the recipient where they encounter profoundly damaged tissues from the effects of the underlying disease, prior infections, and the transplant conditioning regimen. The allogeneic donor cells encounter a foreign environment that has been altered to promote the activation and proliferation of inflammatory cells. Thus, acute GVHD reflects an exaggerated response of the normal inflammatory mechanisms that involves donor T cells as well as multiple innate and adaptive cells and mediators. Three sequential phases can be conceptualized to illustrate the complex cellular interactions and inflammatory cascades that ultimately evolve to acute GVHD: (1) activation of antigen presenting cells (APCs); (2) donor T cell activation, proliferation, differentiation and migration; and (3) target tissue destruction [62].
Phase 1: Activation of APCs
In the first phase, APCs are activated by the underlying disease and the HCT conditioning regimen [63]. Both animal models [63, 64] and clinical HCT [65] have supported the observation that increased risk of GVHD is associated with intensive conditioning regimens which contribute to extensive tissue injury and subsequent release of inflammatory cytokines. Damage to host tissues leads to the secretion of proinflammatory cytokines, such as TNF- α and IL-1, and chemokines, such as CCL2-5 and CXCL9-11, thereby producing increased expression of adhesion molecules, MHC antigens and costimulatory molecules on host APCs. For example, elevation of plasma TNF-α receptor 1 levels, a surrogate marker for TNF-α, at one week after HCT strongly correlates with the later development of GVHD [66]. Systemic translocation of immuno-stimulatory microbial products, such as lipopolysaccharide (LPS), as a result of conditioning regimen induced damage to the gastrointenstinal (GI) tract, enhances the activation of host APCs [67, 68]. The initial site of interaction between activated APCs and donor T cells is likely the secondary lymphoid tissue in the GI tract [69]. Different distinct subsets of APCs, including host and donor type APCs [68, 70, 71], dendritic cells [72, 73], Langerhans cells [74], and monocytes/macrophages [75], have been implicated in this phase. However, the relative contributions of these various APCs remain to be elucidated. The intensity of the conditioning regimen and the degree of tissue injury appear to be associated with the risk of GVHD. Reduced intensity conditioning regimens have thus become more widely employed in an effort to reduce acute GVHD by decreasing the damage to host tissues [65, 76].
Phase 2: Donor T Cell Activation
Donor T cell activation, proliferation, differentiation and migration in response to primed APCs occurs during the second phase of acute GVHD. The T cell receptors (TCR) of donor T cells recognize alloantigens on both host and donor type APCs that are present in secondary lymphoid organs [77, 78]. During direct presentation, donor T cells recognize either the peptide bound to host MHC molecules, or the foreign MHC molecules themselves [79]. During indirect presentation, donor T cells respond to the peptides generated by degradation of the host MHC molecules that are presented on donor-derived MHC [80].
Following antigen recognition, signaling through the TCR induces a conformational change in adhesion molecules, resulting in high affinity binding to the APC [81]. The complex interaction between T cell co-stimulatory molecules and their ligands on APCs facilitates full T cell activation. A large number of T cell costimulatory molecules display both unique and overlapping functions [82]. Receptors of the B7 family and the TNF family play especially critical roles in GVHD, and are known to deliver both positive and negative signals to T cells [83]. Blockade of costimulatory and inhibitory pathways can reduce acute GVHD in murine models, but this approach has not yet been tested in clinical trials [2].
Murine studies have shown that control of alloreactive responses responsible for GVHD depends at least in part on CD4+ CD25+ regulatory T cells. Studies in mice suggest that regulatory T cells added to donor grafts can prevent or delay GVHD [84]. However, the role of regulatory T cells in clinical allogeneic HCT has not been well established in part due to the lack of clear identification of human regulatory T cell phenotype. In contrast to murine studies, more severe acute GVHD developed clinically when donor grafts contained larger numbers of donor CD4+ CD25+ T cells [85]. One recent study found that HCT recipients with higher absolute numbers of FOXP3+ CD4+ T cells were associated with a reduced risk of developing GVHD [86]. However, FOXP3 expression in humans is not specific for regulatory T cell phenotype [87], and improved techniques to identify and expand regulatory T cells are required for its wider application in clinical BMT.
Several intracellular biochemical pathways are rapidly amplified following T cell activation. Activated T cells secrete cytokines that are generally classified as Th1 (IFN-γ, IL-2 and TNF-α) or Th2 (IL-4, IL-5, IL-10 and IL-13) and Th17. Although Th1 cytokines induce GVHD efficiently, the balance of Th1 and Th2 cytokines is important in the immunopathogenesis of GVHD, but remains incompletely understood [88]. TNF-α, already discussed as an inducer of APC activation in phase I, functions to amplify T cell activation and proliferation in this second phase of GVHD. IL-2 production is also integral in this early development of acute GVHD [89], and remains the principal target of many current clinical therapeutic and prophylactic approaches to GVHD, such as cyclosporine, tacrolimus and monoclonal antibodies against IL-2 and its receptor [44]. However, recent data suggest that regulatory T cell function is dependent on the presence of calcineurin-dependent IL-2, and that interference with IL-2 may possibly antagonize the induction of tolerance [90].
IFN-γ is a cytokine with diverse effects in vivo that can both amplify and suppress acute GVHD. With regards to amplification, IFN-γ increases the expression of molecules such as chemokine receptors, MHC proteins, and adhesion molecules. IFN-γ also sensitizes monocytes and macrophages to stimuli such as LPS, thereby accelerating intracellular cascades in response to these stimuli [88]. IFN-γ may also amplify GVHD by directly damaging target cells in the GI tract and in the skin while conversely inducing nitric oxide-mediated immunosuppression [91]. Interestingly, IFN-γ itself can prevent experimental GVHD by hastening the apoptosis of activated donor T cells [92]. Thus, this complexity makes it a challenging target with respect to therapeutic intervention.
IL-10 can suppress the expression of proinflammatory cytokines, chemokines, and adhesion molecules, as well as antigen-presenting and costimulatory molecules in monocytes/macrophages, neutrophils, and T cells [93]. Recent clinical data suggest that genetic polymorphism in the IL-10 promoter region of the recipient has a significant impact on the risk of developing acute GVHD [94]. Increased IL-10 production by recipient cells stimulated ex vivo has been associated with a reduced risk of acute GVHD [95]. Experimental data have also demonstrated that TGF-β, another suppressive cytokine, attenuates acute GVHD, but may lead to chronic GVHD [96]. Thus multiple cytokines are important in GVHD pathogenesis and regulation. Furthermore, the timing and duration of cytokine expression may be a critical factor determining the induction of the GVH reaction, and cytokine dysregulation could potentially contribute to the severity of acute GVHD [62].
Phase 3: Cellular and Inflammatory Effector Phase
A complex cascade of cellular and inflammatory mediators occurs during the effector phase of acute GVHD. These mediators synergize to amplify local tissue injury and damage target tissues. As such, the effector phase of GVHD involves aspects of both the innate and adaptive immune response as well as interactions with the proinflammatory cells and cytokines generated during phase 1 and 2.
Cellular Effectors
Cytotoxic T cells (CTLs) are the major cellular effectors of acute GVHD and lyse target cells using principally the Fas/Fas ligand (FasL) and the perforin/granzyme pathways [97]. The Fas-FasL pathway appears to predominate in hepatic GVHD whereas the perforin/granzyme pathways are more important in the GI tract and skin [2]. The “danger” signals generated in phase 1, augmented by the expression of costimulatory molecules in phase 2, induce the production of chemokines. The migration of donor T cells from lymphoid tissues to the target organs where damage occurs is directed by these chemokine gradients and adhesion molecules such as L-selectin and αLB2 integrin. The up-regulation of chemokines, such as CCL2, CCL3, CCL4, CCL5 and CXCL9, CXCL10, and CXCL11 in GVHD target organs (skin, liver, and gut), play primary roles in this homing process [98]. Furthermore, integrin receptors, which are regulated by chemokines, mediate many adhesive interactions that are critical for successful T cell migration [99].
