Abstract
Aminoglycoside antibiotics are highly effective agents against gram-negative bacterial infections, but they cause adverse effects on hearing and balance dysfunction as a result of toxicity to hair cells of the cochlea and vestibular organs. While ototoxicity has been comprehensively studied, the contributions of the immune system, which controls the host response to infection, have not been studied in antibiotic ototoxicity. Recently, it has been shown that an inflammatory response is induced by hair cell injury. In this study, we found that lipopolysaccharide (LPS), an important component of bacterial endotoxin, when given in combination with kanamycin and furosemide, augmented the inflammatory response to hair cell injury and exacerbated hearing loss and hair cell injury. LPS injected into the peritoneum of experimental mice induced a brisk cochlear inflammatory response with recruitment of mononuclear phagocytes into the spiral ligament, even in the absence of ototoxic agents. While LPS alone did not affect hearing, animals that received LPS prior to ototoxic agents had worse hearing loss compared to those that did not receive LPS pretreatment. The poorer hearing outcome in LPS-treated mice did not correlate to changes in endocochlear potential. However, LPS-treated mice demonstrated an increased number of CCR2+ inflammatory monocytes in the inner ear when compared with mice treated with ototoxic agents alone. We conclude that LPS and its associated inflammatory response are harmful to the inner ear when coupled with ototoxic medications and that the immune system may contribute to the final hearing outcome in subjects treated with ototoxic agents.
Keywords: LPS, monocyte, macrophage, cochlea, inflammation, ototoxicity
INTRODUCTION
Cochlear hair cells are sensory cells of the inner ear that are known to be susceptible to specific insults including noise exposure and ototoxic medications. Hair cells are not replaced after damage, and the loss of cochlear hair cells results in permanent loss of hearing. Exacerbating factors that contribute to the likelihood of ototoxic injury after use of aminoglycoside antibiotics have been described. Certain medications when given together with aminoglycosides demonstrate synergy and potentiate the ototoxic effects of either drug given alone. Loop diuretics when given in combination with aminoglycoside antibiotics induce an increased likelihood of hearing loss, and the synergistic effects of loop diuretics and aminoglycosides have been well established (Hirose and Sato 2011; Oesterle and Campbell 2009; Oesterle et al. 2008; Rybak 1993; Komune and Snow 1982). In experiments using mice, the use of aminoglycoside antibiotics in combination with loop diuretics has become popular because mice are highly resistant to aminoglycoside ototoxicity (Wu et al. 2001; Oesterle et al. 2008; Taylor et al. 2008). However, interactions between ototoxic antibiotics and infectious agents have not been studied. Because aminoglycoside antibiotics are used specifically to treat infections, the effect of these infections in ototoxicity is important to investigate.
While infections can be modeled by injection of live bacteria into animals, the mortality associated with true bacterial sepsis is high. Experimental models using isolated components of bacterial cell wall, such as lipopolysaccharide, permit a higher rate of survival and initiate many of the events associated with systemic infection. Lipopolysaccharide (LPS) comprises an important component of the cell wall in gram-negative bacteria and is a large molecule consisting of lipids, proteins, and oligosaccharides. LPS plays a critical role in signaling the presence of an infection to the immune system. Activating immunity generally results in control of infection, but sometimes, bystander injury resulting from immune function can cause injury to host tissues. With high levels of LPS that mimic sepsis, deleterious effects have been noted in the central nervous system (Nishioku et al. 2009; Lustig et al. 1992; Shi et al. 2010). However, in studies that use LPS at a lower dose (1 mg/kg/day or less for 2–4 days), beneficial effects have been observed in studies of brain injury (Nadeau and Rivest 2002; Rosenzweig et al. 2004; Simard and Rivest 2007; Kang and Rivest 2007; Marsh et al. 2009). LPS at low doses before ligation of the middle cerebral artery, an experimental model for stroke, has been shown to be protective. In the majority of studies exploring the effect of LPS priming, low-level inflammation resulted in protection from subsequent injuries. We used low-dose-LPS pretreatment (0.5 mg/kg × 2 days) to stimulate the immune system prior to exposing mice to ototoxic medication. In this study, we found that preconditioning with low-dose LPS before ototoxicity caused worsening and acceleration of hearing loss, not protection.
