Abstract
Mutations in the glucocerebrosidase (GBA) gene, which encodes the lysosomal enzyme that is deficient in Gaucher's disease, are important and common risk factors for Parkinson’s disease and related disorders. This association was first recognised in the clinic, where parkinsonism was noted, albeit rarely, in patients with Gaucher's disease and more frequently in relatives who were obligate carriers. Subsequently, findings from large studies showed that patients with Parkinson’s disease and associated Lewy body disorders had an increased frequency of GBA mutations when compared with control individuals. Patients with GBA-associated parkinsonism exhibit varying parkinsonian phenotypes but tend to have an earlier age of onset and more associated cognitive changes than patients with parkinsonism without GBA mutations. Hypotheses proposed to explain this association include a gain-of-function due to mutations in glucocerebrosidase that promotes α-synuclein aggregation; substrate accumulation due to enzymatic loss-of-function, which affects α-synuclein processing and clearance; and a bidirectional feedback loop. Identification of the pathological mechanisms underlying GBA-associated parkinsonism will improve our understanding of the genetics, pathophysiology, and treatment for both rare and common neurological diseases.
Introduction
Recent progress in human genetics has resulted in dramatic advances in our understanding of Parkinson’s disease and related disorders. Different genetic techniques have proven useful in unravelling the complexity of these multifactorial diseases. In some instances, the analysis of a pedigree with several affected members has led to the identification of linkage, and ultimately to a causative gene. Whole-genome investigations, such as genome-wide association studies of large cohorts, have directed investigators to important candidate genes. Recently, whole-exome and whole-genome sequencing have resulted in the identification of unanticipated genes and pathways involved in the cause of disease. However, in the case of Parkinson’s disease, the most common genetic risk factor identified to date came about from an unanticipated clinical finding made in the genetics clinic during studies of patients with the rare lysosomal storage disorder Gaucher's disease. In most populations with Parkinson’s disease, mutations in the glucocerebrosidase (GBA) gene are more frequent than in other implicated genes including dardarin (LRKK2),α-synuclein (SNCA), and parkin (PARK2). In this Review, we detail how glucocerebrosidase was identified as a risk factor for parkinsonism, discuss the clinical relevance of this association, and describe new areas for research and treatment that have resulted from this discovery.
Gaucher's disease
Gaucher's disease, the inherited deficiency of the enzyme glucocerebrosidase, is the most common lysosomal storage disorder. First described by Philippe Gaucher in 1882,1 Gaucher's disease is an autosomal recessive disorder that primarily affects the mononuclear phagocyte system where lysosomes within cells of the macrophage lineage become engorged with stored lipid. Patients typically manifest with hepatosplenomegaly, anaemia, thrombocytopenia, and bony involvement.2,3 A subgroup of patients with neuronopathic forms of Gaucher's disease also have brain involvement of varying severity (panel 1). Although the disorder is panethnic, it is more frequent in the Ashkenazi Jewish population.
Panel 1.
The three types of Gaucher's disease
Type 1: non-neuronopathic
Panethnic disorder, although more common in Ashkenazi Jews
Associated with clinical heterogeneity
Wide range of symptom severity
Hepatosplenomegaly, anaemia, and thrombocytopenia are common
Bone disease is a frequent cause of morbidity
Treated with enzyme replacement therapy
Type 2: acute neuronopathic
Rare, panethnic disorder
Can present prenatally, at birth, or in the first year of life
Rapidly progressive neurological deterioration
Enzyme replacement therapy does not reverse or halt neurological progression
Early death within days to years
Type 3: chronic neuronopathic
Includes several different phenotypes with variable longevity
Accompanied by a specific disorder of horizontal saccadic eye movements
Some patients develop myoclonic epilepsy
A subgroup of patients develop cardiac calcification, hydrocephalus, and other abnormalities
Associated with distinct learning disabilities in some patients
The gene encoding glucocerebrosidase, GBA, is located in a gene-rich region on chromosome 1q21.4,5 A nearby pseudogene, which shares 96% exonic sequence homology with GBA, complicates sequencing and detection of mutations. So far, about 300 different mutations have been identified in GBA, including point mutations, frameshift mutations, splice-site alterations, and recombinant alleles that encompass segments of the pseudogene sequence.6,7 Eight mutant alleles frequently occur in patients of Ashkenazi Jewish ancestry, the most common of which is N370S. Among Ashkenazi Jews, one in 14–18 individuals harbour GBA mutations, and N370S accounts for 70% of the mutant alleles.8,9 The carrier frequency in other ethnic groups is less than 1%, with a vast range of GBA mutations reported. Although some phenotypic predictions can be made from the identified genotype, genotype–phenotype correlation in this disorder is incomplete. Differing clinical presentations in patients and even siblings sharing the same genotype suggest a role for disease modifiers.10,11
Because of the wide spectrum of phenotypes encountered, Gaucher's disease is typically divided into three types (panel 1) on the basis of the absence (type 1) or rate of progression (types 2 and 3) of neuronopathic involvement.2,3,12 However, the associated phenotypes can also be considered a continuum, ranging from asymptomatic individuals identified inadvertently through family screenings to infants who have Gaucher's disease-associated hydrops fetalis in utero.13,14 The major distinction between these types is CNS involvement, which, in some cases, results in progressive neurodegeneration.
Gaucher's disease with parkinsonism
Among the more atypical and rare Gaucher phenotypes described are patients who develop progressive parkinsonian features, dementia, or both. Initially, case reports of patients were documented, although in 1996 a small series of patients from Italy and Israel was described,15 and additional reports were subsequently published.16–18 In 2003, a group of 17 patients with Gaucher's disease and parkinsonism who were of different ethnic origins, including Ashkenazi Jewish patients, were described.19 The parkinsonian manifestations were similar to those noted in sporadic Parkinson’s disease, although many had an age of onset in their 40s and most, although not all, responded favourably to levodopa.19 Sequencing of GBA was done in these 17 patients and revealed 12 different genotypes, with the common type 1 N370S allele identified in 14 patients (82%), including five N370S homozygotes. Autopsies were done on four patients and showed Lewy body inclusions, especially in the cerebral cortex and hippocampus.
Parkinson’s disease in GBA mutation carriers
Relatives of several Gaucher probands with parkinsonism have been reported to have Parkinson’s disease and to be either obligate or confirmed GBA mutation carriers.19 This finding prompted a prospective survey of patients assessed at the Gaucher clinics at the National Institutes of Health, which confirmed the initial finding, with 25% of patients reporting a first-degree or second-degree relative with parkinsonism.20 A survey in a Gaucher clinic in Jerusalem yielded similar results.21
The unexpected finding of decreased glucocerebrosidase activity in neuropathological specimens from patients with sporadic Parkinson’s disease, confirmed by the finding of heterozygous GBA mutations in these cases, launched the investigation in a new direction.22 Brain bank samples from 57 patients with Parkinson’s disease were genotyped, and heterozygous GBA mutations were identified in 14% of patients.22 Subsequently, investigators screened DNA samples from 99 patients with idiopathic Parkinson’s disease from northern Israel who were of Ashkenazi Jewish ancestry and 1543 Ashkenazi Jewish control individuals for six common GBA mutations, and reported that 31·3% of the patients with Parkinson’s disease carried a GBA mutation compared with 6·2% of the control individuals (p<0·0001; table).23
Table.