Inflammatory Effectors
The secretion of inflammatory cytokines, such as TNF-α or IL-1 also results in GVHD target organ injury. The secretion signal may be provided through toll-like receptors (TLRs) by LPS and other microbial products that have leaked through damaged intestinal mucosa by the conditioning regimen in phase 1 [100]. TNF-α is produced by both donor and host cells and is a critical component of GVHD pathophysiology. TNF-α can (1) activate APCs and enhance alloantigen presentation, (2) recruit effector cells to target organs through the induction of inflammatory chemokines, and (3) directly cause tissue damage via apoptosis and necrosis. IL-1 is also involved in the pathogenesis of acute GVHD [17]. Its secretion occurs predominantly in the spleen and skin during the effector phase of experimental GVHD [101]. Increased expression of mononuclear IL-1 mRNA has been associated with the development of GVHD [102]. However, the blockade of IL-1 using recombinant human IL-1 receptor antagonist (IL-1Ra) in patients undergoing HCT did not reduce the risk of GVHD [103].
Clinical Features
The three main organs involved in acute GVHD are the skin, liver, or gastrointestinal (GI) tract. GVHD presents clinically in an acute or chronic form. Historically, the acute and chronic forms were arbitrarily defined based on the timeframe post-transplant (less than or greater than 100 days, respectively) [104]. However, current consensus is that clinical manifestations guide whether the signs and symptoms of GVHD are acute, chronic, or an overlap syndrome (wherein diagnostic or distinctive features of acute and chronic GVHD appear together) [105].
The extent (stage) of involvement of the three principal target organs determines the overall severity (grade) of acute GVHD (Table 1) [106]. The overall grades are classified as I (mild), II (moderate), III (severe) and IV (very severe). A poor prognosis is associated with severe grade III or IV GVHD, with 25% and 5% overall survival, respectively [107].
Table 1. Acute GVHD Grading Criteria.
The standard GVHD grading criteria was developed by Glucksberg in 1974 [146], and then revised at the 1994 Consensus Conference in Keystone [147]. The grading system did not initially take into consideration the staging of the GI tract for pediatric patients. However, most pediatric centers have adopted the modified Glucksberg grading scale and adjusted the staging of the GI tract to be based on volume per kilogram of body weight.
| Stage | Skin | Liver (bilirubin) | GI tract (stool output/day) |
|---|---|---|---|
| 0 | No GVHD rash | < 2 mg/dl | Adult: < 500 ml/day Child: < 10 ml kg/day |
| 1 | Maculopapular rash < 25% BSA |
2–3 mg/dl | Adult: 500–999 ml/day Child: 10–19.9 ml/kg/day or persistent nausea, vomiting, or anorexia, with a positive upper GI biopsy |
| 2 | Maculopapular rash 25–50% BSA |
3.1–6 mg/dl | Adult: 1000–1500 ml/day Child: 20–30 ml/kg/day |
| 3 | Maculopapular rash > 50% BSA |
6.1–15 mg/dl | Adult: >1500 ml/day Child: > 30 ml/kg/day |
| 4 | Generalized erythroderma with bullous formation | > 15 mg/dl | Severe abdominal pain with or without ileus |
BSA = body surface area, GI = gastrointestinal, GVHD = graft versus host disease.
Overall Clinical Grade
| 0 | No stage 1–4 of any organ |
| 1 | Stage 1–2 skin rash and no liver or GI involvement |
| 2 | Stage 3 skin rash, or Stage 1 liver involvement, or Stage 1 GI involvement |
| 3 | Stage 0–3 skin rash, with Stage 2-3 liver involvement, and/or Stage 2–3 GI involvement |
| 4 | Stage 4 skin rash, liver, and/or GI involvement |
Skin is generally the first and most commonly affected organ [104]. The presentation of skin involvement generally coincides with donor cell engraftment and is characterized by an erythematous, maculopapular rash that is often pruritic. In severe cases the skin may blister and ulcerate [108]. The pathognomonic histopathologic finding is apoptosis at the base of epidermal rete ridges. Other features include dyskeratosis, exocytosis of lymphocytes, satellite lymphocytes adjacent to dyskeratotic epidermal keratinocytes, and a perivascular lymphocytic infiltration in the dermis [1, 109].
Liver GVHD can be a challenging diagnosis. The initial feature typically includes the development of jaundice or an increase in the alkaline phosphatase and bilirubin; hepatomegaly may be noted. However, it is often difficult to distinguish from other causes of liver dysfunction following allogeneic HCT, such as drug toxicity, veno-occlusive disease, opportunistic (bacterial, viral and fungal) infections, TPN, acalculous cholecystitis, and iron overload, because of the overlapping patterns of clinical history, physical examination, and laboratory and imaging data. Thus, a biopsy is often required to confirm the diagnosis of liver GVHD [110]. The histologic features are endothelialitis, lymphocytic infiltration of the portal areas, pericholangitis, and bile duct destruction [111]. However, the increased risk of bleeding associated with thrombocytopenia in the immediate posttransplant period can preclude obtaining a biopsy. As such, the diagnosis of liver GVHD is often a clinical diagnosis of exclusion.
GI tract involvement of acute GVHD may present as nausea, vomiting, anorexia, diarrhea, and/or abdominal pain. It is a pan-intestinal process with focal lesions of widely varying intensity. Gastric involvement gives rise to postprandial vomiting that is not always preceded by nausea. The diarrhea of GVHD is secretory and may be accompanied by significant GI bleeding as a result of mucosal ulceration, which is a prognostic factor for poor outcome [112]. In advanced disease, diffuse, severe abdominal pain and distension is accompanied by voluminous diarrhea. The histologic features include patchy ulcerations apoptotic bodies in the base of crypts, crypt abscesses, and loss as well as flattening of the epithelium surface [113].