We also studied the effects of systemic LPS pretreatment on the specific mononuclear phagocytes attracted into the inner ear during ototoxic injury. Previously, we have shown that large numbers of cochlear monocytes and macrophages enter the inner ear after ototoxic injury (Hirose et al. 2005; Sato et al. 2010). While in the past, leukocytes were thought to be excluded from the membranous labyrinth and inner ear fluids, it is clear now that macrophages and monocytes are abundantly recruited into the inner ear after noise and other injuries (Fredelius and Rask-Andersen 1990; Warchol 1997; Hirose et al. 2005; Tornabene et al. 2006; Sato et al. 2010; Tan et al. 2008; Sato et al. 2008). Monocytes and macrophages congregate in the spiral ligament, particularly in the region of the type IV fibrocytes, in the spiral ganglion, and the scala tympani. Monocytes and macrophages represent the majority of leukocytes that migrate to the inner ear after noise and after ototoxicity. When LPS is used as a pretreatment, CC chemokine receptor 2 (CCR2)-expressing inflammatory monocytes enter the inner ear. These inflammatory monocytes are a different breed of monocyte when compared with the previously observed CX3CR1-expressing monocyte in inner ear injury (Geissmann et al. 2003; Saha and Geissmann 2011; Auffray et al. 2007). CCR2+ monocytes spend less time in peripheral tissues, are more rapidly exchanging from the blood into the periphery, and are quickly replaced by hematopoietic precursors from the bone marrow. This lesion with LPS pretreatment is the first example where CCR2-expressing cells have been shown definitively to enter the inner ear with the potential to play an important role for inflammatory cells in inner ear injury.
MATERIALS AND METHODS
Experimental animals
CX3CR1+/GFP mice on a C57Bl6 (B6) background were bred in our animal facility from founders acquired from Dan Littman, NYU. CX3CR1 (also known as fractalkine receptor) is a G protein-coupled receptor that binds exclusively to fractalkine (CX3CL1). In this mouse, the coding sequence for CX3CR1 was replaced by green fluorescent protein (GFP), and thus GFP is expressed in all cells that normally express CX3CR1. CX3CR1 is expressed in macrophages, monocytes, microglia, activated T cells, and NK cells and is endogenously green fluorescent in these mice (Jung et al. 2000).
In prior experiments, we have demonstrated that CX3CR1+/GFP mice (CX3CR1 heterozygotes) have similar responses to ototoxicity as wild-type C57Bl6 mice with respect to threshold shift and hair cell loss and these mice provide a convenient method to track monocytes and macrophages in the inner ear (Sato et al. 2008). CCR2+/RFP CX3CR1+/GFP mice (double heterozygous, B6 background) were raised from CCR2+/RFP founders obtained from Israel Charo, UCSF and crossed to mice possessing the CX3CR1GFP allele to provide reporters of both CCR2 (red) and CX3CR1 (green). The CCR2+/RFP mice express endogenous red fluorescent signal in all cells expressing CCR2 by a similar knock-in construct where one copy of CCR2 is replaced by the coding sequence for monomeric red fluorescent protein (Saederup et al. 2010). Inflammatory monocytes and dendritic cells express CCR2 (CCR2+); thus, this double het mouse can be used to distinguish inflammatory monocytes (red CCR2+) from patrolling monocytes (green CX3CR1+) (Geissmann et al. 2003). All mice were evaluated at 14 weeks of age before the onset of high-frequency age-related hearing loss observed in B6 mice (Hequembourg and Liberman 2001). Initial treatment with ototoxic agents was performed at 7–8 weeks of life. The Institute for Animal Care and Use Committee at Washington University School of Medicine has approved all the experiments described here.
Drug administration
Mice were genotyped shortly after birth and uniquely identified. ABR testing was completed before initiating drug treatment to ensure normal hearing thresholds at baseline. Four different treatments were used: One group of CX3CR1+/GFP mice received saline each day for 3 days, a second group received LPS for 2 days, a third group received saline pretreatment for 2 days and kanamycin and furosemide on the third day, and a fourth group received LPS pretreatment for 2 days followed by kanamycin and furosemide on the third day. A combination of kanamycin and furosemide was used in order to produce a reliable threshold shift in mice with a single-day dosing regimen to facilitate a dynamic study of structural and functional changes in the inner ear in the first few days after injury. All groups were tested for hearing thresholds and cochlear damage 5 days after kanamycin-furosemide treatment (see Fig. 1). A separate group of experiments was performed using CCR2+/RFPCX3CR1+/GFP mice, which were generated from CCR2RFP/RFP and CX3CR1GFP/GFP breeders. These mice were also divided into four groups and given either saline, LPS only, kanamycin and furosemide, or LPS followed by kanamycin-furosemide. These mice were sacrificed at day 5 after kanamycin-furosemide and used for assessment of monocyte migration and expressions of CCR2 and CX3CR1. For groups 2 and 4, LPS was given by intraperitoneal (IP) injection at a dose of 0.5 mg/kg for 2 consecutive days (see injection protocol, Fig. 1). This dose of LPS is 10–20-fold lower than a typical dose of LPS to mimic sepsis (5–10 mg/kg). Mice in groups 1 and 3 were given comparable volumes of saline IP. For groups 3 and 4, kanamycin (1,000 mg/kg) was given once by IP injection. Furosemide (180 mg/kg IP) was then given 40 min after the injection of kanamycin (titrated to produce moderate threshold shift within 2 weeks of administration). Daily weights were obtained for all mice. Two days of LPS at this dose induced a weight loss of approximately 10 % over the period of 3 days. Weights returned to baseline within 1 to 2 days after LPS treatment. Kanamycin-furosemide combination therapy did not appear to affect the total body weight significantly. All mice were 7–8 weeks of age at the initiation of treatment. Kanamycin sulfate was purchased as a powder from USB Corporation (Cleveland, OH, USA) and prepared at 45 mg/ml in sterile saline. Furosemide (10 mg/ml, Hospira Inc., Lake Forest, IL, USA) was diluted to 5 mg/ml in sterile saline. CX3CR1+/GFP mice were used in experiments for auditory brainstem response (ABR), endocochlear potential (EP), and cytocochleograms, and CCR2+/RFPCX3CR1+/GFP mice were used for CCR2/CX3CR1 cell counts and confocal imaging.