Frequency of GBA mutations in patients with Parkinson’s disease
| Population | Screened mutations | Number of participants |
Carrier frequency (%) |
p value | Most common variants | |||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |||||
| Lwin et al 200422 | Mixed | GBA exons | 57 | 44 | 21·0% | 0·0% | ·· | N370S |
| Aharon-Peretz et al 200423 | Ashkenazi | N370S, L444P, c.84dupG, IVS2+A>G, V394L, R496H |
99 | 1543 | 31·3% | 6·2% | <0·0001 | N370S |
| Clark et al 200524 | Ashkenazi | N370S | 160 | 92 | 10.7% | 4·3% | 0·20 | N370S |
| Sato et al 200525 | Caucasians (Canadian origin) | N370S, L444P, IVS2+A>G, K198T, R329C, c.84dupG, RecNci1 |
88 | 122 | 5.7% | 0·8% | 0.48 | RecNci1 |
| Toft et al 200626 | Norwegian | N370S, L444P | 311 | 474 | 2·3% | 1.7% | 0·58 | N370S |
| Eblan et al 200627 | Mixed (non-Jewish) | GBA exons | 33 | 31 | 12·0% | 3·2% | ·· | RecNci1, L444P |
| Tan et al 200728 | Chinese | L444P, N370S | 331 | 347 | 2.4% | 0·0% | 0·06 | L444P |
| Ziegler et al 200729 | Chinese | GBA exons | 92 | 92 | 4·3% | 1.1% | ·· | L444P |
| Wu et al 200730 | Taiwanese | L444P, RecNci1, R120W | 518 | 339 | 3·1% | 1·2% | 0·07 | L444P, RecNci1 |
| Clark et al 200731 | Mixed (64% Jewish)* | GBA exons | 278 (178) | 179 (85) | 13.7% | 4·5% | ·· | N370S, c.84dupG |
| De Marco et al 200832 | Italian | N370S, L444P | 395 | 483 | 2·8% | 0·2% | 0·0018 | L444P |
| Spitz et al 200833 | Brazilian | N370S, L444P, G377S | 65 | 267 | 3·0% | 0·0% | 0·037 | L444P |
| Mata et al 200834 | Mixed | N370S, L444P | 721 | 554 | 2·9% | 0.4% | 0·001 | N370S, L444P |
| Gan-Or et al 200835 | Ashkenazi | N370S, R496H, L444P, c.84dupG, IVS2+1, V394L, D409H, RecTL |
420 | 333 | 17·9% | 4·2% | <0·0001 | N370S |
| Bras et al 200936 | Portuguese | GBA exons | 230 | 430 | 6·1% | 0.7% | ·· | N370S, N396T |
| Kalinderi et al 200937 | Greek | GBA exons | 172 | 132 | 47% | 0·8% | 0·048 | H255Q, L444P |
| Nichols et al 200938 | Mixed (<10% Jewish) | N370S, T369M, L444P, IVS6, IVS10, E326K, K303K, R262H, RecNci1 |
1325 | 359 | 12·6% | 5.3% | ·· | E326K, T369M, N370S, L444P |
| Neumann et al 200939 | British | GBA exons | 790 | 257 | 4·2% | 1·2% | 0·01 | L444P, N370S |
| Mitsui et al 200940 | Japanese | GBA exons | 534 | 544 | 9.4% | <0·1% | <0·0001 | R120W, RecNci1 |
| Emelyanov et al 201041 | Russian | N370S, L444P | 330 | 240 | 2.7% | 0.4% | 0·038 | N370S |
| Saunders-Pullman et al 201042 |
Ashkenazi | N370S, L444P, 84GG, IVS2+1G>A, V394L, del55bp, D409H, R496H |
250 | ·· | 12·8% | ·· | ·· | N370S |
| Mao et al 201043 | Chinese | L444P | 616 | 411 | 3·2% | 0·2% | 0·001 | ·· |
| Sun et al 201044 | Chinese | L444P, F213I, R353W, N370S | 402 | 413 | 2.7% | 0·0% | 0·0007 | L444P |
| Hu et al 201045 | Chinese | N370S | 328 | 300 | 1·8% | 0.7% | 0·29 | ·· |
| Lesage et al 201146 | North African (Algeria, Morocco, Tunisia, Libya) | GBA exons, LRRK2(G2019S) | 194 | 177 | 4·6% | 0·5% | 0·01 | N370S, L444P, RecNci1 |
| Noreau et al 201147 | French-Canadian | GBA exons | 212 | 189 | 3·8% | 0·5% | 0·10 | L444P, p.L236F, p.S378L, p.W417G |
| Lesage et al 201148 | European | GBA exons | 1130 | 391 | 6.7% | 1·0% | <0·0001 | N370S |
| Huang et al 201149 | Chinese | GBA exons | 967 | 780 | 3.7% | 0.3% | 0·0001 | L444P |
| Choi et al 201250 | Korean | GBA exons | 277 | 291 | 3·2% | 0·0% | 0·01 | N188S, P201H, R257Q, S271G, L444P |
Number of Jewish participants shown in parentheses.
Since these initial studies, the frequency of mutations in GBA has been assessed in different Parkinson’s disease centres worldwide. Some centres screened for specific commonly encountered GBA mutations, such as N370S and L444P, while others fully sequenced all GBA exons (panel 2). The methods used for detection of mutations and the ethnic origins of the people studied differed among centres, which contributed to the variability in the number of mutations identified. However, the frequency of GBA mutations was consistently higher in patients with Parkinson’s disease than in control individuals matched for ethnic origin, age, and sex (table).22–50 Heterozygous GBA mutations were reported in 10·7–31·3% of Ashkenazi Jewish patients with Parkinson’s disease, whereas the frequency ranged from 2·3% to 9·4% in patients of other ethnic origins.
Panel 2.
Screening patients for GBA mutations
GBA is located on chromosome 1q21 and there is a highly homologous pseudogene sequence located only 16 kb downstream. There are approximately 300 described mutations in GBA, some of which originate from pseudogene sequences. The N370S mutation accounts for about 70% of mutant alleles in the Ashkenazi Jewish population. In Caucasian populations, N370S and L444P mutations are common. Sequencing of all exons, using primers specific for the GBA gene, and not the pseudogene, is the most reliable means to screen for GBA mutations. However, many centres continue to screen only for several specific mutant alleles.
GBA mutations in familial Parkinson’s disease were assessed in a large cohort in the USA, in which the mutation rate was 4·1% in cases compared with 1·1% in control individuals.38 A study from Japan identified GBA mutations in eight of 34 families with more than one affected individual and in 14·7% of probands.40 All affected family members had concordant variants. Two GBA variants, E326K and T369M, were frequently encountered.
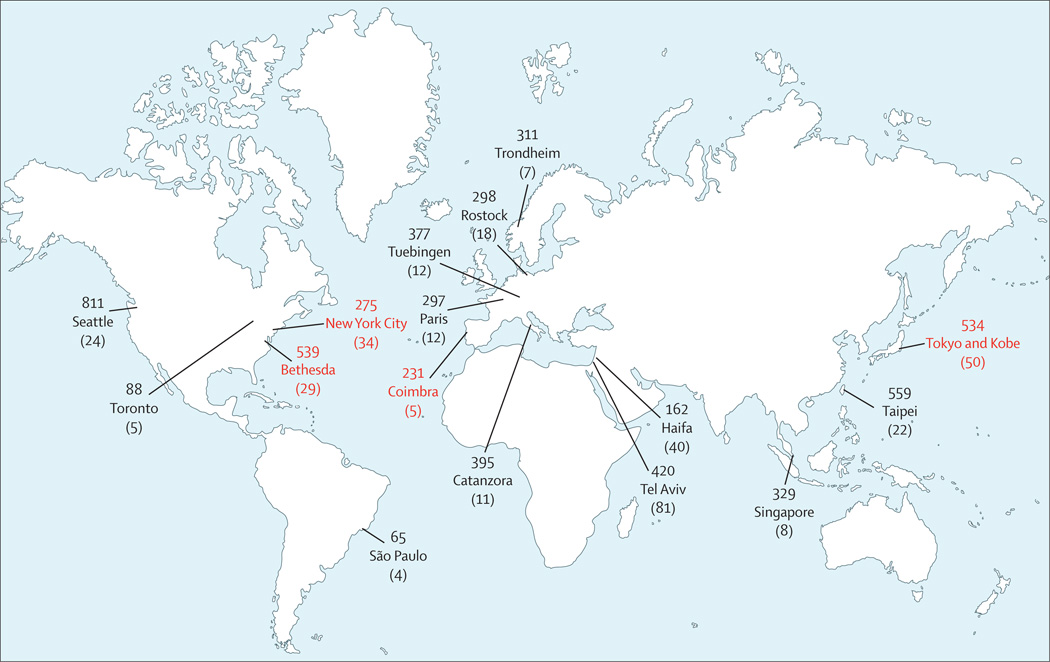
Because most single-centre studies had limitations in sample size, GBA screening strategies, inclusion of appropriate control individuals, or the extent of data on ethnic origin, a large multicentre collaborative study was done that incorporated 16 research centres from four continents, with 5691 patients with Parkinson’s disease (780 Ashkenazi Jews) and 4898 control individuals without Parkinson’s disease (387 Ashkenazi Jews; figure 1).51 All of the patients included were screened for at least two mutations, N370S and L444P, which were identified in 15% of Ashkenazi Jewish patients and 3% of Ashkenazi Jewish control individuals (for N370S odds ratio [OR] 5·62, 95% CI 3·04–10·39; for L444P 4·95, 0·62–39·38) and in 3% of patients with Parkinson’s disease with other ethnic ancestries (6·52, 3·62–11·74). In a subset of 1700 participants in whom GBA was fully sequenced, 7% of patients with Parkinson’s disease who were not of Ashkenazi Jewish origin were mutation carriers (for N370S 3·3, 1.79–6.10; for L444P 9·68, 4·98–18·83). The study concluded that in patients with Parkinson’s disease, the OR for carrying a GBA mutation was 5·43 (95% CI 3·89–7·57), confirming that mutations in this gene are a common risk factor for Parkinson’s disease.
Figure 1. The 16 centres that participated in the international collaborative study on the frequency of GBA mutations in Parkinson’s disease.
The numbers shown are the number of patients from each centre. Numbers in parentheses are the number of GBA mutation carriers identified at each site. Numbers in red show centres that sequenced the GBA gene for genotyping.