Diagnostic Approach
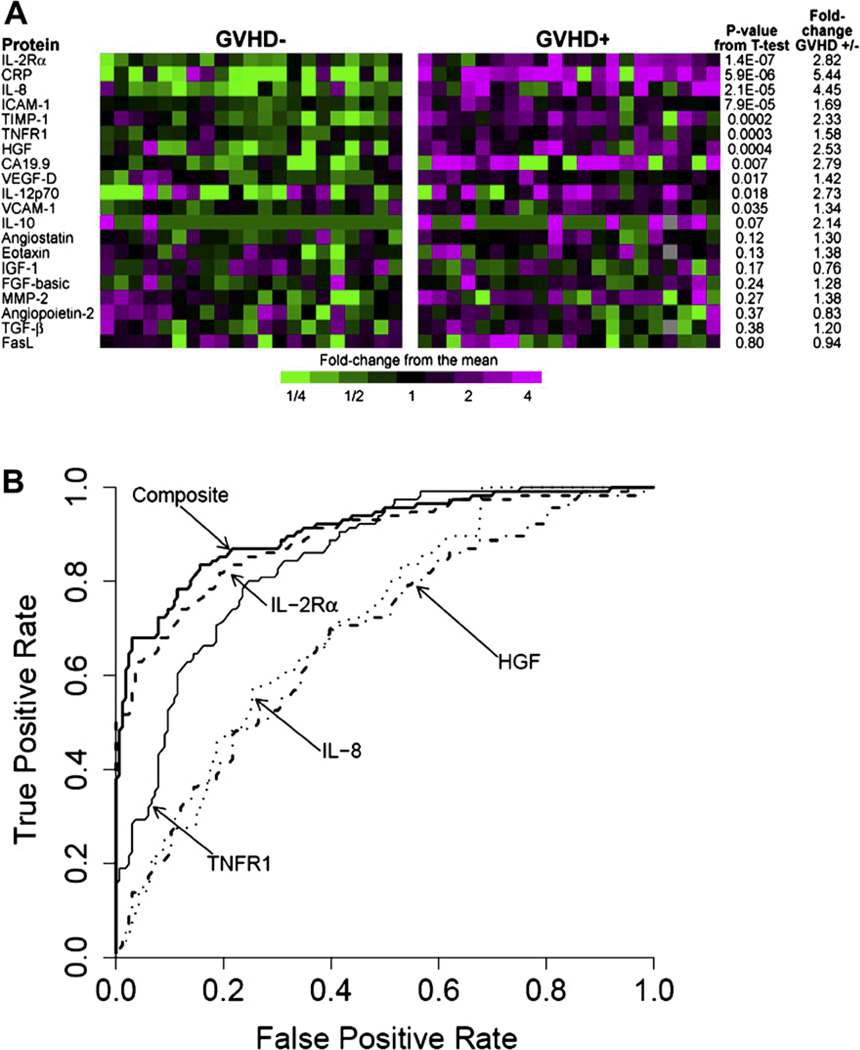
The diagnosis of acute GVHD is based entirely on clinical criteria that can be confirmed by biopsy of one of the three target organs. Laboratory data and/or imaging studies are also useful tests in the diagnostic approach to GVHD. However, a major challenge with the diagnosis is the absence of laboratory tests that can reliably predict or screen for the condition prior to its onset: establish a diagnosis in real-time; distinguish it from other conditions that present with similar symptoms, such as infection; or stratify patients according to response to therapy. Thus, experimental blood tests with predictive value for GVHD such as a four biomarker panel [114, 115] may ultimately be useful to further identify high-risk groups and their outcomes (Figure 1). These biomarkers could potentially result in immunosuppressive treatment schemas tailored to patients in several risk groups.
Figure 1. From discovery to validation of plasma biomarkers of acute graft versus host disease.
(This research was originally published in Blood. [115]
Panel A shows the heatmap of proteins levels measured sequential ELISA in the discovery set samples. Samples from 21 GVHD– patients (left) and 21 GVHD+ patients (right) are represented. Eleven proteins gave a P values for differences between GVHD+ and GVHD– patient plasma< 0.05. Panel B shows the receiver operating characteristic (ROC) curves of four individual discriminator proteins and the composite panel in the training set. Individual ROC curves for IL-2Ra,TNFR1, HGF, and IL-8 and the composite panel.
Treatment
Despite routine treatment with calcineurin-based prophylaxis, GVHD remains a major complication of allogeneic HCT. The most important predictor of long-term survival in patients with acute GVHD is the primary response to the first-line of treatment [116]. Many centers treat mild skin GVHD (grade I) with topical corticosteroids alone, but for more severe skin GVHD and/or visceral GVHD involvement (grade II or greater), systemic corticosteroids are the mainstay of therapy, typically starting at 1–2 mg/kg/day. Unfortunately, durable responses occur in less than half of patients with grade II to IV GVHD [117], and with increasing severity of the disease, the likelihood of response decreases [104]. Treatment with high-dose steroids often continues for 7 days or more, with a gradual dose-reduction depending on the clinical response. In a recent retrospective analysis, low-dose (1 mg/kg/day) versus high-dose (2 mg/kg/day) prednisone was compared for initial treatment of acute GVHD. In patients with grades I-II GVHD, the non-relapse mortality and overall survival were similar between regimens, with a reduced risk of invasive fungal infections and shorter hospitalizations in the low-dose prednisone group. The number of patients with grades III-IV at onset was too few to draw any significant conclusions.
In addition to topical corticosteroid therapy for skin GVHD, non-absorbed steroid therapy is commonly used in GI GVHD. Oral budesonide in combination with systemic corticosteroids in patients with ≥ grade II acute GI GVHD has shown complete responses in 77% of patients compared to 32% of historical controls [118]. In a more recent randomized, placebo-controlled trial of oral beclamethasone for GI GVHD, oral BDP reduced GVHD flares following a prednisone taper and resulted in superior survival at 1 year posttransplant [119]. Intra-arterial administration of steroids for GI and hepatic GVHD has also been attempted in order to deliver steroids directly to the target organ [120].
Prolonged therapy with steroids invariably increases the risk of patients developing muscle weakness and wasting, avascular necrosis, compression fractures, hypertension, hyperglycemia, behavior disturbances, and life-threatening infections. Furthermore, the optimal dose and duration of high-dose steroids, as well as the choice and timing of when to institute second therapy remain unclear [121]. Thus, the management of a patient who develops steroidrefractory acute GVHD is challenging. Generally, steroid-refractoriness is defined as disease progression or lack of response following 3–7 days of systemic therapy with corticosteroids and a calcineurin inhibitor. Once steroidrefractory GVHD develops, a variety of agents may be used and there is currently no standard approach practiced uniformly by the transplant community.
Table 2 summarizes the various drugs that have been studied in addition to steroids for treatment of acute GVHD. Among the agents discussed, mycophenolate mofeitil (MMF), etanercept, denileukin diftitox and pentostatin were most recently studied in a clinical investigation through the BMT CTN (study 0302). In this phase II, randomized trial of 180 patients with newly diagnosed acute GVHD, patients were treated with standard corticosteroids plus one of the four agents. MMF produced the best results with regards to toxicity profile, response, survival, incidence of chronic GVHD, and infections [122]. A randomized phase III study to validate these findings is currently being planned.
Table 2.
Selected studies of novel agents as secondary or first line therapy in acute GVHD
| Novel Therapy |
Type of trial | Type of treatment | Sample size | Overall Response at 4 weeks |
Survival at 3–6 months |
Study | Reference |
|---|---|---|---|---|---|---|---|
| Denileukin diftitox | Phase I | steroid refractory | 30 | 71% | 33% | Ho et al. | [148] |
| Denileukin diftitox | Phase II | steroid refractory | 22 | 41% | ND | Shaughnessy et al. | [149] |
| Denileukin diftitox | Phase II randomized | Initial therapy | 60 | 60% | 49% | Alousi et al. | [122] |
| Etanercept | Phase II | Initial therapy | 61 | 69% | 69% | Levine et al. | [150] |
| Etanercept | Phase II randomized | Initial therapy | 60 | 48% | 56% | Alousi et al. | [122] |
| Pentostatin | Phase I | steroid refractory | 23 | 78% | ND | Bolanos-Meade et al. | [151] |
| Pentostatin | Phase II randomized | Initial therapy | 60 | 62% | 52% | Alousi et al. | [122] |
| MMF | Phase I/II | steroid refractory | 48 | 72% | 78% | Basara et al. | [152] |
| MMF | Phase I/II | steroid refractory | 10 | 60% | 70% | Krejci et al. | [153] |
| MMF | Phase I/II | steroid refractory | 6 | 67% | 64% | Takami et al. | [154] |
| MMF | Phase II randomized | Initial therapy | 60 | 78% | 64% | Alousi et al. | [122] |
| Sirolimus | Phase I/II | steroid refractory | 21 | 57% | 28% | Benito et al. | [155] |
| BDP | Phase II randomized | Initial therapy | 129 | 71% | 87% | Hockenbery et al. | [119] |
| ECP | Phase II | steroid refractory | 59 | 68% | ND | Greinix et al. | [125] |
| ECP | Phase II | steroid refractory | 23 | 52% | 48% | Perfetti et al. | [126] |
| ECP | Phase II | steroid refractory | 41 (children) | 73% | 100% | Calore et al. | [127] |
| ECP | Phase II | steroid refractory | 15 (children) | 0% for grade IV GVHD | ND | Berger et al. | [128] |
| MSC | Phase II | steroid refractory | 55 | 71% | ND | Le Blanc et al. | [130] |
| MSC | Phase II | steroid refractory | 13 | 15% | 31% | von Bonin et al. | [156] |
| Alefacept | Phase I | steroid refractory | 16 | 75% | ND | Shapira et al. | [157] |
GVHD indicates graft versus host disease, MMF, mycophenolate mofetil, BDP, beclomethasone diproprionate, ECP, extracorporeal photopheresis, MSC, mesenchymal stromal cell
Extracorporeal photopheresis (ECP) is increasingly used in the management of acute and chronic GVHD in an effort to minimize corticosteroid exposure. During ECP, the patient’s peripheral blood mononuclear cells are exposed to photo-activated 8 methoxypsoralen (8-MOP) and ultraviolet A radiation, which covalently binds and cross-links DNA, and then returned to the patient. Experimental studies in humans and mice suggest ECP induces apoptosis of the mononuclear cells and increases the number of regulatory T cells [123, 124]. A phase II study of steroidrefractory GVHD showed complete response rates of 60% with overall survival of 59% at 4 years [125]. These data are similar to other studies which have demonstrated complete response rates between 52% and 83% [126–128].