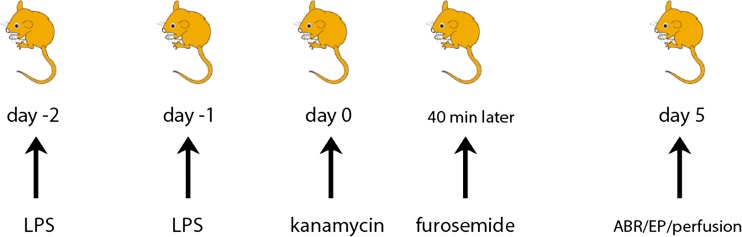
FIG. 1.
Treatment protocol. Mice were treated according to the following protocol: On 2 consecutive days, 0.5 mg/kg LPS was injected IP (saline for control mice), and 24 h after the second LPS injection, mice were injected with kanamycin (1,000 mg/kg) and 40 min later, with furosemide (180 mg/kg). Auditory brainstem responses (ABRs) and endocochlear potentials (EPs) were tested on day 5 after kanamycin-furosemide administration. After physiologic testing, the mice were perfused through the heart with fixative, and cochleas were harvested for histologic analysis.
Auditory brainstem response
At 5 days after treatment with ototoxic agents and/or LPS, ABRs were performed on all of the mice. Mice were anesthetized with intraperitoneal xylazine (20 mg/kg) and ketamine (100 mg/kg). ABRs were evoked through tone pips and recorded via subcutaneous electrodes placed behind the pinna, the vertex of the head, and the ground electrode placed on the back. Stimuli were 5-msec tone pips (0.5-msec rise-fall with a cos2 envelope, delivered at 40 s). The response was amplified (×10,000), filtered (100 Hz to 3 kHz), and averaged using custom computer software (System 3; Tucker-Davis Technologies). The sound level was raised in 5-dB steps from 15- to 95-dB SPL. At each level, 1,024 responses were averaged, with stimulus polarity alternated. Response waveforms were rejected if the peak-to-peak voltage exceeded 15 μV. We tested responses at 5.6, 8, 11, 16, 22.63, 32, 45.2, and 64 kHz to obtain thresholds. Threshold was determined by a single observer, who noted the lowest sound level at which a recognizable waveform was present in a series of tracings stacked from lowest to highest sound pressure level. Waveforms were confirmed as auditory-evoked responses by their increasing amplitude and decreasing latency with increasing intensity of the stimulus. If hearing threshold was not detected at 95 dB, a threshold value of 100 dB was assigned. After analysis of hearing threshold for 5 days posttreatment was completed, additional animals were prepared and tested for evaluation 14 and 28 days after treatment. Differences in hearing thresholds between mouse groups were compared using nonparametric tests. The Kruskal-Wallis test was used to evaluate differences between treatment groups. Post hoc analysis was performed with the Mann-Whitney U test to explore pairwise differences in groups where the Kruskal-Wallis test demonstrated significance. To adjust for multiple comparisons, the Mann-Whitney U test was evaluated at an alpha level of 0.05/3 = 0.017 (using Bonferroni’s correction). Statistical analysis was performed using IBM SPSS statistics for Windows, version 19.0, IBM Corp., Armonk, NY.
Histologic preparation
Immediately after ABR, all animals were injected with a lethal dose of anesthetic agent, and a thoracotomy was performed. Mice were perfused with 4.0 % paraformaldehyde in 10-mM phosphate buffer through the left ventricle. Both petrous temporal bones were extracted, and the round and oval windows opened to allow intralabyrinthine perfusion of fixative. After overnight fixation at 4 °C, cochleas were decalcified in a saturated solution of EDTA in phosphate buffer for 5 days at 4 °C.
Fluorescent cell counting
Decalcified cochleas from the left temporal bones were immersed in cryoprotection solution (20 % glycerol in phosphate buffer), frozen in 30 % sucrose on dry ice, and cut into 30-μm serial sections from round window to oval window on a horizontal sliding microtome. Sections were washed in phosphate-buffered saline (PBS; pH 7.4), dried onto slides, and coverslipped.
For counting CCR2+ and CX3CR1+ cells, five to six sections from each cochlea were selected from serial sections of the bony labyrinth on the basis of their proximity to the modiolus and the histologic quality of the section. These cochlear sections were viewed on an epifluorescence microscope with a 40× objective. Approximately 20 % of the total organ was sampled in these counts. Only leukocytes present in the membranous labyrinth were counted, and cells within the bone marrow, the otic capsule, and within blood vessels were excluded. The vestibule and semicircular canals were not included in these counts. The observer examined the entire thickness of the specimen by focusing through the depth of the section and counted the number of CCR2+ (red-labeled) or CX3CR1+ (green-labeled) cells.