However, findings from large concurrent genome-wide association studies undertaken on thousands of patients with Parkinson’s disease worldwide initially failed to identify GBA as a susceptibility gene for parkinsonism, probably because of the presence of several rare variants with incomplete penetrance.52–54 Moreover, these variants occur on different haplotypes, and thus might not be detected in a study of common variants.7 The glucocerebrosidase story was described as “an illustration of how an important risk factor for a complex disease can evade detection by systematic analysis; it only came into the radar because of astute clinical observation”.55 Subsequent genome-wide association studies specifically looking at GBA variants confirmed the glucocerebrosidase locus as a risk factor for Parkinson’s disease by focusing on specific single nucleotide polymorphisms in the gene.56–58
Clinical features of GBA-associated parkinsonism
Age at onset
Parkinsonian phenotypes in GBA mutation carriers and GBA homozygotes with parkinsonism seem to be similar. Overall, the onset of motor impairment among carriers occurred 1·7–6·0 years earlier than in those without mutations.23,28,30,31,38,39 Screening showed that in patients with an early onset of Parkinson’s disease (<50 years) GBA mutations were twice as common as in late-onset cases.30,38 Among 951 patients screened for N370S and L444P with an onset of Parkinson’s disease before age 51 years, 6·7% carried either GBA mutation, which is equivalent to the frequency of patients with homozygous or heterozygous PARK2 mutations.59 Among the patients who developed Par kinson’s disease before age 50 years, GBA mutation carriers developed clinical symptoms at an earlier age than non-mutation carriers.31,35
Clinical characteristics of patients with Gaucher's disease who develop parkinsonism
Generally, patients with Gaucher's disease and Parkinson’s disease have mild Gaucher's symptoms. No specific Gaucher's genotype is associated with parkinsonian features, although the mutation most frequently identified in most American, European, and Ashkenazi Jewish cohorts is N370S.19,60
A review of the International Collaborative Gaucher Group (ICGG) Gaucher Registry,60 which included data on 4051 patients with Gaucher's disease, revealed that 68 patients had been diagnosed with Parkinson’s disease. Those with Parkinson’s disease had no specific pattern of associated Gaucher manifestations, and their mean age at symptom onset was 57 years. 54 of the 68 patients had received enzyme replacement therapy for their Gaucher manifestations, which suggests that this treatment did not prevent Parkinson’s disease. In another study of 444 patients with Gaucher's disease undertaken in New York (NY, USA) and New Haven (CT, USA), the age at onset of Gaucher-related symptoms, age at Gaucher diagnosis, and sex distribution of patients with parkinsonism were similar to those in patients with Gaucher's disease without Parkinson’s disease.61
Although most patients with Gaucher's disease and Parkinson’s disease present with Gaucher symptoms many years before the development of parkinsonian manifestations, some develop Parkinson’s disease be fore Gaucher's disease is diagnosed.16,42,60 Screening of 250 Ashkenazi Jewish patients with Parkinson’s disease in New York identified four GBA homozygotes who were unaware of their Gaucher's disease.42 In the initial series of 17 patients with Gaucher's disease and Parkinson’s disease,19 most patients had typical Parkinson’s disease features, including asymmetric onset, bradykinesia, rigidity, and resting tremor. Characterisation of Parkinson’s disease manifestations associated with GBA mutations in ten patients revealed nine with an asymmetric onset.62 Tremor and bradykinesia were the most common initial symptoms, and motor fluctuations were frequent. In a review of 49 patients with Gaucher's disease and Parkinson’s disease, including ten new patients from France, resting tremor was noted in 69% of patients.63 Atypical features such as perceptive deafness18 and supranuclear oculomotor signs19 have been reported, but whether these manifestations are attributable to the parkinsonian phenotype as opposed to Gaucher symptoms is difficult to establish. Alonso-Canovas and colleagues64 described a 66-year-old Ashkenazi Jewish patient with Gaucher's disease and atypical parkinsonism who developed apraxia and supra nuclear gaze abnormalities. One non-Jewish patient with Gaucher's disease from Poland who had a 24-year history of parkinsonism was assessed 12 years after the initial report. This patient ultimately developed cognitive decline, akinetic mutism, and cortical atrophy.65
Reports regarding the efficacy of levodopa treatment in GBA mutation carriers have been inconsistent. Although parkinsonian symptoms in patients with Gaucher's disease were initially thought to be poorly responsive to levodopa treatment,19 most subsequent studies have reported good or excellent responses, similar to those in patients with sporadic Parkinson’s disease.17,30,34,39,41,62
A group of 30 adult patients with Gaucher's disease from the Lysosomal Disorders Unit at the Royal Free Hospital (London, UK) were screened for potential prodromal symptoms of early neurodegeneration.66 Although an increased occurrence of hyposmia and cognitive impairment was reported, larger prospective studies are needed to identify the specific cognitive areas most affected in these patients.
The effect of treatment for Gaucher's disease on parkinsonian manifestations
Two types of treatments are approved for Gaucher's disease. Enzyme replacement therapy using recombinant glucocerebrosidase can be used to control visceral and haematological complications of Gaucher's disease, but does not cross the blood–brain barrier and does not affect neurological manifestations of Gaucher's disease. The treatment does not seem to have any effect on the progression of parkinsonian symptoms.18,60,62,63 Thus, using the development of parkinsonian manifestations as a criterion to start this costly treatment is not recommended. An alternative treatment for Gaucher's disease that is less widely used is designed to reduce glucosylceramide synthesis with an iminosugar inhibitor. However, substrate reduction therapy with the iminosugar miglustat was used in two patients with Gaucher's disease and Parkinson’s disease without any effect on the progression of their parkinsonism.63
Parkinsonian features in GBA mutation carriers
Findings from several studies of patients with Parkinson’s disease who were being managed at movement disorders clinics have confirmed the earlier age at onset of Parkinson’s disease symptoms in patients heterozygous for GBA mutations compared with those without mutations. Neumann and colleagues39 described clinical features of patients with Parkinson’s disease in a large non-Jewish cohort. They screened 790 patients with Parkinson’s disease for GBA mutations and examined the age at disease onset, sex, levodopa response, and motor and non-motor symptoms (cognition and visual hallucinations). GBA carriers were predominantly men, were more likely to have cognitive dysfunction or dementia, and had more frequent hallucinations (not associated with drug treatment) compared with patients without GBA mutations. Another study from Spain reported that dementia was more frequent among GBA mutation carriers with Parkinson’s disease than non-carriers.67 Scales including the Unified Parkinson’s disease rating scale, mini-mental state examination, and Hoehn and Yahr staging have not detected significant differences in either the extent of Parkinson’s disease manifestations or the rate of progression of disease between GBA carriers and non-carriers.51 However, some studies have reported a higher frequency of cognitive decline,39,62 bradykinesia,68 and olfactory dysfunction,62 and less rigidity31 associated with GBA mutations.
Non-motor symptoms have also been described in GBA mutation carriers with Parkinson’s disease. In one prospective study, the most consistent non-motor feature was olfactory dysfunction. Other associated features were variable, including depression, anxiety, and cognitive decline.62 A German study of Parkinson’s disease that compared 20 patients with GBA mutations with 20 patients without concluded that mutation carriers were more likely to have dementia, neuropsychological disturbances, and autonomic dysfunction.69
Neuroimaging
PET scanning has been used to look for possible dopaminergic dysfunction in patients with Parkinson’s disease and GBA mutations. Two sisters with Parkinson’s disease from South Korea, one with Gaucher's disease and one a carrier, were assessed with 18F-N-fluoropropyl–2β-carbomethoxy-3β-(4-iodophenyl)nortropane PET at ages 44 years and 55 years, respectively.70 Both showed decreased reuptake of dopamine in the posterior putamen, in a pattern similar to that noted in sporadic Parkinson’s disease. Four additional patients assessed with 18F-fluorodopa PET also showed decreased striatal uptake.42 Findings from a larger study showed that although the pattern of dopamine loss in patients with Gaucher's disease and Parkinson’s disease was similar to that in patients with sporadic Parkinson’s disease, H215O PET showed decreased resting regional cerebral blood flow activity in a pattern characteristic of diffuse Lewy body disease.71
Findings from preliminary studies with transcranial sonography suggest increased echogenicity in the substantia nigra of patients with GBA-associated Par kinson’s disease.69 Three other patients with Gaucher's disease and Parkinson’s disease exhibited increased nigral echogenicity compared with control individuals.42
Studies of CSF
CSF concentrations of 13 fatty acids were assessed by gas chromatography in eight patients with Parkinson’s disease carrying a GBA mutation and were compared with concentrations from 41 patients with idiopathic Parkinson’s disease.72 Findings from this preliminary study suggested specific abnormalities of fatty acid metabolism in GBA-associated Parkinson’s disease, which merits further assessment in larger cohorts.
In one patient with Gaucher's disease with a long history of Parkinson’s disease, reverse-phase high-performance liquid chromatography of CSF revealed low concentrations of monoamine metabolites, suggesting the involvement of norepinephrinergic and serotonergic neurons. Concentrations of tau and amyloid-β, markers of dementia, were normal.65
Genetic counselling
Most patients with GBA mutations never develop Parkinson’s disease. Care should be taken when counselling patients with Gaucher's disease and carrier relatives about risk factors for neurodegenerative disorders. In particular, the reported increased incidence of dementia in GBA-associated Parkinson’s disease66,67 can be a source of great concern for patients with Gaucher's disease and their families. Careful clinical assessments should be done and detailed histories taken in patients with Gaucher's disease and their relatives, and any evidence of parkinsonian features or cognitive difficulties should be investigated further. As more is known about the pathophysiological mechanisms underlying GBA-associated parkinsonism and as potential neuroprotective drugs become available, early identification of those at highest risk will be important.
Based on published data from the ICGG Gaucher Registry,60 among patients with Gaucher's disease the probability of developing Parkinson’s disease before age 70 years was 5–7% and before age 80 years was 9–12%. However, of 203 patients in the registry who were born before 1929, only ten had developed Par kinson’s disease. Thus, the enzymatic deficiency in most cases was not predictive of Parkinson’s disease. Bultron and colleagues61 attempted to estimate the risk of Par kinson’s disease in a series of 444 consecutively assessed patients from the USA who had type 1 Gaucher's disease. 11 patients developed Parkinson’s disease over a 12-year follow-up period. Despite this limited sample size, the authors attempted to compare the lifetime risk ratio for developing Parkinson’s disease in patients with Gaucher's disease to that in the general population, and estimated it to be increased by 20 times. However, this finding is not substantiated by the ICGG Gaucher Registry,60 and larger, better-designed studies are needed to address this question accurately.