Mesenchymal stromal cell infusion is another non-pharmacologic approach, but little is known about its mechanism of action. In vitro, mesenchymal stromal cells have various effects on immune cells, including T-cells, antigenpresenting cells, natural-killer cells, and B-cells. In phase I and II trials, HLA-identical mesenchymal stromal cells expanded ex vivo have been infused to promote hematopoietic recovery after autologous and allogeneic HCT [129, 130]. In a phase II study, mesenchymal stem cells were used for the treatment of corticosteroid-resistant, severe, acute GVHD [130]. Thirty-nine of 55 patients with corticosteroid-resistant, severe, acute GVHD responded to treatment with mesenchymal stem cells. In those who achieved a complete response, survival was significantly higher and transplant-related mortality was lower compared to those with partial or no response. No major toxicities were observed, and treatment with mesenchymal stem cells seemed to be safe. This therapy may be a promising treatment for corticosteroid-refractory acute GVHD, but further investigation is required.
CHRONIC GVHD
Epidemiology and Risk Factors
Chronic GVHD is the major cause of late non-relapse death following allogeneic HCT [131], and is associated with decreased quality of life and impaired physical and functional status [132]. It remains the most common problem in long-term survivors. However, chronic GVHD has been associated with beneficial GVL/GVT effects [133]. The median time of diagnosis of chronic GVHD is 4.5 months after HLA-identical sibling transplantation and 4 months after unrelated donor transplantation [134]. The incidence of chronic GVHD has been increasing with the wider availability of PBSCs and the increased age of transplant recipients. The development of chronic GVHD ranges from 30% in recipients of fully histocompatible sibling donor transplants to 60% to 70% in recipients of mismatched hematopoietic cells or hematopoietic cells from an unrelated donor. Other factors that likely increase the development of chronic GVHD include prior acute GVHD, older recipient age, female donor (multiparous, in particular) with a male recipient, and use of peripheral blood stem cells [132].
Prevention and Pathophysiology
Although prevention of acute GVHD has improved during the past three decades, no effective prophylaxis regimen currently exists for chronic GVHD. Management of chronic GVHD is guided by a multidisciplinary approach to treatment, including recent adjustment of immunosuppressive medications and aggressive supportive care.
Our understanding of the pathophysiology of chronic GVHD is not as advanced as that of acute GVHD. Chronic GVHD is a complex, multisystem disorder with myriad manifestations that involves many different organs (see below). It is characterized by immune dysregulation, immunodeficiency, impaired organ function and decreased survival [135]. Alloreactive T cells have been implicated in the pathogenesis; however, the precise role of specific T cell subsets, autoantigens, alloantigens, and B cells, as well as interactions of chemokines and cytokines has not been fully elucidated. The clinical manifestations of chronic GVHD are often similar to an autoimmune process, suggesting similar pathophysiology.
Clinical Features
The diagnosis of chronic GVHD is based upon specific signs, degree of organ involvement (mild, moderate, severe), laboratory data, and/or histopathologic confirmation rather than time of onset posttransplant (i.e. > 100 days). Historically, a classification of “limited” (localized skin involvement with or without limited hepatic dysfunction) versus “extensive” (generalized skin involvement or limited disease plus eye, oral, liver, or other target organ involvement) was the most commonly adopted staging system [136]. However, a new consensus criteria for the diagnosis and staging of chronic GVHD has been proposed by the National Institutes of Health Consensus Development Project (Table 3) [105]. Chronic GVHD may present as acute GVHD merging into chronic (progressive type), develop following a period of resolution from acute GVHD (quiescent or interrupted type), or occur de novo. An overlap syndrome includes clinical features in which diagnostic or distinctive features of chronic GVHD and acute GVHD appear together. Specific signs and symptoms, including erythematous skin rash, nausea, vomiting, diarrhea, and liver dysfunction are shared between the two.
Table 3.
Signs and Symptoms of Chronic GVHD (reproduced with permission) [105]
| Organ or Site |
Diagnostic (Sufficient to Establish the Diagnosis of Chronic GVHD) |
Distinctive (Seen in Chronic GVHD, but Insufficient Alone to Establish a Diagnosis of Chronic GVHD) |
Other Features* | Common (Seen with Both Acute and Chronic GVHD) |
|---|---|---|---|---|
| Skin | Poikiloderma Lichen planus-like features Morphea-like features Lichen sclerosus-like features |
Depigmentation | Sweat impairment Ichthyosis Keratosis pilaris Hypopigmentation Hyperpigmentation |
Erythema Maculopapular rash Pruritis |
| Nails | Dystrophy Longitudinal ridging, splitting, or brittle features Onycholysis Pterygium unguis Nail loss (usually symmetric; affects most nails)† |
|||
| Scalp and body hair | New onset of scarring or nonscarring scallop alopecia (after recovery from chemoradiotherapy) Scaling, papulosquamous lesions |
Thinning scalp hair, typically patchy, coarse, or dull (not explained by endocrine or other causes) Premature gray hair |
||
| Mouth | Lichen-type features Hyperkeratotic plaques Restriction of mouth opening from sclerosis |
Xerostomia Mucocele Mucosal atrophy Pseudomembranes† Ulcers† |
Gingivitis Mucositis Erythema Pain |
|
| Eyes | New onset dry, gritty, or painful eyes‡Cicatricial conjunctivitis Keratoconjunctivitis sicca‡Confluent areas of punctate keratopathy |
Photophobia Periorbital hyperpigmentation Blepharitis (erythema of the eyelids with edema) |
||
| Genitalia | Lichen planus-like features Vaginal scarring or stenosis |
Erosions† Fissures† Ulcers† |
||
| GI tract | Esophageal web Strictures of stenosis in the upper to mid third of the esophagus† |
Exocrine pancreatic insufficiency | Anorexia Nausea Vomiting Diarrhea Weight loss Failure to thrive (infants and children) Total bilirubin, alkaline phosphatase > 2×upper limit of normal†ALT or AST > 2×upper limit of normal† |
|
| Lung | Bronchiolitis obliterans diagnosed with lung bioopsy | Bronchiolitis obliterans diagnosed with PFTs and radiology‡ | BOOP | |
| Muscles, fascia, joints | Fasciitis Joint stiffness or contractures secondary to sclerosis |
Myositis of polymyositis‡ | Edema Muscle cramps Arthralgia or arthritis |
|
| Hematopoietic and immune | Thrombocytopenia Eosinophilia Lymphopenia Hypo- or hypergammaglobulinemia Autoantibodies (AIGA and ITP) |
|||
| Other | Pericardial or pleural effusions Ascites Peripheral neuropathy Nephrotic syndrome Myasthema gravis Cardiac conduction abnormality or cardiomyopathy |
GVHD indicated graft-versus-host disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BOOP, bronchiolitis obliterans-organizing pneumonia; PFTs, pulmonary function tests; AIGA, autoimmune hemolytic anemia; ITP, idiopathic thrombocytopenic purpura.