Morphometric analysis
Decalcified cochleas from the right temporal bones were postfixed in osmium (1 % OsO4 in dH2O) for 60 min. Cochleas were dehydrated through a series of graded ethanols ending in 100 % propylene oxide and subsequently embedded in Araldite resin (see protocol in Wang et al. 2002). The plastic-embedded cochleas were serially sectioned at 40 μm in a plane parallel to the axis of the modiolus. Each section was numbered, oriented, mounted, and coverslipped. For each subject, the cochlear spiral was reconstructed using Neurolucida software (MicroBrightField, Colchester, VT). Using the tunnel of Corti as the reference point, we mapped each profile of the cochlear duct that contained sensory cells in three-dimensional space. From these three-dimensional reconstructed data, the distance of each cochlear profile from the basal tip was computed using custom software. The cochlear location in space was then converted into frequency according to frequency map data described by Ehret (1983) which was fit to a mathematical equation f (kHz) = 3.109 × (10(100 − d) × 0.0142 − 0.7719), where d is the percent distance from the cochlear base.
Hair cells
A standard cytocochleogram was prepared for each ear using high-power oil immersion objectives and Nomarski optics. In every section through the cochlear duct, the number of present and absent hair cells was assessed throughout the entire section thickness. Evaluation of both the nuclear and cuticular regions was used to make these assessments. Outer hair cell stereocilia could not reliably be resolved at the light microscopic level, but inner hair cell stereocilia could clearly be seen. The outer hair cell was deemed present by an intact nucleus, cuticular plate, and the integrity of the reticular lamina across the apical surface of the organ of Corti. Cells in which the nucleus and the cuticular plate were present were counted as present. Cells that lacked a nucleus were considered to be missing. The presence of Deiter cells and pillar cells facilitated the assessment of how many cells should have been present in any given section. Statistical analysis was performed on the effect of the different drug regimens using one-way ANOVA. P values of <0.05 were considered statistically significant.
Endocochlear potentials
The EP was measured in mice 5 days after they received ototoxic drugs. The mice were anesthetized, and a tracheostomy was performed. The airway was secured, and the head was stabilized. An incision was made in the postauricular soft tissue, the bulla was opened, and the lateral surface of the cochlea was exposed. A hole was created with a fine drill in the left otic capsule directly lateral to the stria vascularis of the basal turn. Glass capillary pipettes (40–80 MΩ) filled with 0.15 M KCl were mounted on a hydraulic microdrive (Frederick Haer) and advanced until a stable positive potential was obtained that did not change with increased electrode depth. The signal from the recording electrode was recorded on an AM Systems Model 1600 intracellular amplifier. EP changes were compared across experimental groups using Kruskal-Wallis testing (nonparametric equivalent of the one-way ANOVA), which showed a difference between groups (P = 0.006), and post hoc analysis was performed using the Mann-Whitney U test with Bonferroni correction.
RESULTS
LPS pretreatment exacerbates hearing loss caused by combined ototoxic agents: kanamycin-furosemide
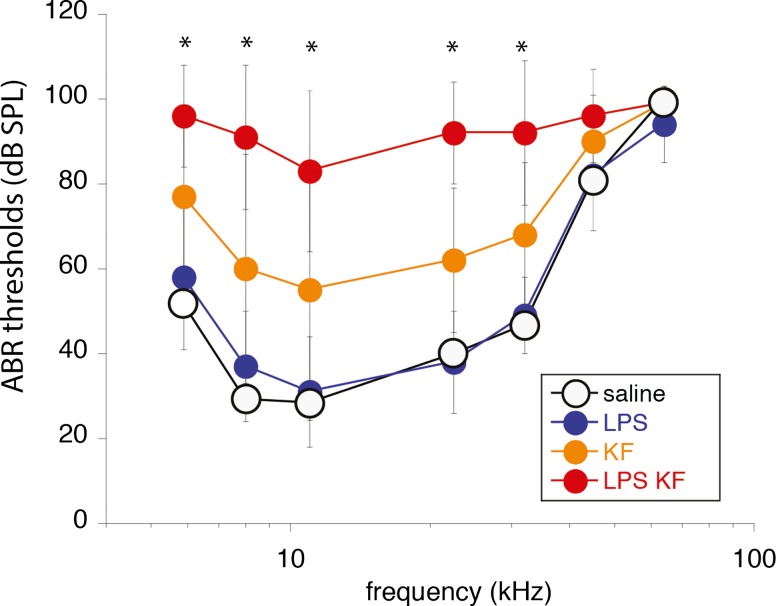
Exposure to kanamycin-furosemide caused a moderate threshold shift across all frequencies. Threshold shift was noted at day 5 and persisted through 4-week postinjection. Kanamycin-furosemide-treated animals had a flat threshold shift of 30–40 dB (Fig. 2). LPS given alone did not cause changes in hearing thresholds. LPS -kanamycin-furosemide treated mice demonstrated threshold shifts of approximately 40–60 dB. ABR threshold testing at days 14 and 28 showed that hearing thresholds remained higher in the LPS-pretreated group when compared with kanamycin-furosemide-treated animals without LPS. Statistical analysis was performed at day 5 on three groups: LPS controls, kanamycin-furosemide, LPS-kanamycin-furosemide. Nonparametric testing (Kruskal-Wallis) was used to assess differences among treatment groups at each frequency. Post hoc analysis (Mann-Whitney U test) was applied to pairwise differences among the three groups where Kruskal-Wallis testing demonstrated significance. To adjust for multiple comparisons, the Mann-Whitney U test was evaluated at alpha level of 0.05/3 = 0.017 using Bonferroni’s correction. Each group was shown to be statistically significantly different from the other two at five of seven frequencies (asterisks, Fig. 2). Differences in hearing threshold persisted at days 14 and 28 after treatment with LPS-kanamycin-furosemide causing the highest threshold shift.