A study from France attempted to estimate the Parkinson’s disease penetrance in families of GBA mutation carriers.73 Focusing on 24 GBA mutation carriers with Parkinson’s disease, the authors found that 26 of 32 relatives with Parkinson’s disease carried GBA mutations, whereas 31 of 71 tested relatives who did not have Parkinson’s disease had GBA mutations.73 The authors concluded that this high penetrance estimate suggested that GBA is a dominant causal Parkinson’s disease gene with reduced penetrance. This interpretation is of concern, because it could lead to inappropriate genetic counselling in settings where the counsellor is not fully aware of the overlap between Mendelian inheritance with reduced penetrance and complex multigenic inheritance.
Thus, at present, genetic counselling about the risk of Parkinson’s disease in patients with a GBA mutation is complicated. Most patients with Gaucher's disease and GBA mutation carriers never develop Parkinson’s disease. Taking a careful family history to investigate other possible risk factors for Parkinson’s disease is essential. Although GBA mutation carriers do seem to be at an increased risk for Parkinson’s disease, further studies might reveal other genetic or non-genetic factors that modify Parkinson’s disease risk in such patients.
Neuropathological findings in GBA-associated parkinsonism
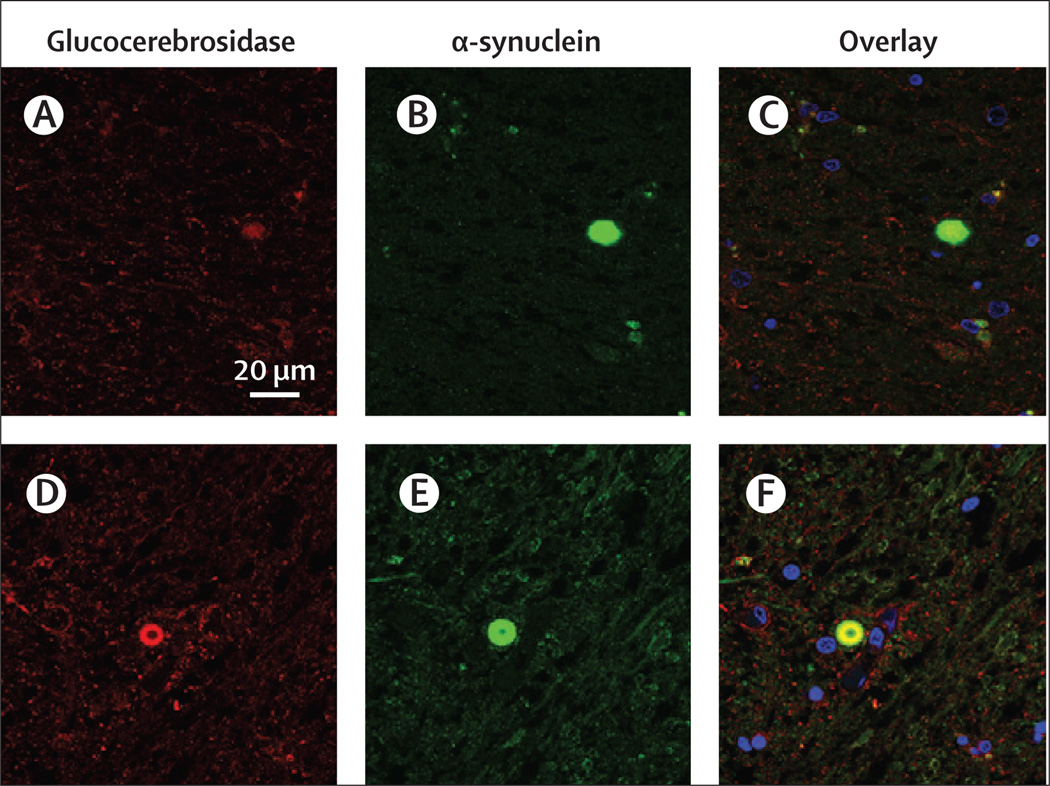
Typically, patients with type 1 Gaucher's disease have few neuropathological findings. However, findings from autopsies of brains from patients with GBA-associated parkinsonism show α-synuclein-immuno reactive cortical-type and brain-stem-type Lewy bodies and Lewy neurites. Additionally, Lewy bodies have been found in more atypical locations, such as in hippocampal regions CA2–4, which are areas of vulnerability in neuronopathic Gaucher's disease and dementia with Lewy bodies.19,74 In one study, patients with GBA mutations had a more diffuse distribution of Lewy bodies than the brainstem and limbic distribution noted in patients with typical Parkinson’s disease.75 Moreover, α-synuclein inclusions were found in the cortical areas, which corresponded to Braak stages 5–6, resembling findings reported in patients with dementia with Lewy bodies.76 However, in another study of brain samples from 33 patients with Parkinson’s disease, including 17 with heterozygous GBA mutations, no association was identified between GBA status and the density of cortical Lewy bodies when corrected for age, sex, duration of Parkinson’s disease, and the presence of dementia.77 Seven brain samples from patients carrying GBA mutations who had pathological diagnoses of Parkinson’s disease or dementia with Lewy bodies were used to assess the contribution of glucocerebrosidase to the development of parkinsonian pathological abnormalities with immunofluorescence and confocal microscopy.78 Glucocerebrosidase was present in 32–90% of both ubiquitinated and non-ubiquitinated Lewy bodies, whereas in samples from patients without mutations, less than 10% of Lewy bodies were glucocerebrosidase positive (figure 2). Whether or how the presence of the enzyme in Lewy inclusions contributes to disease pathogenesis is still not known.
Figure 2. Immunofluorescence assessment of glucocerebrosidase in Lewy bodies in patients with parkinsonism without and with GBA mutations.
(A, B, and C) The substantia nigra area from a patient without GBA mutations stained for glucocerebrosidase (A, red) and α-synuclein (B, green). The merged image with the nuclear stain (blue) shows a paucity of glucocerebrosidase staining in the Lewy body structure (C). (D, E, and F) The substantia nigra area from a GBA mutation carrier stained for glucocerebrosidase (D, red) and α-synuclein (E, green) showing colocalisation and accumulation in the Lewy body structure (F).
GBA mutations in other Lewy body disorders
Studies of GBA have been extended to other Lewy body disorders, including dementia with Lewy bodies and multiple systems atrophy. Deposition of fibrillated α-synuclein, either in the brainstem or in cortical inclusion bodies, is characteristic of such disorders.79–81 GBA mutations were first identified in 23% of 35 cases with pathologically confirmed dementia with Lewy bodies.82 In another study, screening for N370S and L444P detected GBA alterations in 3·5% of 57 patients with dementia with Lewy bodies compared with 0·4% of 554 control individuals (p=0·045).34 Farrer and colleagues83 studied 50 brain samples from individuals with pathologically confirmed dementia with Lewy bodies and identified GBA mutations in 6%; whereas Clark and colleagues76 reported the presence of GBA mutations in 28% of 95 patients with dementia with Lewy bodies, 10% of 60 patients with Alzheimer’s disease, and 3% of 35 control individuals (p<0·001). In these studies, GBA carriers were significantly more likely than non-carriers to have Lewy bodies at autopsy. Findings from a large multicentre analysis of GBA mutations in patients with dementia with Lewy bodies,84 which was similar to the international study in Parkinson’s disease,51 suggested that the frequency of GBA mutations in patients with dementia with Lewy bodies is even higher than in patients with Parkinson’s disease, with an OR of 8·28 (95% CI 4·78–14·88)
The association between GBA mutations and multiple systems atrophy seems to be different from that in dementia with Lewy bodies. A study of 12 cases of multiple systems atrophy did not identify GBA mutations.82 In a study of 108 autopsy-verified cases of multiple systems atrophy and 257 control individuals from the UK,85 all GBA exons were screened and no significant differences were noted in cases compared with control individuals (p=0·66). A Polish study that examined 66 patients diagnosed with multiple systems atrophy also failed to identify GBA mutations.86 Since in multiple systems atrophy α-synuclein is deposited mostly in oligo dendroglial cytoplasmic inclusions, rather than in neurons, this distinction might be relevant to disease pathogenesis.81
Proposed mechanisms for GBA-associated parkinsonism
The mechanisms underlying the relation between GBA mutations and the development of Parkinson’s disease and associated disorders remain elusive. However, several recent studies provide some new perspectives. Generally, in autosomal recessive forms of Parkinson’s disease, such as those involving PARK2, DJ-1,and PINK1,loss-of-function mutations are implicated, and these patients have an early onset of disease manifestations. By contrast, gain-of-function mutations are usually associated with autosomal dominant forms of Parkinson’s disease, such as with SCNA and LRRK2.87 Although Gaucher's disease is an autosomal recessive disorder, the inheritance of parkinsonism associated with GBA mutations does not follow a strict Mendelian pattern, leading investigators to propose both gain-of-function and loss-of-function theories (panel 3).
Panel 3.