Can be acknowledged as part of the chronic GVHD symptomatology if the diagnosis is confirmed.
In all cases, infection, drug effects, malignancy, or other causes must be excluded.
Diagnosis of chronic GVHD required biopsy or radiology confirmation (or Schirmer test for eyes).
Diagnostic Approach
The potential clinical manifestations of chronic GVHD are many and varied, involving multiple organs and sites. At least one distinctive manifestation for chronic GVHD is required to diagnose chronic GVHD, such as oral or vaginal lichenoid findings, ocular sicca, skin dyspigmentation, scleroderma, or bronchiolitis obliterans. Biopsies or other diagnostic tests (e.g., laboratory tests, radiographic tests, and/or pulmonary tests) are recommended to confirm the diagnosis of chronic GVHD. Definitive diagnosis of chronic GVHD requires excluding other possible diagnoses such as infection, drug effects, malignancies and residual post-inflammatory damage and scarring.
Treatment
Definitive treatment of chronic GVHD, including the implementation and withdrawal of specific therapies, remains elusive. The prior use of prophylaxis agents and/or the history of therapies used in acute GVHD ultimately affect the choice of treatment for chronic GVHD, as do the specific patient characteristics and preferences of the treating physician and center. Generally, treatment for chronic GVHD is less intense and less aggressive than that used for acute GVHD, but may require a prolonged duration of multimodal immunosuppressive therapy. The most widely used first-line therapy for patients with chronic GVHD is a combination of systemic corticosteroids and a calcineurin inhibitor. The recently published National Institutes of Health (NIH) guidelines recommend this treatment if 3 or more organs are involved or any single organ has a severity score of more than 2 [105]. The NIH Working Group defines failure of initial therapy or requirement of additional secondary therapy as follows: i) progression of chronic GVHD despite optimal first-line therapy (including >1 mg/kg/day of prednisolone for two weeks), or ii) no improvement after 4–8 weeks of sustained therapy, or iii) inability to taper corticosteroid dose [105].
Chronic immunosuppressants, especially those containing steroids, are toxic and often result in life-threatening infectious complications. Many second-line therapies for chronic GVHD have been studied, but none has achieved widespread acceptance. Agents studied in chronic GVHD include ECP, MMF, and rituximab. ECP has demonstrated significant response rates in high risk chronic GVHD patients, particularly in the skin, liver, oral mucosa, eye, and lung [137]. However, the response of chronic GVHD to treatment is often unpredictable, and mixed responses in different organs can occur in the same patient. MMF showed initial promise, but in a multicenter placebo-controlled randomized trial, the addition of MMF to the initial systemic treatment regimen for chronic GVHD did not show any benefit [138]. In small phase II trials, rituximab, an anti-CD20 chimeric monoclonal antibody, has demonstrated an overall response rate of 65–70% for its use in refractory chronic GVHD [139, 140]. More recently, it has been shown that patients with fibrotic, chronic GVHD have antibodies activating the plateletderived growth factor receptor pathway [141]. In this light, a pilot study in 19 patients with refractory chronic GVHD investigated imatinib, which inhibits this pathway. At six months, the overall response rate was 79% with 7 complete remissions and 8 partial remissions [142]. These promising results deserve larger exploration and longer study.
Supportive Care in the Management of GVHD Patients
Supportive care and routine monitoring are critical in the management of chronic GVHD. Early recognition of highrisk features (such as thrombocytopenia, progressive onset chronic GVHD, extensive skin involvement with sclerodermatous features, and multi-organ involvement) and appropriate early intervention are important [105]. The Ancillary Therapy and Supportive Care Working Group as part of the 2005 NIH-sponsored Consensus Conference in chronic GVHD, recently established guidelines for ancillary and supportive care therapies [137]. Routine interval history, physical examination with assessment of symptoms, review of medications, laboratory monitoring (complete blood cell counts with differential, chemistry panel to assess kidney and liver functions, drug monitoring, IgG levels, lipid profiles, and endocrine evaluations to assess thyroid function, calcium and vitamin D levels) and the consultation of appropriate sub-specialists should be performed. It is important that transplant physicians and primary hematologists recognize the increased incidence of second malignancies in transplant recipients, particularly squamous cell carcinoma in chronic GVHD patients [143]. Furthermore, specific attention to the surveillance of infection prophylaxis (anti-bacterial, -viral, and -fungal), vaccinations, general hygiene, physical therapy, nutritional status, pain control, and monitoring of drug-drug interactions and the associated drug-related side effects, is required. There is little supporting evidence for the routine use of intravenous immunoglobulin as prophylaxis [144], but patients should receive routine antibiotic prophylaxis (penicillin or its equivalent) due to the increased risk of streptococcal sepsis [145]. The sites of any indwelling catheter should be assessed regularly. Early recognition of signs or symptoms of septic shock requires prompt evaluation with blood cultures and initiation of broad spectrum antibiotics.
Future Directions
The population of patients with GVHD has steadily grown as the number of allogeneic transplantations being performed annually has increased. Newer treatment approaches and wider availability of supportive care and ancillary therapies have led to improved survival. Despite these recent advances, however, GVHD remains a leading cause of morbidity and mortality in survivors of allogeneic transplantation. Novel approaches to GVHD are thus urgently needed. In this light, it is important to recognize that the management of patients with GVHD is complex and requires a multidisciplinary approach. A major barrier to current therapies is life-threatening infectious complications due to broad non-specific immune suppression. Future strategies are likely to include modulation of cell types that play key roles in the GVHD process, including regulatory T cells, dendritic cells, NKT cells and B cells. As such, targeted therapies are preferable in order to preserve the beneficial anti-tumor effect while eliminating the debilitating consequences of GVHD. Identification of biomarkers for GVHD with diagnostic and prognostic significance may further clarify groups of patients at highest risk and eventually improve the management of GVHD.