FIG. 2.
ABR thresholds after LPS pretreatment and ototoxic medications. Hearing thresholds are shown at day 5 after ototoxic treatment in four experimental groups, saline controls (saline), LPS alone (no ototoxic agents), kanamycin-furosemide (no LPS pretreatment), and LPS pretreatment with kanamycin-furosemide. Mice that received LPS alone (LPS) had no significant change in hearing thresholds compared with saline controls. Those treated with kanamycin-furosemide (KF) showed a moderate hearing loss (30–40-dB threshold shift). LPS-kanamycin-furosemide mice (LPS-KF) had the highest hearing thresholds (40–60-dB threshold shift). Statistical analysis: asterisks demonstrate frequencies at which significant differences were present between the three groups (LPS vs KF, LPS vs LPS-KF, KF vs LPS-KF). Mice tested at days 14 and 28 showed similar threshold shifts as shown at day 5.
Endocochlear potential is decreased in both mouse groups treated with kanamycin-furosemide
Furosemide has been shown to affect hearing thresholds by inhibiting the Na+K+/2Cl− cotransporter in marginal cells of the stria vascularis causing temporary decrease in EP (Sewell 1984; Rybak et al. 1991; Lang et al. 2003). The effect of furosemide on the EP when given together with aminoglycoside antibiotics has also been the subject of careful study (Komune and Snow 1982; Asakuma and Snow 1980). However, the role of LPS and cochlear inflammation in maintenance or recovery of the EP with combined kanamycin-furosemide has not been evaluated. Because the lateral wall is located where most macrophages collect within the inner ear, we considered the possibility that the EP could be affected by the presence of cochlear inflammatory cells or by perturbations of the blood labyrinth barrier associated with LPS-induced inflammation and macrophage activation. To test this hypothesis, we measured the EP in the basal turn of the cochlea of LPS-pretreated and saline-pretreated mice 5 days after kanamycin-furosemide. We observed a decrement of the EP in the mice treated with kanamycin-furosemide, as expected, but the variance was high. LPS alone appeared to have no effect on the EP, as hearing thresholds by ABR testing were normal. LPS given before kanamycin-furosemide did not have a consistent or substantial effect on EP when we compared these animals to those that received kanamycin-furosemide alone.
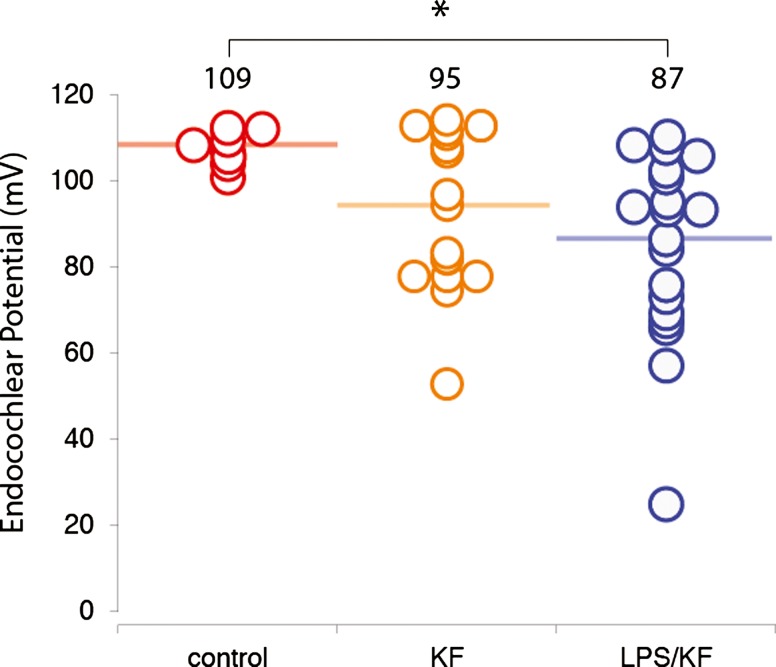
Control mice had an average EP of 109 mV (range 95–110 mV, Fig. 3). Prior studies have shown an EP of 95–115 mV in normal C57Bl6 mice (Ohlemiller 2009). In animals that had received kanamycin-furosemide, the average EP was about 15 mV lower than controls (95 mV), and the variance was considerably greater (range 53–114 mV). The group that received both LPS and kanamycin-furosemide had a decreased EP also (average 87 mV, range 25–110 mV), and while this difference was significant when compared with controls, it was not statistically significantly different when compared to saline-pretreated kanamycin-furosemide-exposed mice.