Arguments for a gain-of-function versus a loss-of-function mechanism for GBA associated parkinsonism
Gain of function
Parkinson’s disease occurs in GBA heterozygotes
Most GBA mutations are missense, leading to a misfolded protein
Mutant glucocerebrosidase is found in Lewy bodies, suggesting a role in α-synuclein oligomerisation or impaired degradation
Most patients with Gaucher's disease do not develop Parkinson’s disease, despite low glucocerebrosidase activity
Mutant glucocerebrosidase can accumulate in the endoplasmic reticulum and burden the proteasomal and lysosomal systems responsible for degrading proteins
Loss of function
Null GBA alleles (eg, c.84dupG and IVS2+1G>A) have been reported in patients with Parkinson’s disease
Mouse models of Gaucher's disease show increased levels of α-synuclein
Lipid rafts and ceramides play a part in Parkinson’s disease pathogenesis
Chemical inhibition of glucocerebrosidase in mice and in cells increases concentrations of α-synuclein
The lipids glucocerebroside and glucosylsphingosine can accumulate in the lysosome, affecting its function
Support for gain-of-function and loss-of function hypotheses
The finding that most mutant alleles identified result in a misfolded protein supports a gain-of-function role for mutations in GBA. This abnormal conformation could contribute to the development of parkinsonism by increasing α-synuclein aggregation. Alternatively, the misfolded protein could lead to lysosomal dysfunction, overwhelming the ubiquitin-proteasome pathway or causing impairment of autophagy.
Additionally, parkinsonism could arise as a consequence of glucocerebrosidase deficiency, due to the loss of function of the enzyme. A misfolded protein can be degraded, leading to enzymatic deficiency and substrate accumulation. The potential role of lipid homoeostasis and lysosomal function in the cause of Parkinson’s disease is a topic of great interest, and other ceramide-related genes have been implicated in Parkinson’s disease.88 Furthermore, α-synuclein can bind to the lipid-raft-associated ganglioside GM1.89 Lipid rafts have an essential role in the proper trafficking of α-synuclein to presynaptic membranes; hence, lipid storage could cause changes in lipid homoeostasis, with subsequent alterations in α-synuclein processing.90
Although both theories have some support, there are serious deficiencies with each. First, some GBA mutations encountered in patients with parkinsonism are null mutations—a finding in conflict with the gain-of-function hypothesis. In fact, carriers of null alleles might have an even higher risk of development of parkinsonism.68 Second, the clinical finding that most patients with Gaucher's disease never develop Parkinson’s disease, despite having a deficiency of glucocerebrosidase exceeding that of heterozygotes, argues against a solely loss-of-function hypothesis. This theory is supported by the low frequency of Parkinson’s disease reported in the large Gaucher cohort in the ICGG Gaucher Registry.60 Thus, in most cases, the enzymatic deficiency is not predictive of Parkinson’s disease.
In another study, Cullen and colleagues91 concluded that both loss-of-function and gain-of-function mechanisms were consistent with their data. In culture, neuronal cell lines stably overexpressing α-synuclein and different GBA mutations showed a gain-of-function effect; however, findings from the sensitive sandwich ELISA assay, developed by the authors to measure α-synuclein, showed that raised concentrations of α-synuclein were dependent on the amount of expressed mutant GBA,but not its activity. Using a point mutation knock-in mouse model of Gaucher's disease with genotype D409V/D409V, the authors noted that both mutant GBA and downregulation of glucocerebrosidase led to upregulation of membrane-associated α-synuclein in the hippocampus; in heterozygote mice (D409V/+) this was not identified.
Using this same mouse model, other investigators reported that α-synuclein accumulates in hippocampal neurons and that this accumulation increases with age.92 Moreover, these mice developed a corresponding memory deficit that was reversed when recombinant glucocerebrosidase was administered directly into the brain. The α-synuclein accumulation was associated more with increased concentrations of glucosylsphingosine than glucosylceramide. However, although this mouse model is used because it has decreased enzymatic activity, it is not ideal because the D409V mutation is not reported in Gaucher's disease, and this mouse model does not manifest typical Gaucher features. Although the authors concluded that the reported pathological abnormalities are a combination of loss of activity and toxicity related to the mutant enzyme, neuropathological changes in both D409V homozygote and heterozygote (but not heterozygous knockout) mice suggest that α-synuclein aggregation is not associated with glucocerebrosidase activity or substrate accumulation.
Other attempts to model the association between Gaucher's disease and parkinsonism in animals have yielded similarly conflicting results. Treatment of wild-type mice with the glucocerebrosidase inhibitor conduritol β epoxide moderately increased concentrations of α-synuclein.93 Gaucher mice homozygous for V394L or D409H, which have a normal lifespan and do not show storage of glucosylceramide in the CNS, were crossed with mice deficient in prosaposin, including the peptide saposin C, which is necessary for glucocerebrosidase activation, yielding mice that developed α-synuclein aggregation in cortical neurons. However, this aggregation was not related to glucosylceramide accumulation.94
The prion hypothesis
Another hypothesis that has been proposed to explain this association is that α-synuclein aggregates might have a prion-like mechanism of cell-to-cell transmission, and that macrophages, the cells most affected in Gaucher's disease, might serve as a carrier of such aggregates.95 The author suggested that both endogenous and exogenous α-synuclein might accumulate and spread in the lipid-rich environment found in Gaucher's cells. However, this suggestion does not explain why carriers would be at risk, because they generally do not have storage in macrophages. One possible explanation is that a second hit—a new somatic GBA mutation— could occur in isolated macrophages, which would initiate α-synuclein accumulation and spread.
The role of α-synuclein
Findings from recent studies have further implicated α-synuclein in the pathogenesis of GBA-associated parkinsonism. Yap and colleagues96 reported a physical interaction between α-synuclein and purified recombinant glucocerebrosidase, occurring only at the acidic pH present in the lysosomal compartment, using four different methodologies. First, when incubated with glucocerebrosidase at pH 5·5, labelled Dns-136-α-synuclein caused a marked spectral shift with in creased intensity, suggesting a physical interaction between the two molecules in lysosomes. However, this shift did not occur when the experiment was done at pH 7·4 or when the label was placed on the N-terminal of α-synuclein. Residue level mapping by nuclear MRI at pH 5·5 was done to examine the relative spectra intensity ratio of α-synuclein-glucocerebrosidase to α-synuclein alone as a function of α-synuclein residues and showed that the interaction occurred at residues 118–137 of α-synuclein. Again, no significant interaction was noted at pH 7·4. In brain samples from patients with Parkinson’s disease, glucocerebrosidase co-immunoprecipitated with native α-synuclein at pH 5·5 but not at pH 7·4. Lastly, confocal microscopy of M17 neuroblastoma cells stably over-expressing glucocerebrosidase and α-synuclein showed colocalisation of the enzyme with α-synuclein in cathepsin-D-positive cellular structures. The C-terminal region of α-synuclein seems to interact with glucocerebrosidase, whereas the N-terminal helix is bound to a glycolipid-rich vesicle inside the lysosome. This binding at lysosomal pH might facilitate α-synuclein degradation or prevent aggregation. Because much of the processing of α-synuclein occurs in the cytoplasm, this interaction was unexpected, but it supports a role for lysosomal pathways in α-synuclein degradation. That glucocerebrosidase might have a beneficial role in α-synuclein processing that is disrupted when GBA is mutated or absent is intriguing.