REFERENCES
- 1.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324(10):667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 2.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 3.Reddy P, Arora M, Guimond M, et al. GVHD: a continuing barrier to the safety of allogeneic transplantation. Biol Blood Marrow Transplant. 2008;15(1 Suppl):162–168. doi: 10.1016/j.bbmt.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966–67;62:21–78. [PubMed] [Google Scholar]
- 5.Korngold R, Sprent J. Purified T cell subsets and lethal graft-versus-host disease in mice. In: Gale RP, Champlin R, editors. Progress in Bone Marrow Transplant. New York: Alan R, Liss, Inc.; 1987. pp. 213–218. [Google Scholar]
- 6.Kernan NA, Collins NH, Juliano LL, et al. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-v-host disease. Blood. 1986;68(3):770–773. [PubMed] [Google Scholar]
- 7.Erlich HA, Opelz G, Hansen J. HLA DNA typing and transplantation. Immunity. 2001;14(4):347–356. doi: 10.1016/s1074-7613(01)00115-7. [DOI] [PubMed] [Google Scholar]
- 8.Loiseau P, Busson M, Balere ML, et al. HLA Association with hematopoietic stem cell transplantation outcome: the number of mismatches at HLA-A, -B, -C, -DRB1, or -DQB1 is strongly associated with overall survival. Biol Blood Marrow Transpl. 2007;13(8):965–974. doi: 10.1016/j.bbmt.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Hahn T, McCarthy PL, Jr, Zhang MJ, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26(35):5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kernan NA, Bartsch G, Ash RC, et al. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. N Engl J Med. 1993;328(9):593–602. doi: 10.1056/NEJM199303043280901. [DOI] [PubMed] [Google Scholar]
- 11.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 12.Bray RA, Hurley CK, Kamani NR, et al. National marrow donor program HLA matching guidelines for unrelated adult donor hematopoietic cell transplants. Biol Blood Marrow Transpl. 2008;14(9 Suppl):45–53. doi: 10.1016/j.bbmt.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 14.Goulmy E, Schipper R, Pool J, et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. 1996;334(5):281–285. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 15.Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4(5):371–380. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 16.Goulmy E. Human minor histocompatibility antigens: new concepts for marrow transplantation and adoptive immunotherapy. Immunol Rev. 1997;157:125–140. doi: 10.1111/j.1600-065x.1997.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 17.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80(12):2964–2968. [PubMed] [Google Scholar]
- 18.Cavet J, Middleton PG, Segall M, et al. Recipient tumor necrosis factor-alpha and interleukin-10 gene polymorphisms associate with early mortality and acute graft-versus-host disease severity in HLA-matched sibling bone marrow transplants. Blood. 1999;94(11):3941–3946. [PubMed] [Google Scholar]
- 19.Lin MT, Storer B, Martin PJ, et al. Relation of an interleukin-10 promoter polymorphism to graftversus- host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349(23):2201–2210. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson AM, Charron D. Non-HLA immunogenetics in hematopoietic stem cell transplantation. Curr Opin Immunol. 2005;17(5):517–525. doi: 10.1016/j.coi.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 22.Miller JS, Cooley S, Parham P, et al. Missing KIR-ligands is associated with less relapse and increased graft versus host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007 doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velardi A, Ruggeri L, Alessandro NK, et al. cells: a lesson from mismatched hematopoietic transplantation. Trends Immunol. 2002;23(9):438–444. doi: 10.1016/s1471-4906(02)02284-6. [DOI] [PubMed] [Google Scholar]
- 24.Holler E, Rogler G, Brenmoehl J, et al. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood. 2006;107(10):4189–4193. doi: 10.1182/blood-2005-09-3741. [DOI] [PubMed] [Google Scholar]
- 25.Weisdorf D, Hakke R, Blazar B, et al. Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation. 1991;51(6):1197–1203. doi: 10.1097/00007890-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Eisner MD, August CS. Impact of donor and recipient characteristics on the development of acute and chronic graft-versus-host disease following pediatric bone marrow transplantation. Bone Marrow Transplant. 1995;15(5):663–668. [PubMed] [Google Scholar]
- 27.Martin P, Bleyzac N, Souillet G, et al. Clinical and pharmacological risk factors for acute graft-versushost disease after paediatric bone marrow transplantation from matched-sibling or unrelated donors. Bone Marrow Transplant. 2003;32(9):881–887. doi: 10.1038/sj.bmt.1704239. [DOI] [PubMed] [Google Scholar]
- 28.Gale RP, Bortin MM, van Bekkum DW, et al. Risk factors for acute graft-versus-host disease. Br J Haematol. 1987;67(4):397–406. doi: 10.1111/j.1365-2141.1987.tb06160.x. [DOI] [PubMed] [Google Scholar]
- 29.Nash RA, Pepe MS, Storb R, et al. Acute graft-versus-host disease: analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood. 1992;80(7):1838–1845. [PubMed] [Google Scholar]
- 30.Vigorito AC, Azevedo WM, Marques JF, et al. A randomised, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation in the treatment of haematological malignancies. Bone Marrow Transpl. 1998;22(12):1145–1151. doi: 10.1038/sj.bmt.1701510. [DOI] [PubMed] [Google Scholar]
- 31.Cutler C, Giri S, Jeyapalan S, et al. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19(16):3685–3691. doi: 10.1200/JCO.2001.19.16.3685. [DOI] [PubMed] [Google Scholar]
- 32.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577):1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 33.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335(3):157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 34.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337(6):373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 35.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344(24):1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 36.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 37.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351(22):2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 38.Grewal SS, Barker JN, Davies SM, et al. Unrelated donor hematopoietic cell transplantation: marrow or umbilical cord blood? Blood. 2003;101(11):4233–4244. doi: 10.1182/blood-2002-08-2510. [DOI] [PubMed] [Google Scholar]
- 39.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 40.Macmillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: Analysis of risk factors. Blood. 2008 doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314(12):729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 42.Hiraoka A, Ohashi Y, Okamoto S, et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transpl. 2001;28(2):181–185. doi: 10.1038/sj.bmt.1703097. [DOI] [PubMed] [Google Scholar]
- 43.Horowitz MM, Przepiorka D, Bartels P, et al. Tacrolimus vs. cyclosporine immunosuppression: results in advanced-stage disease compared with historical controls treated exclusively with cyclosporine. Biol Blood Marrow Transpl. 1999;5(3):180–186. doi: 10.1053/bbmt.1999.v5.pm10392964. [DOI] [PubMed] [Google Scholar]
- 44.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–2314. [PubMed] [Google Scholar]
- 45.Ram R, Gafter-Gvili A, Yeshurun M, et al. Prophylaxis regimens for GVHD: systematic review and meta-analysis. Bone Marrow Transplant. 2009;43(8):643–653. doi: 10.1038/bmt.2008.373. [DOI] [PubMed] [Google Scholar]
- 46.Nash RA, Antin JH, Karanes C, et al. Phase III study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–2068. [PubMed] [Google Scholar]
- 47.van Hooff JP, Squifflet JP, Wlodarczyk Z, et al. A prospective randomized multicenter study of tacrolimus in combination with sirolimus in renal-transplant recipients. Transplantation. 2003;75(12):1934–1939. doi: 10.1097/01.TP.0000071301.86299.75. [DOI] [PubMed] [Google Scholar]
- 48.Kirken RA, Wang YL. Molecular actions of sirolimus: sirolimus and mTor. Transplant Proc. 2003;35(3 Suppl):227S–230S. doi: 10.1016/s0041-1345(03)00230-6. [DOI] [PubMed] [Google Scholar]
- 49.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolwell B, Sobecks R, Pohlman B, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transpl. 2004;34(7):621–625. doi: 10.1038/sj.bmt.1704647. [DOI] [PubMed] [Google Scholar]
- 51.Bornhauser M, Schuler U, Porksen G, et al. Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation. 1999;67(4):499–504. doi: 10.1097/00007890-199902270-00001. [DOI] [PubMed] [Google Scholar]
- 52.Kasper C, Sayer HG, Mugge LO, et al. Combined standard graft-versus-host disease (GvHD) prophylaxis with mycophenolate mofetil (MMF) in allogeneic peripheral blood stem cell transplantation from unrelated donors. Bone Marrow Transpl. 2004;33(1):65–69. doi: 10.1038/sj.bmt.1704299. [DOI] [PubMed] [Google Scholar]