FIG. 3.
Endocochlear potential 5 days after treatment. Endocochlear potential (EP) was measured in the basal turn of the cochlea in control, kanamycin-furosemide, and LPS-kanamycin-furosemide-treated mice 5 days after exposure to ototoxic agents. The control mice showed an average endocochlear potential of 109 mV (bar) with a range of 101–112 mV (individual data plotted in open circles). The kanamycin-furosemide-treated mice demonstrated a reduction in the average EP (average 94 mV). Mice treated with LPS-kanamycin-furosemide also showed decline in EP (average 86 mV). The difference between KF and LPS-KF was not statistically significant (Mann-Whitney U test). Controls demonstrated a statistically significant difference when compared with LPS-KF (asterisk).
Nonparametric testing (Kruskal-Wallis) was used to assess differences among the three groups, which showed that there was a significant difference among the three groups at day 5 (P value = 0.006). Post hoc analysis (Mann-Whitney U test) was applied to pairwise differences among the three groups where Kruskal-Wallis testing demonstrated significance. To adjust for multiple comparisons, the Mann-Whitney U test was evaluated at alpha = 0.017 (Bonferroni’s correction 0.05/3 = 0.017). This showed that there was a significant difference between controls and LPS-kanamycin-furosemide (KF) group (P < 0.001) but not between controls and KF group (P = 0.215) or KF and LPS-KF groups (P = 0.155). Compared to control mice, both groups exposed to ototoxins demonstrated a large range in EP. Thus, if LPS-induced inflammation contributes to hearing loss after ototoxicity by exacerbating changes in EP, those effects are too small to detect in this experiment.
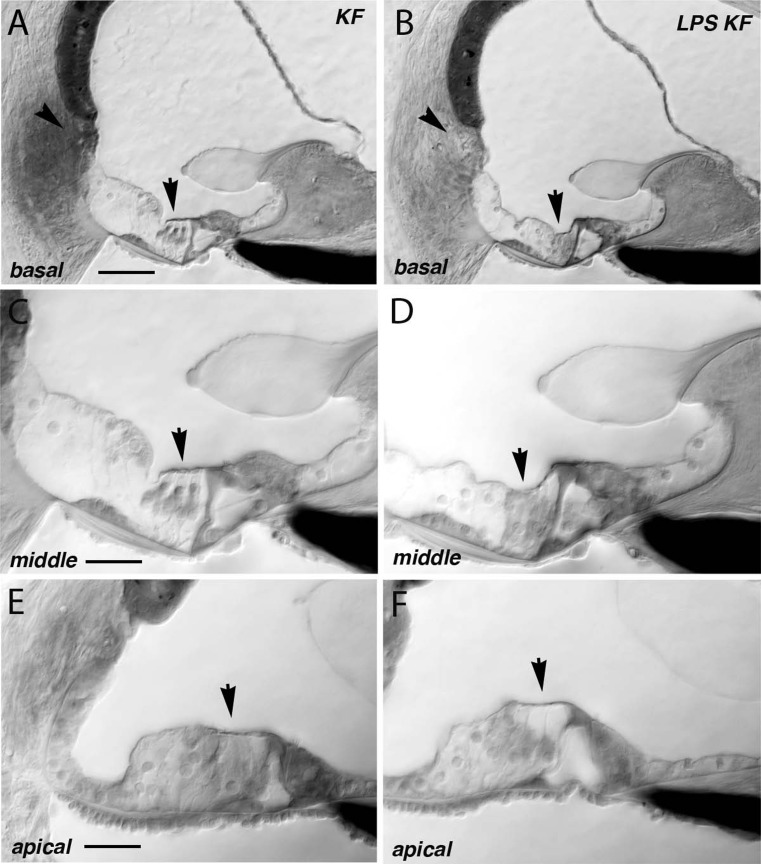
The stria vascularis swells after treatment with kanamycin-furosemide, irrespective of LPS pretreatment
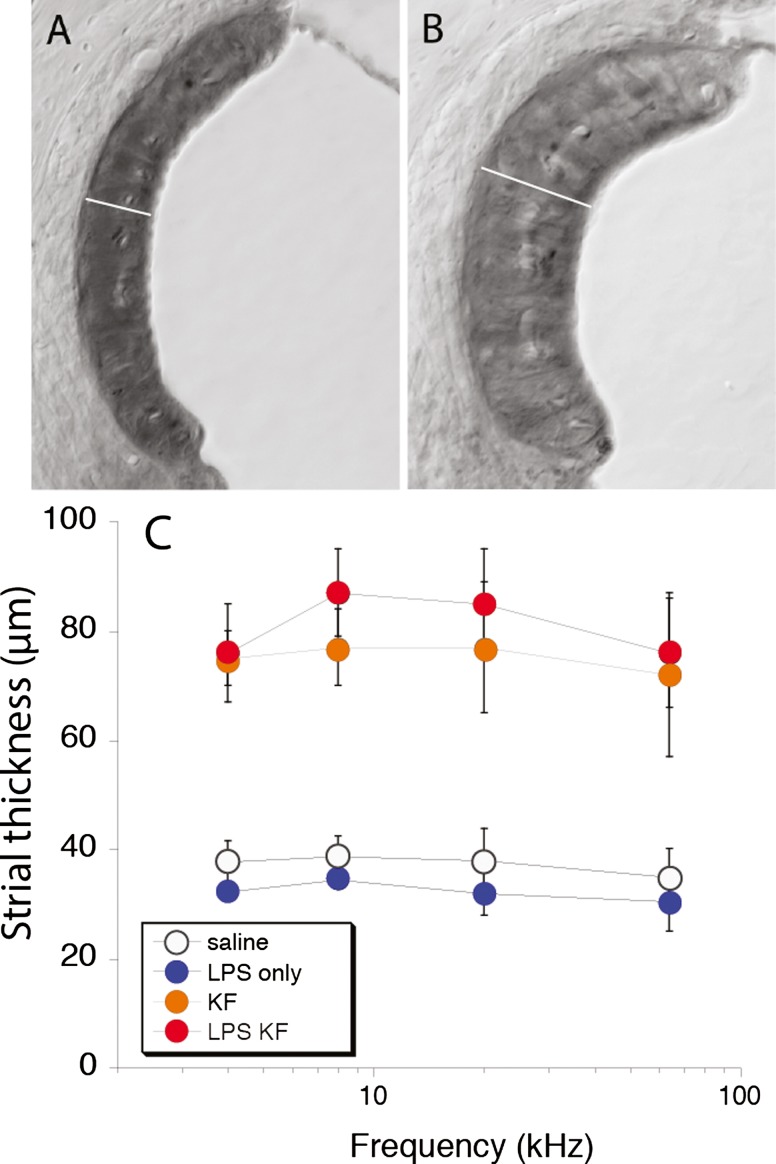
Histologic analysis of the stria vascularis in plastic-embedded sections demonstrated abnormalities in both groups treated with ototoxic agents, and LPS did not appear to influence strial histopathology. The thickness of the stria vascularis in the midmodiolar plane observed by light microscopy shows that edema of the intrastrial space is present 5 days after aminoglycoside-diuretic treatment (see Fig. 4). This finding is consistent with what has been reported in the literature after furosemide administration. The mice treated with kanamycin-furosemide systematically demonstrated larger strial volumes when compared to control mice that received no ototoxic medications. The stria vascularis was enlarged in both groups receiving ototoxic medications irrespective of LPS pretreatment. Clearly, the effect of these combined ototoxic agents includes changes in the EP and in strial volume, but the mechanism that accounts for