Studies of brain samples have shed some light on the association between α-synuclein pathology and glucocerebrosidase. Proteins extracted from cerebral cortex samples from patients with synucleinopathies with and without GBA mutations, as well as samples from control individuals and patients with Gaucher's disease, were studied by western blotting. Patients with GBA-associated parkinsonism showed aggregated oligomeric forms of α-synuclein in the insoluble fraction, whereas only monomeric α-synuclein was noted in patients with GBA mutations without parkinsonism, including samples from patients with neuronopathic Gaucher's disease. Thus, aggregation of α-synuclein is not related to glucocerebrosidase deficiency alone.97
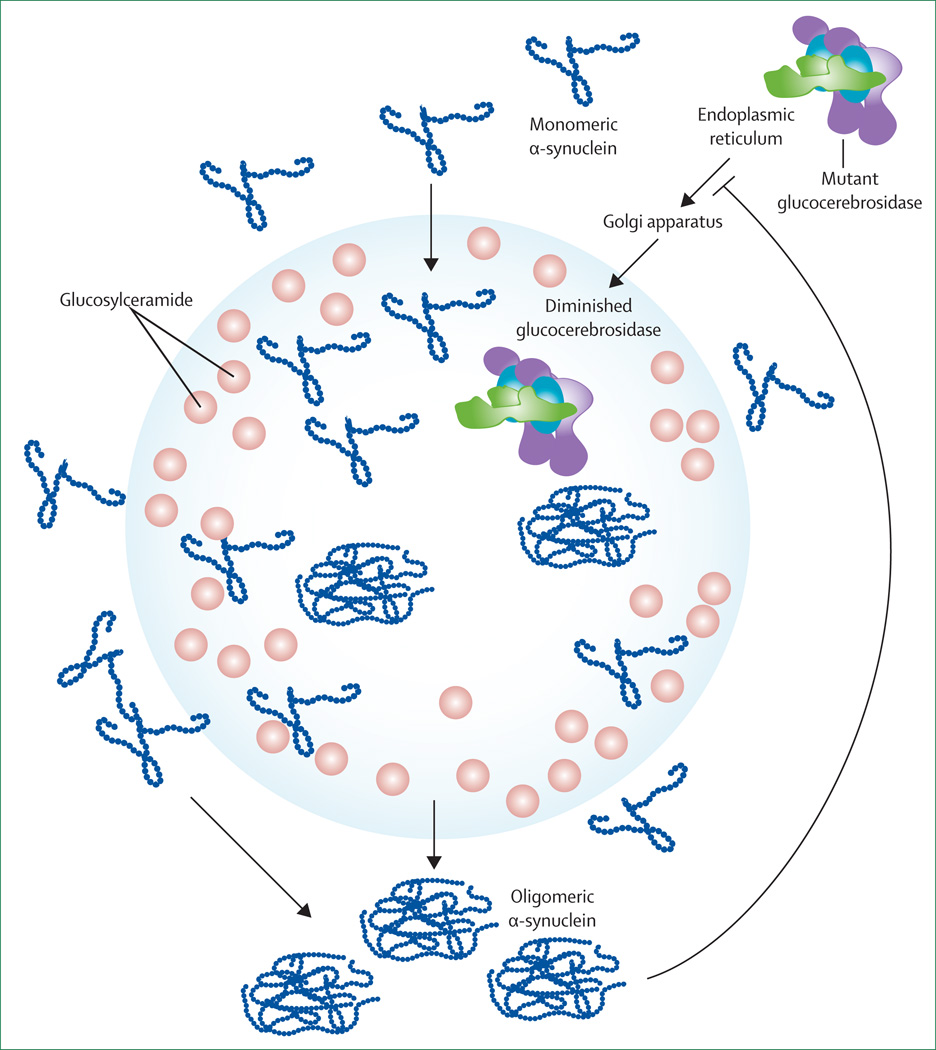
The association between α-synuclein pathology and glucocerebrosidase was recently assessed from a different perspective in a complex study by Mazzulli and colleagues.98 Using cultured primary neurons treated with short hairpin RNA by lentiviral infection to knock down the expression of glucocerebrosidase, a 50% reduction in glucocerebrosidase and reduced concentrations of endoglycosidase H-resistant glucocerebrosidase were reported and were ascribed to the depletion of the lysosomal form of the enzyme. This glucocerebrosidase knock-down also increased α-synuclein concentrations by 1·8 times. The authors then showed that human induced-pluripotent-stem-cell-derived neurons made from Gaucher fibroblasts also had an accumulation of α-synuclein that resulted in neurotoxicity attributed to aggregation-dependent mechanisms. They concluded that deficiency of glucocerebrosidase resulted in impaired lysosomal proteolysis that preferentially affected α-synuclein. Working with a mouse model, Caenorhabditis elegans, and human brain samples, an attempt was made to establish that increased concentrations of lysosomal glucosylceramides resulting from diminished enzymatic activity promoted the formation of soluble α-synuclein oligomers and fibrils. Further more, they noted that increased concentrations of cytoplasmic α-synuclein blocked endoplasmic reticulum–Golgi apparatus trafficking of glucocerebrosidase, thus compounding the problem (figure 3). This further decrease in lysosomal glucocerebrosidase amplified the increase in glucosylceramide and the production of additional stable α-synuclein oligomers. The authors concluded that a bidirectional pathogenic loop had formed, leading to self-propagating disease. The exciting aspect of this hypothesis is that even without mutations, decreased glucocerebrosidase concentrations might have a role in Parkinson’s disease pathogenesis. However, how the 50% reduction in glucocerebrosidase reported in their cell-based model resulted in glucosylceramide accumulation and complete lysosomal dysfunction is difficult to understand. This finding does not typically occur in patients with type 1 Gaucher's disease, who have a 70–90% reduction in glucocerebrosidase activity.2 Thus, although this work provides ideas for future research, it does not completely explain what occurs clinically.
Figure 3. The bidirectional loop theory.
Decreased glucocerebrosidase increases the lysosomal concentrations of glucosylceramide, which increases the formation of soluble α-synuclein oligomers. These oligomers also disrupt transport of newly synthesised glucocerebrosidase between the endoplasmic reticulum and Golgi apparatus, further compounding the problem.
Does mutated GBA affect autophagy?
Lysosomes are involved in the degradation of substrates both through enzymatic degradation and through different autophagic pathways that can include chaperone-mediated autophagy, macroautophagy, or micro autophagy.99 In addition to proteasomal pathways, α-synuclein is also degraded via chaperone-mediated autophagy.99,100 Alterations in autophagy have been reported in several neurodegenerative lysosomal storage disorders and in a Gaucher mouse model.101 In a murine study, overexpression of D409V glucocerebrosidase resulted in α-synuclein accumulation that could be reversed using rapamycin, an inducer of macroautophagy.91 Parkin, an E3 ubiquitin ligase, might also play a part in the degradation and accumulation of mutant glucocerebrosidase.102
Conclusions
Further studies using techniques in cell biology, neuropathology, and genetics are needed to better piece together the mechanisms that contribute to GBA-associated parkinsonism and to identify other risk factors working in concert with GBA that favour the development of parkinsonism. Since Parkinson’s disease is a disorder of ageing, factors that change during the ageing process probably play a part.103 As we age, cellular concentrations of α-synuclein increase.104,105 In parallel, ageing is associated with diminished numbers of lysosomes and poorer lysosomal function.106 Thus, one might speculate that there is a crucial balance between the amount of α-synuclein needing to be degraded and the lysosomal capacity, which becomes compromised as one ages, or as the lysosomes become engorged with storage products. Mutant or absent glucocerebrosidase might somehow contribute to this scenario by further compromising the ability of the lysosome to function normally. Either the absence of this protein, which in this case might have a totally different purpose than its known enzymatic function, or altered lipid metabolism might affect the normal lysosomal breakdown of α-synuclein aggregates, resulting in the increased risk of Parkinson’s disease (figure 4).
Figure 4. How mutant glucocerebrosidase might result in enhanced α-synuclein aggregation.
(A) In the normally functioning lysosome, wild-type glucocerebrosidase might interact with α-synuclein, facilitating the lysosomal component of α-synuclein degradation. (B) In most cases, when glucocerebrosidase is mutated, α-synuclein remains in the monomeric form and other processes are active in its degradation. (C) In some patients, glucocerebrosidase is mutated and the cell is unable to degrade α-synuclein. Lysosomal function is compromised and increased oligomeric forms of α-synuclein lead to neuronal cell death and the development of parkinsonism. This situation might enhanced by ageing, when the number of lysosomes and lysosome function decrease and cellular α-synuclein concentrations increase.
The identification of glucocerebrosidase as a risk factor for parkinsonism is, and will continue to be, a source of considerable excitement in the specialty. In contrast to most other identified genes associated with Parkinson’s disease, a great deal is known about this protein, its processing, and its function. This knowledge provides the opportunity for new hypotheses and research directions for years to come. Moreover, different therapeutic strategies under consideration and development for the treatment of Gaucher's disease could lead to stabilisation of the protein or enhancement of enzymatic activity and affect the course of the neurodegeneration that occurs in parkinsonism.
However, despite the substantial progress that has been made, the basis for GBA-associated parkinsonism remains enigmatic. Unravelling the factors underlying this association is imperative to enable improved genetic counselling for people who carry mutations in this gene. Moreover, a better understanding of the role of this lysosomal enzyme in the development of parkinsonism will contribute to new and improved therapeutic strategies for GBA-associated parkinsonism, which might affect the treatment of all individuals with Parkinson’s disease, a common and complex disease.
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed and the Web of Science from 1990 to May, 2012, with the search items “glucocerebrosidase”, “GBA”, “Gaucher”, “Parkinson”, and “parkinsonism”. The final reference list was selected on the basis of relevance to the subject of this review.
Acknowledgments
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Institutes of Health. We thank Julia Fekecs, Wendy Westbroek, and Darryl Leja for their assistance with the preparation of the figures.