- 53.Mohty M, de Lavallade H, Faucher C, et al. Mycophenolate mofetil and cyclosporine for graft-versushost disease prophylaxis following reduced intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transpl. 2004;34(6):527–530. doi: 10.1038/sj.bmt.1704640. [DOI] [PubMed] [Google Scholar]
- 54.Vogelsang GB, Arai S. Mycophenolate mofetil for the prevention and treatment of graft-versus-host disease following stem cell transplantation: preliminary findings. Bone Marrow Transpl. 2001;27(12):1255–1262. doi: 10.1038/sj.bmt.1703076. [DOI] [PubMed] [Google Scholar]
- 55.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101(4):1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 56.Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78(8):2120–2130. [PubMed] [Google Scholar]
- 57.Martin PJ, Hansen JA, Torok-Storb B, et al. Graft failure in patients receiving T cell-depleted HLA-identical allogeneic marrow transplants. Bone Marrow Transplant. 1988;3(5):445–456. [PubMed] [Google Scholar]
- 58.O'Reilly RJ. T-cell depletion and allogeneic bone marrow transplantation. Semin Hematol. 1992;29(2 Suppl 1):20–26. [PubMed] [Google Scholar]
- 59.Kottaridis PD, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96(7):2419–2425. [PubMed] [Google Scholar]
- 60.Barge RMY, Starrenburg CWJ, Falkenburg JHF, et al. Long-term follow-up of myeloablative allogeneic stem cell transplantation using Campath "in the bag" as T-cell depletion: the Leiden experience. Bone Marrow Transpl. 2006;37(12):1129–1134. doi: 10.1038/sj.bmt.1705385. [DOI] [PubMed] [Google Scholar]
- 61.Bacigalupo A, Lamparelli T, Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98(10):2942–2947. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 62.Reddy P, Ferrara JL. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003;17(4):187–194. doi: 10.1016/s0268-960x(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 63.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: Rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–2759. [PubMed] [Google Scholar]
- 64.Xun CQ, Thompson JS, Jennings CD, et al. Effect of total body irradiation, busulfancyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83(8):2360–2367. [PubMed] [Google Scholar]
- 65.Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10(3):178–185. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Choi SW, Kitko CL, Braun T, et al. Change in plasma tumor necrosis factor receptor 1 levels in the first week after myeloablative allogeneic transplantation correlates with severity and incidence of GVHD and survival. Blood. 2008;112(4):1539–1542. doi: 10.1182/blood-2008-02-138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 68.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285(5426):412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 69.Murai M, Yoneyama H, Ezaki T, et al. Peyer's patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003;4(2):154–160. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 70.Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8(6):575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 71.Reddy P, Maeda Y, Liu C, et al. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11(11):1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 72.Duffner UA, Maeda Y, Cooke KR, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172(12):7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 73.Maeda Y, Reddy P, Lowler KP, et al. Critical role of host gammadelta T cells in experimental acute graft-versus-host disease. Blood. 2005;106(2):749–755. doi: 10.1182/blood-2004-10-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merad M, Hoffmann P, Ranheim E, et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10(5):510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nachbaur D, Kircher B, Eisendle K, et al. Phenotype, function and chimaerism of monocyte-derived blood dendritic cells after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2003;123(1):119–126. doi: 10.1046/j.1365-2141.2003.04588.x. [DOI] [PubMed] [Google Scholar]
- 76.Sorror ML, Maris MB, Storer B, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004;104(4):961–968. doi: 10.1182/blood-2004-02-0545. [DOI] [PubMed] [Google Scholar]
- 77.Korngold R, Sprent J. Negative selection of T cells causing lethal graft-versus-host disease across minor histocompatibility barriers. Role of the H-2 complex. J Exp Med. 1980;151(5):1114–1124. doi: 10.1084/jem.151.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beilhack A, Schulz S, Baker J, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106(3):1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kloosterman TC, Tielemans MJ, Martens AC, et al. Quantitative studies on graft-versus-leukemia after allogeneic bone marrow transplantation in rat models for acute myelocytic and lymphocytic leukemia. Bone Marrow Transplant. 1994;14(1):15–22. [PubMed] [Google Scholar]
- 80.Weijtens M, van Spronsen A, Hagenbeek A, et al. Reduced graft-versus-host disease-inducing capacity of T cells after activation, culturing, and magnetic cell sorting selection in an allogeneic bone marrow transplantation model in rats. Hum Gene Ther. 2002;13(2):187–198. doi: 10.1089/10430340252769725. [DOI] [PubMed] [Google Scholar]
- 81.Cobbold S, Martin G, Waldmann H. Monoclonal antibodies for the prevention of graft-versus-host disease and marrow graft rejection. The depletion of T cell subsets in vitro and in vivo. Transplantation. 1986;42(3):239–247. doi: 10.1097/00007890-198609000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Sun Y, Tawara I, Toubai T, et al. Pathophysiology of acute graft-versus-host disease: recent advances. Transl Res. 2007;150(4):197–214. doi: 10.1016/j.trsl.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 84.Cohen JL, Boyer O. The role of CD4+CD25hi regulatory T cells in the physiopathogeny of graft-versus-host disease. Curr Opin Immunol. 2006;18(5):580–585. doi: 10.1016/j.coi.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Stanzani M, Martins SL, Saliba RM, et al. CD25 expression on donor CD4+ or CD8+ T cells is associated with an increased risk of graft-versus-host disease following HLA-identical stem cell transplantation in humans. Blood. 2003 doi: 10.1182/blood-2003-06-2085. [DOI] [PubMed] [Google Scholar]
- 86.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108(4):1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 88.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43(1):3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Rolink AG, Gleichmann E. Allosuppressor- and allohelper-T cells in acute and chronic graft-vs.-host (GVH) disease. III. Different Lyt subsets of donor T cells induce different pathological syndromes. J Exp Med. 1983;158(2):546–558. doi: 10.1084/jem.158.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krenger W, Falzarano G, Delmonte J, Jr, et al. Interferon-gamma suppresses T-cell proliferation to mitogen via the nitric oxide pathway during experimental acute graft-versus-host disease. Blood. 1996;88(3):1113–1121. [PubMed] [Google Scholar]
- 92.Yang YG, Dey BR, Sergio JJ, et al. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102(12):2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 94.Lin MT, Storer B, Martin PJ, et al. Genetic variation in the IL-10 pathway modulates severity of acute graft-versus-host disease following hematopoietic cell transplantation: synergism between IL-10 genotype of patient and IL-10 receptor beta genotype of donor. Blood. 2005;106(12):3995–4001. doi: 10.1182/blood-2004-11-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holler E, Roncarolo MG, Hintermeier-Knabe R, et al. Prognostic significance of increased IL-10 production in patients prior to allogeneic bone marrow transplantation. Bone Marrow Transpl. 2000;25(3):237–241. doi: 10.1038/sj.bmt.1702126. [DOI] [PubMed] [Google Scholar]
- 96.Banovic T, MacDonald KP, Morris ES, et al. TGF-beta in allogeneic stem cell transplantation: friend or foe? Blood. 2005;106(6):2206–2214. doi: 10.1182/blood-2005-01-0062. [DOI] [PubMed] [Google Scholar]
- 97.van den Brink MR, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol. 2002;2(4):273–281. doi: 10.1038/nri775. [DOI] [PubMed] [Google Scholar]
- 98.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, et al. Leukocyte migration and graft-versus-host disease. Blood. 2005;105(11):4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pribila JT, Quale AC, Mueller KL, et al. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–180. doi: 10.1146/annurev.immunol.22.012703.104649. [DOI] [PubMed] [Google Scholar]
- 100.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 101.Abhyankar S, Gilliland DG, Ferrara JL. Interleukin-1 is a critical effector molecule during cytokine dysregulation in graft versus host disease to minor histocompatibility antigens. Transplantation. 1993;56(6):1518–1523. doi: 10.1097/00007890-199312000-00045. [DOI] [PubMed] [Google Scholar]
- 102.Tanaka J, Imamura M, Kasai M, et al. Cytokine gene expression in peripheral blood mononuclear cells during graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 1993;85(3):558–565. doi: 10.1111/j.1365-2141.1993.tb03348.x. [DOI] [PubMed] [Google Scholar]