worsening of hearing threshold after LPS exposure could not be identified by evaluating strial volume by histology or the measuring of the EP. Statistical analysis of differences in strial thickness among the four groups was performed with the Kruskal-Wallis test, which showed a significant difference between at least two of the four groups. Post hoc analysis with the Mann-Whitney U test (alpha level of 0.05/6 = 0.0083 using Bonferroni’s correction) showed a statistically significant difference between control and KF and a difference between control and LPS-KF and no difference between KF and LPS-KF.
FIG. 4.
Morphometry of the stria vascularis. Strial thickness was markedly increased 5 days after antibiotic-diuretic treatment (B) when compared to controls (A). The strial thickness was measured in the upper basal turn in the midmodiolar plane in all cochlear sections, and the three experimental groups were compared with saline controls (C). Strial thickness was significantly different in control mice when compared to KF or LPS-KF mice. There was no significant difference between KF and LPS-KF mice. The width of the SV marked by white line in A is 30 μm, in B is 80 μm. Scale bar 30 μm.
Systemic LPS results in enhanced recruitment of monocytes and macrophages into the inner ear
Inflammatory cells, including monocytes and macrophages, enter the cochlea after ototoxic injury. When animal subjects are exposed to LPS prior to injury, a greater diversity and a larger number of inflammatory cells arrive into the cochlea. Monocytes and macrophages, sorted by chemokine receptors, CCR2 and CX3CR1, were counted in mouse cochlear sections in each of the four experimental groups: saline-treated, LPS only, kanamycin-furosemide only, and LPS-kanamycin-furosemide-treated mice. Double knock-in mice were used in these experiments (CX3CR1+/GFPCCR2+/RFP). Green fluorescent protein, driven by the promoter for CX3CR1 (fractalkine receptor), endogenously labels monocytes, macrophages, microglia, NK cells, and some activated T cells. Similarly, red fluorescent protein controlled by the CCR2 promoter identifies CCR2-expressing cells as endogenously red, depicted here in magenta, which include inflammatory monocytes, neutrophils, B cells, and activated T cells. In the inner ear, CX3CR1+ cells are patrolling monocytes and resident macrophages that reside in the cochlea and increase in number under conditions of cochlear injury. CCR2 plays a diversity of roles but is particularly well studied for its expression in a subset of monocytes, which constitute the inflammatory monocytes that are recruited from the vasculature to injured end organs. CCR2+ cells traffic rapidly into and out of organs and spend most of their time within the vascular space. CCR2+ monocytes are observed rarely in the inner ear of control subjects.