Footnotes
Contributors
ES and GL reviewed the published work and wrote the manuscript.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Gaucher P. De l’epithelioma primitif de la rate, hypertrophie idiopathique de la rate sans leucemie. PhD thesis: University of Paris; 1882. [Google Scholar]
- 2.Beutler E, Grabowski G. Gaucher disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, et al., editors. The metabolic and molecular bases of inherited disease. 8th edn. New York: McGraw-Hill; 2001. pp. 3635–3668. [Google Scholar]
- 3.Zimran A. How I treat Gaucher disease. Blood. 2011;118:1463–1471. doi: 10.1182/blood-2011-04-308890. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz M, Wilder S, Horowitz Z, Reiner O, Gelbart T, Beutler E. The human glucocerebrosidase gene and pseudogene: structure and evolution. Genomics. 1989;4:87–96. doi: 10.1016/0888-7543(89)90319-4. [DOI] [PubMed] [Google Scholar]
- 5.Winfield SL, Tayebi N, Martin BM, Ginns EI, Sidransky E. Identification of three additional genes contiguous to the glucocerebrosidase locus on chromosome 1q21: implications for Gaucher disease. Genome Res. 1997;7:1020–1026. doi: 10.1101/gr.7.10.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tayebi N, Stubblefield BK, Park JK, et al. Reciprocal and nonreciprocal recombination at the glucocerebrosidase gene region: implications for complexity in Gaucher disease. Am J Hum Genet. 2003;72:519–534. doi: 10.1086/367850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum Mutat. 2008;29:567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 8.Beutler E, Gelbart T, Kuhl W, Zimran A, West C. Mutations in Jewish patients with Gaucher disease. Blood. 1992;79:1662–1666. [PubMed] [Google Scholar]
- 9.Zuckerman S, Lahad A, Shmueli A, et al. Carrier screening for Gaucher disease: lessons for low-penetrance, treatable diseases. JAMA. 2007;298:1281–1290. doi: 10.1001/jama.298.11.1281. [DOI] [PubMed] [Google Scholar]
- 10.Koprivica V, Stone DL, Park JK, et al. Analysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher disease. Am J Hum Genet. 2000;66:1777–1786. doi: 10.1086/302925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goker-Alpan O, Hruska KS, Orvisky E, et al. Divergent phenotypes in Gaucher disease implicate the role of modifiers. J Med Genet. 2005;42:e37. doi: 10.1136/jmg.2004.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sidransky E, Ginns EI. Clinical heterogeneity among patients with Gaucher’s disease. JAMA. 1993;269:1154–1157. [PubMed] [Google Scholar]
- 13.Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Goker-Alpan O, Schiffmann R, Park JK, Stubblefield BK, Tayebi N, Sidransky E. Phenotypic continuum in neuronopathic Gaucher disease: an intermediate phenotype between type 2 and type 3. J Pediatr. 2003;143:273–76. doi: 10.1067/S0022-3476(03)00302-0. [DOI] [PubMed] [Google Scholar]
- 15.Neudorfer O, Giladi N, Elstein D, et al. Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM. 1996;89:691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 16.Machaczka M, Rucinska M, Skotnicki AB, Jurczak W. Parkinson’s syndrome preceding clinical manifestation of Gaucher’s disease. Am J Hematol. 1999;61:216–217. doi: 10.1002/(sici)1096-8652(199907)61:3<216::aid-ajh12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Tayebi N, Callahan M, Madike V, et al. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol Genet Metab. 2001;73:313–321. doi: 10.1006/mgme.2001.3201. [DOI] [PubMed] [Google Scholar]
- 18.Bembi B, Zambito Marsala S, Sidransky E, et al. Gaucher’s disease with Parkinson’s disease: clinical and pathological aspects. Neurology. 2003;61:99–101. doi: 10.1212/01.wnl.0000072482.70963.d7. [DOI] [PubMed] [Google Scholar]
- 19.Tayebi N, Walker J, Stubblefield B, et al. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol Genet Metab. 2003;79:104–109. doi: 10.1016/s1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 20.Goker-Alpan O, Schiffmann R, LaMarca ME, Nussbaum RL, McInerney-Leo A, Sidransky E. Parkinsonism among Gaucher disease carriers. J Med Genet. 2004;41:937–940. doi: 10.1136/jmg.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halperin A, Elstein D, Zimran A. Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood Cells Mol Dis. 2006;36:426–428. doi: 10.1016/j.bcmd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004;81:70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 24.Clark LN, Nicolai A, Afridi S, et al. Pilot association study of the beta-glucocerebrosidase N370S allele and Parkinson’s disease in subjects of Jewish ethnicity. Mov Disord. 2005;20:100–103. doi: 10.1002/mds.20320. [DOI] [PubMed] [Google Scholar]
- 25.Sato C, Morgan A, Lang AE, et al. Analysis of the glucocerebrosidase gene in Parkinson’s disease. Mov Disord. 2005;20:367–370. doi: 10.1002/mds.20319. [DOI] [PubMed] [Google Scholar]
- 26.Toft M, Pielsticker L, Ross OA, Aasly JO, Farrer MJ. Glucocerebrosidase gene mutations and Parkinson disease in the Norwegian population. Neurology. 2006;66:415–417. doi: 10.1212/01.wnl.0000196492.80676.7c. [DOI] [PubMed] [Google Scholar]
- 27.Eblan MJ, Scholz S, Stubblefield B, et al. Glucocerebrosidase mutations are not found in association with LRRK2 G2019S in subjects with parkinsonism. Neurosci Lett. 2006;404:163–165. doi: 10.1016/j.neulet.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Tan EK, Tong J, Fook-Chong S, et al. Glucocerebrosidase mutations and risk of Parkinson disease in Chinese patients. Arch Neurol. 2007;64:1056–1058. doi: 10.1001/archneur.64.7.1056. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler SG, Eblan MJ, Gutti U, et al. Glucocerebrosidase mutations in Chinese subjects from Taiwan with sporadic Parkinson disease. Mol Genet Metab. 2007;91:195–200. doi: 10.1016/j.ymgme.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu YR, Chen CM, Chao CY, et al. Glucocerebrosidase gene mutation is a risk factor for early onset of Parkinson disease among Taiwanese. J Neurol Neurosurg Psychiatry. 2007;78:977–979. doi: 10.1136/jnnp.2006.105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark LN, Ross BM, Wang Y, et al. Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease. Neurology. 2007;69:1270–1277. doi: 10.1212/01.wnl.0000276989.17578.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Marco EV, Annesi G, Tarantino P, et al. Glucocerebrosidase gene mutations are associated with Parkinson’s disease in southern Italy. Mov Disord. 2008;23:460–463. doi: 10.1002/mds.21892. [DOI] [PubMed] [Google Scholar]
- 33.Spitz M, Rozenberg R, da Veiga Pereira L, Barbosa ER. Association between Parkinson’s disease and glucocerebrosidase mutations in Brazil. Parkinsonism Relat Disord. 2008;14:58–62. doi: 10.1016/j.parkreldis.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Mata IF, Samii A, Schneer SH, et al. Glucocerebrosidase gene mutations—a risk factor for Lewy body disorders. Arch Neurol. 2008;65:379–382. doi: 10.1001/archneurol.2007.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gan-Or Z, Giladi N, Rozovski U, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70:2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 36.Bras JM, Singleton A. Genetic susceptibility in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:597–603. doi: 10.1016/j.bbadis.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalinderi K, Bostantjopoulou S, Paisan-Ruiz C, Katsarou Z, Hardy J, Fidani L. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Greece. Neurosci Lett. 2009;452:87–89. doi: 10.1016/j.neulet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Nichols WC, Pankratz N, Marek DK, et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009;72:310–316. doi: 10.1212/01.wnl.0000327823.81237.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitsui J, Mizuta I, Toyoda A, et al. Mutations for Gaucher disease confer high susceptibility to Parkinson disease. Arch Neurol. 2009;66:571–576. doi: 10.1001/archneurol.2009.72. [DOI] [PubMed] [Google Scholar]
- 41.Emelyanov A, Boukina T, Yakimovskii A, et al. Glucocerebrosidase gene mutations are associated with Parkinson’s disease in Russia. Mov Disord. 2012;27:158–159. doi: 10.1002/mds.23950. [DOI] [PubMed] [Google Scholar]
- 42.Saunders-Pullman R, Hagenah J, Dhawan V, et al. Gaucher disease ascertained through a Parkinson’s center: imaging and clinical characterization. Mov Disord. 2010;25:1364–1372. doi: 10.1002/mds.23046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao XY, Burgunder JM, Zhang ZJ, et al. Association between GBA L444P mutation and sporadic Parkinson’s disease from mainland China. Neurosci Lett. 2010;469:256–259. doi: 10.1016/j.neulet.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Sun QY, Guo JF, Wang L, et al. Glucocerebrosidase gene L444P mutation is a risk factor for Parkinson's disease in Chinese population. Mov Disord. 2010;25:1005–1011. doi: 10.1002/mds.23009. [DOI] [PubMed] [Google Scholar]
- 45.Hu FY, Xi J, Guo J, et al. Association of the glucocerebrosidase N370S allele with Parkinson’s disease in two separate Chinese Han populations of mainland China. Eur J Neurol. 2010;17:1476–1478. doi: 10.1111/j.1468-1331.2010.03097.x. [DOI] [PubMed] [Google Scholar]
- 46.Lesage S, Condroyer C, Hecham N, et al. Mutations in the glucocerebrosidase gene confer a risk for Parkinson disease in north Africa. Neurology. 2011;76:301–303. doi: 10.1212/WNL.0b013e318207b01e. [DOI] [PubMed] [Google Scholar]
- 47.Noreau A, Riviere JB, Diab S, et al. Glucocerebrosidase mutations in a French-Canadian Parkinson’s disease cohort. Can J Neurol Sci. 2011;38:772–773. doi: 10.1017/s0317167100012300. [DOI] [PubMed] [Google Scholar]
- 48.Lesage S, Anheim M, Condroyer C, et al. Large-scale screening of the Gaucher’s disease-related glucocerebrosidase gene in Europeans with Parkinson’s disease. Hum Mol Genet. 2011;20:202–210. doi: 10.1093/hmg/ddq454. [DOI] [PubMed] [Google Scholar]
- 49.Huang CL, Wu-Chou YH, Lai SC, et al. Contribution of glucocerebrosidase mutation in a large cohort of sporadic Parkinson’s disease in Taiwan. Eur J Neurol. 2011;18:1227–1232. doi: 10.1111/j.1468-1331.2011.03362.x. [DOI] [PubMed] [Google Scholar]
- 50.Choi JM, Kim WC, Lyoo CH, et al. Association of mutations in the glucocerebrosidase gene with Parkinson disease in a Korean population. Neurosci Lett. 2012;514:12–15. doi: 10.1016/j.neulet.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 51.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satake W, Nakabayashi Y, Mizuta I, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 54.Pankratz N, Wilk JB, Latourelle JC, et al. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogaeva E, Hardy J. Gaucher and Parkinson diseases: unexpectedly related. Neurology. 2008;70:2272–2273. doi: 10.1212/01.wnl.0000314657.92762.0f. [DOI] [PubMed] [Google Scholar]