- 103.Antin JH, Weisdorf D, Neuberg D, et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood. 2002;100(10):3479–3482. doi: 10.1182/blood-2002-03-0985. [DOI] [PubMed] [Google Scholar]
- 104.Martin P, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76(8):1464–1472. [PubMed] [Google Scholar]
- 105.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 106.Przepiorka D, Weisdorf D, Martin P. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 107.Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: A joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106(4):1495–1500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29–52. doi: 10.1146/annurev.med.54.101601.152339. [DOI] [PubMed] [Google Scholar]
- 109.Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29(3):259–277. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- 110.Choi SW, Islam S, Greenson JK, et al. The use of laparoscopic liver biopsies in pediatric patients with hepatic dysfunction following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2005;36(10):891–896. doi: 10.1038/sj.bmt.1705158. [DOI] [PubMed] [Google Scholar]
- 111.Snover DC, Weisdorf SA, Ramsay NK, et al. Hepatic graft versus host disease: a study of the predictive value of liver biopsy in diagnosis. Hepatology. 1984;4(1):123–130. doi: 10.1002/hep.1840040122. [DOI] [PubMed] [Google Scholar]
- 112.Nevo S, Enger C, Swan V, et al. Acute bleeding after allogeneic bone marrow transplantation: association with graft versus host disease and effect on survival. Transplantation. 1999;67(5):681–689. doi: 10.1097/00007890-199903150-00007. [DOI] [PubMed] [Google Scholar]
- 113.Snover DC, Weisdorf SA, Vercellotti GM, et al. A histopathologic study of gastric and small intestinal graft-versus-host disease following allogeneic bone marrow transplantation. Hum Pathol. 1985;16(4):387–392. doi: 10.1016/s0046-8177(85)80232-x. [DOI] [PubMed] [Google Scholar]
- 114.Paczesny S, Levine JE, Braun TM, et al. Plasma biomarkers in graft-versus-host disease: a new era? Biol Blood Marrow Transpl. 2008;15(1 Suppl):33–38. doi: 10.1016/j.bbmt.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113(2):273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75(4):1024–1030. [PubMed] [Google Scholar]
- 117.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transpl. 2002;8(7):387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 118.Bertz H, Afting M, Kreisel W, et al. Feasibility and response to budesonide as topical corticosteroid therapy for acute intestinal GVHD. Bone Marrow Transpl. 1999;24(11):1185–1189. doi: 10.1038/sj.bmt.1702055. [DOI] [PubMed] [Google Scholar]
- 119.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109(10):4557–4563. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 120.Shapira MY, Bloom AI, Or R, et al. Intra-arterial catheter directed therapy for severe graft-versus-host disease. Br J Haematol. 2002;119(3):760–764. doi: 10.1046/j.1365-2141.2002.03923.x. [DOI] [PubMed] [Google Scholar]
- 121.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–4126. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin or pentostatin plus corticosteroids for acute graft vs. host disease: a randomized phase II trial from the BMT CTN. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lamioni A, Parisi F, Isacchi G, et al. The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation. 2005;79(7):846–850. doi: 10.1097/01.tp.0000157278.02848.c7. [DOI] [PubMed] [Google Scholar]
- 124.Gatza E, Rogers CE, Clouthier SG, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112(4):1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Greinix HT, Knobler RM, Worel N, et al. The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica. 2006;91(3):405–408. [PubMed] [Google Scholar]
- 126.Perfetti P, Carlier P, Strada P, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transpl. 2008;42(9):609–617. doi: 10.1038/bmt.2008.221. [DOI] [PubMed] [Google Scholar]
- 127.Calore E, Calo A, Tridello G, et al. Extracorporeal photochemotherapy may improve outcome in children with acute GVHD. Bone Marrow Transplant. 2008;42(6):421–425. doi: 10.1038/bmt.2008.174. [DOI] [PubMed] [Google Scholar]
- 128.Berger M, Pessolano R, Albiani R, et al. Extracorporeal photopheresis for steroid resistant graft versus host disease in pediatric patients: a pilot single institution report. J Pediatr Hematol Oncol. 2007;29(10):678–687. doi: 10.1097/MPH.0b013e31814d66f5. [DOI] [PubMed] [Google Scholar]
- 129.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 130.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 131.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341(1):14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 132.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 133.Weiden PL, Flournoy N, Sanders JE, et al. Antileukemic effect of graft-versus-host disease contributes to improved survival after allogeneic marrow transplantation. Transplant Proc. 1981;13(1 Pt 1):248–251. [PubMed] [Google Scholar]
- 134.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 135.Pavletic SZ, Smith LM, Bishop MR, et al. Prognostic factors of chronic graft-versus-host disease after allogeneic blood stem-cell transplantation. Am J Hematol. 2005;78(4):265–274. doi: 10.1002/ajh.20275. [DOI] [PubMed] [Google Scholar]
- 136.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 137.Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transpl. 2006;12(4):375–396. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 138.Martin PJ, Storer BE, Rowley SD, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113(21):5074–5082. doi: 10.1182/blood-2009-02-202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zaja F, Bacigalupo A, Patriarca F, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transpl. 2007;40(3):273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 140.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Svegliati S, Olivieri A, Campelli N, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110(1):237–241. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- 142.Olivieri A, Locatelli F, Zecca M, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114(3):709–718. doi: 10.1182/blood-2009-02-204156. [DOI] [PubMed] [Google Scholar]
- 143.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kumar A, Teuber SS, Gershwin ME. Intravenous immunoglobulin: striving for appropriate use. Int Arch Allergy Immunol. 2006;140(3):185–198. doi: 10.1159/000093204. [DOI] [PubMed] [Google Scholar]
- 145.Kulkarni S, Powles R, Treleaven J, et al. Chronic graft versus host disease is associated with long-term risk for pneumococcal infections in recipients of bone marrow transplants. Blood. 2000;95(12):3683–3686. [PubMed] [Google Scholar]
- 146.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 147.Pzrepiorka D, Weisdorf D, Martin P, et al. Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–828. [PubMed] [Google Scholar]
- 148.Przepiorka D, Kernan NA, Ippoliti C, et al. Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood. 2000;95(1):83–89. [PubMed] [Google Scholar]
- 149.Shaughnessy PJ, Bachier C, Grimley M, et al. Denileukin diftitox for the treatment of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transpl. 2005;11(3):188–193. doi: 10.1016/j.bbmt.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 150.Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111(4):2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bolanos-Meade J, Jacobsohn DA, Margolis J, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;23(12):2661–2668. doi: 10.1200/JCO.2005.06.130. [DOI] [PubMed] [Google Scholar]
- 152.Basara N, Kiehl MG, Blau W, et al. Mycophenolate Mofetil in the treatment of acute and chronic GVHD in hematopoietic stem cell transplant patients: four years of experience. Transplant Proc. 2001;33(3):2121–2123. doi: 10.1016/s0041-1345(01)01968-6. [DOI] [PubMed] [Google Scholar]
- 153.Krejci M, Doubek M, Buchler T, et al. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol. 2005;84(10):681–685. doi: 10.1007/s00277-005-1070-0. [DOI] [PubMed] [Google Scholar]
- 154.Takami A, Mochizuki K, Okumura H, et al. Mycophenolate mofetil is effective and well tolerated in the treatment of refractory acute and chronic graft-versus-host disease. Int J Hematol. 2006;83(1):80–85. doi: 10.1532/IJH97.05111. [DOI] [PubMed] [Google Scholar]
- 155.Benito AI, Furlong T, Martin PJ, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72(12):1924–1929. doi: 10.1097/00007890-200112270-00010. [DOI] [PubMed] [Google Scholar]
- 156.von Bonin M, Stolzel F, Goedecke A, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant. 2009;43(3):245–251. doi: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- 157.Shapira MY, Resnick IB, Bitan M, et al. Rapid response to alefacept given to patients with steroid resistant or steroid dependent acute graft-versus-host disease: a preliminary report. Bone Marrow Transpl. 2005;36(12):1097–1101. doi: 10.1038/sj.bmt.1705185. [DOI] [PubMed] [Google Scholar]