- 56.Do CB, Tung JY, Dorfman E, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nalls MA, Plagnol V, Hernandez DG, et al. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Cheng R, Verbitsky M, et al. Genome-wide association study identifies candidate genes for Parkinson’s disease in an Ashkenazi Jewish population. BMC Med Genet. 2011;12:104. doi: 10.1186/1471-2350-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alcalay RN, Mejia-Santana H, Tang MX, et al. Self-report of cognitive impairment and mini-mental state examination performance in PRKN, LRRK2, and GBA carriers with early onset Parkinson’s disease. J Clin Exp Neuropsychol. 2010;32:775–779. doi: 10.1080/13803390903521018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenbloom B, Balwani M, Bronstein JM, et al. The incidence of Parkinsonism in patients with type 1 Gaucher disease: data from the ICGG Gaucher Registry. Blood Cells Mol Dis. 2011;46:95–102. doi: 10.1016/j.bcmd.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bultron G, Kacena K, Pearson D, et al. The risk of Parkinson’s disease in type 1 Gaucher disease. J Inherit Metab Dis. 2010;33:167–173. doi: 10.1007/s10545-010-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goker-Alpan O, Lopez G, Vithayathil J, Davis J, Hallett M, Sidransky E. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol. 2008;65:1353–1357. doi: 10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kraoua I, Sedel F, Caillaud C, et al. A French experience of type 3 Gaucher disease: phenotypic diversity and neurological outcome of 10 patients. Brain Dev. 2011;33:131–139. doi: 10.1016/j.braindev.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Alonso-Canovas A, Katschnig P, Tucci A, et al. Atypical parkinsonism with apraxia and supranuclear gaze abnormalities in type 1 Gaucher disease. Expanding the spectrum: case report and literature review. Mov Disord. 2010;25:1506–1509. doi: 10.1002/mds.23109. [DOI] [PubMed] [Google Scholar]
- 65.Machaczka M, Paucar Arce M, Rucinska M, et al. A twelve-year follow-up study on a case of early-onset parkinsonism preceding clinical manifestation of Gaucher disease. JIMD Reports. 2012;3:53–57. doi: 10.1007/8904_2011_48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McNeill A, Duran R, Proukakis C, et al. Hyposmia and cognitive impairment in Gaucher disease patients and carriers. Mov Disord. 2012;27:526–532. doi: 10.1002/mds.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seto-Salvia N, Pagonabarraga J, Houlden H, et al. Glucocerebrosidase mutations confer a greater risk of dementia during Parkinson’s disease course. Mov Disord. 2012;27:393–399. doi: 10.1002/mds.24045. [DOI] [PubMed] [Google Scholar]
- 68.Gan-Or Z, Giladi N, Orr-Urtreger A. Diff erential phenotype in Parkinson’s disease patients with severe versus mild GBA mutations. Brain. 2009;132:e125. doi: 10.1093/brain/awp161. [DOI] [PubMed] [Google Scholar]
- 69.Brockmann K, Srulijes K, Hauser AK, et al. GBA-associated PD presents with nonmotor characteristics. Neurology. 2011;77:276–280. doi: 10.1212/WNL.0b013e318225ab77. [DOI] [PubMed] [Google Scholar]
- 70.Sunwoo MK, Kim SM, Lee S, Lee PH. Parkinsonism associated with glucocerebrosidase mutation. J Clin Neurol. 2011;7:99–101. doi: 10.3988/jcn.2011.7.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goker-Alpan O, Masdue JC, Kohn PD, et al. The neurobiology of glucocerebrosidase-associated parkinsonism: a PET study of dopamine synthesis and rCBF. Brain. 2012;135:2440–2448. doi: 10.1093/brain/aws174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmid SP, Schleicher ED, Cegan A, et al. Cerebrospinal fluid fatty acids in glucocerebrosidase-associated Parkinson’s disease. Mov Disord. 2012;27:288–292. doi: 10.1002/mds.23984. [DOI] [PubMed] [Google Scholar]
- 73.Anheim M, Elbaz A, Lesage S, et al. Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology. 2012;78:417–420. doi: 10.1212/WNL.0b013e318245f476. [DOI] [PubMed] [Google Scholar]
- 74.Wong KD, Sidransky E, Verma A, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 75.Nishioka K, Ross OA, Vilarino-Guell C, et al. Glucocerebrosidase mutations in diff use Lewy body disease. Parkinsonism Relat Disord. 2011;17:55–57. doi: 10.1016/j.parkreldis.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clark LN, Kartsaklis LA, Wolf Gilbert R, et al. Association of glucocerebrosidase mutations with dementia with Lewy bodies. Arch Neurol. 2009;66:578–583. doi: 10.1001/archneurol.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parkkinen L, Neumann J, O’Sullivan SS, et al. Glucocerebrosidase mutations do not cause increased Lewy body pathology in Parkinson’s disease. Mol Genet Metab. 2011;103:410–412. doi: 10.1016/j.ymgme.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 78.Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 2010;120:641–649. doi: 10.1007/s00401-010-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 80.Wenning GK, Jellinger KA. The role of alpha-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathol. 2005;109:129–140. doi: 10.1007/s00401-004-0935-y. [DOI] [PubMed] [Google Scholar]
- 81.Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122:187–204. doi: 10.1007/s00401-011-0852-9. [DOI] [PubMed] [Google Scholar]
- 82.Goker-Alpan O, Giasson BI, Eblan MJ, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- 83.Farrer MJ, Williams LN, Algom AA, et al. Glucosidase-beta variations and Lewy body disorders. Parkinsonism Relat Disord. 2009;15:414–416. doi: 10.1016/j.parkreldis.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nalls MA, Lopez G, Sidransky E. 16th International Congress of Parkinson Disease and Movement Disorders. Ireland: Dublin; Jun, 2012. The International Working Group on GBA mutations in DLB. A multicenter collaborative study demonstrates the frequency of GBA mutations in dementia with Lewy bodies exceeds that in Parkinson disease; pp. 17–21. Abstract LBA23. [Google Scholar]
- 85.Segarane B, Li A, Paudel R, et al. Glucocerebrosidase mutations in 108 neuropathologically confirmed cases of multiple system atrophy. Neurology. 2009;72:1185–1186. doi: 10.1212/01.wnl.0000345356.40399.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jamrozik Z, Lugowska A, Slawek J, Kwiecinski H. Glucocerebrosidase mutations p.L444P and p.N370S are not associated with multisystem atrophy, progressive supranuclear palsy and corticobasal degeneration in Polish patients. J Neurol. 2010;257:459–460. doi: 10.1007/s00415-009-5363-4. [DOI] [PubMed] [Google Scholar]
- 87.Hardy J, Lewis P, Revesz T, Lees A, Paisan-Ruiz C. The genetics of Parkinson’s syndromes: a critical review. Curr Opin Genet Dev. 2009;19:254–265. doi: 10.1016/j.gde.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 88.Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson’s disease: potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275:5767–5773. doi: 10.1111/j.1742-4658.2008.06709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martinez Z, Zhu M, Han SB, Fink AL. GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry. 2007;46:1868–1877. doi: 10.1021/bi061749a. [DOI] [PubMed] [Google Scholar]
- 90.Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cullen V, Sardi SP, Ng J, et al. Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter alpha-synuclein processing. Ann Neurol. 2011;69:940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- 92.Sardi SP, Clarke J, Kinnecom C, et al. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci USA. 2011;108:12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manning-Bog AB, Schule B, Langston JW. Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology. 2009;30:1127–1132. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 94.Xu YH, Jia L, Quinn B, et al. Global gene expression profile progression in Gaucher disease mouse models. BMC Genomics. 2011;12:20. doi: 10.1186/1471-2164-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldin E. Gaucher disease and parkinsonism, a molecular link theory. Mol Genet Metab. 2010;101:307–310. doi: 10.1016/j.ymgme.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yap TL, Gruschus JM, Velayati A, et al. Alpha-synuclein interacts with glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. J Biol Chem. 2011;286:28080–28088. doi: 10.1074/jbc.M111.237859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi JH, Stubblefield B, Cookson MR, et al. Aggregation of alpha-synuclein in brain samples from subjects with glucocerebrosidase mutations. Mol Genet Metab. 2011;104:185–188. doi: 10.1016/j.ymgme.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mazzulli JR, Xu YH, Sun Y, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Westbroek W, Gustafson AM, Sidransky E. Exploring the link between glucocerebrosidase mutations and parkinsonism. Trends Mol Med. 2011;17:485–493. doi: 10.1016/j.molmed.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 101.Sun Y, Grabowski GA. Impaired autophagosomes and lysosomes in neuronopathic Gaucher disease. Autophagy. 2010;6:648–649. doi: 10.4161/auto.6.5.12047. [DOI] [PubMed] [Google Scholar]
- 102.Ron I, Rapaport D, Horowitz M. Interaction between parkin and mutant glucocerebrosidase variants: a possible link between Parkinson disease and Gaucher disease. Hum Mol Genet. 2010;19:3771–3781. doi: 10.1093/hmg/ddq292. [DOI] [PubMed] [Google Scholar]
- 103.Kinghorn KJ. Pathological looping in the synucleinopathies: investigating the link between Parkinson’s disease and Gaucher disease. Dis Model Mech. 2011;4:713–715. doi: 10.1242/dmm.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xuan Q, Xu SL, Lu DH, et al. Increase expression of alpha-synuclein in aged human brain associated with neuromelanin accumulation. J Neural Transm. 2011;118:1575–1583. doi: 10.1007/s00702-011-0636-3. [DOI] [PubMed] [Google Scholar]
- 105.Alladi PA, Mahadevan A, Vijayalakshmi K, Muthane U, Shankar SK, Raju TR. Ageing enhances alpha-synuclein, ubiquitin and endoplasmic reticular stress protein expression in the nigral neurons of Asian Indians. Neurochem Int. 2010;57:530–539. doi: 10.1016/j.neuint.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 106.Zhang L, Sheng R, Qin Z. The lysosome and neurodegenerative diseases. Acta Biochim Sin (Shanghai) 2009;41:437–445. doi: 10.1093/abbs/gmp031. [DOI] [PubMed] [Google Scholar]