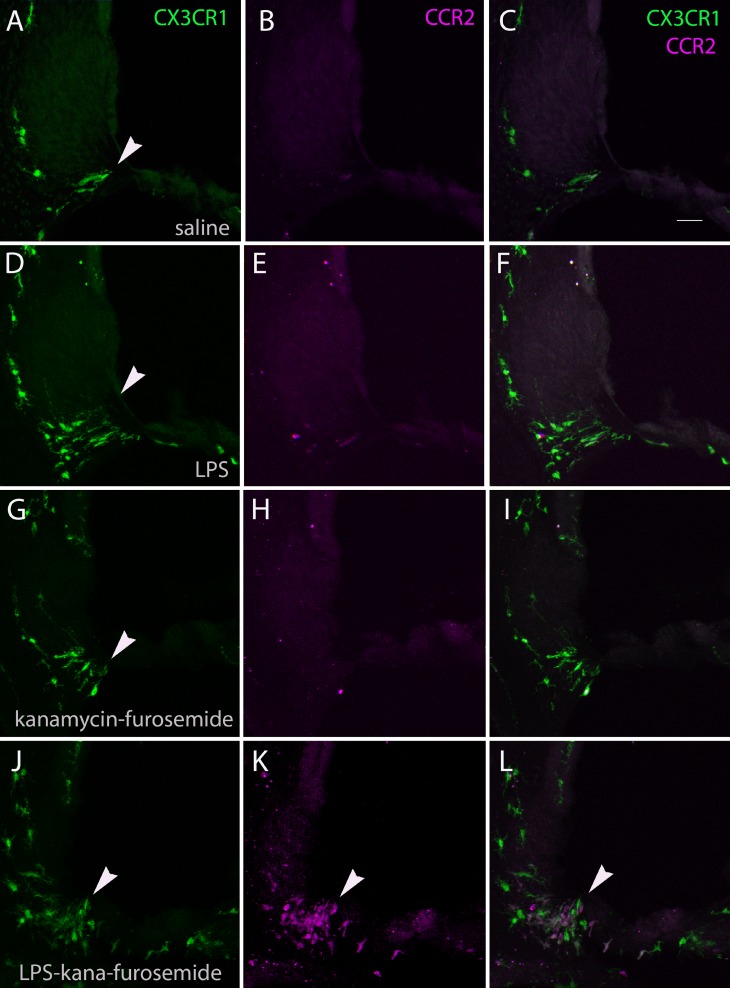
Representative cochlear sections are shown in Figure 5 demonstrating the cochlear lateral wall in control, (top row), LPS control mice (second row), kanamycin-furosemide-treated mice (third row), and LPS-kanamycin-furosemide-treated mice (bottom row). CX3CR1-expressing resident monocytes and macrophages are shown in green in the left column (CX3CR1+/GFP), CCR2+ inflammatory monocytes are observed in magenta in the middle (CCR2+/RFP), and the merged images are shown on the right. Arrowheads indicate the location where inflammatory cells were most consistently observed, in the lower spiral ligament where type IV fibrocytes are usually located. Macrophages were also observed in the osseus spiral lamina crowding into the region where the dendrites of the eighth nerve enter the habenula perforata. There were also some monocytes and macrophages observed in the scala tympani adherent to the bony walls and attached to the surface. Overall, increased numbers of CX3CR1+ cells were observed in the cochleas of drug-treated animals compared to controls. Green CX3CR1+ cells densely populated the spiral ligament and the spiral ganglion and the lining of the scala tympani in injured cochleas. In control animals, there were a few CX3CR1+ macrophages in the lower spiral ligament and in the spiral limbus, but we did not observe these cells in great numbers in the sensory epithelial layer. Cochlear macrophages are present in the stria vascularis but do not increase in number with ototoxic injury.
FIG. 5.
Fluorescent-labeled monocytes and macrophages in the cochlea. Representative sections of the cochlea in saline controls (A, B, C), LPS (D, E, F), kanamycin-furosemide (G, H, I), and LPS-kanamycin-furosemide (J, K, L)-treated ears in double heterozygous knock-in mice (CX3CR1+/GFP CCR2+/RFP). CX3CR1+ cells are shown in green (A, D, G, J), CCR2+ cells in magenta (B, E, H, K), and merged images (C, F, I, L). Arrowheads indicate the lower spiral ligament, in the location of type IV fibrocytes, where inflammatory cells typically appear. Most inflammatory cells in the cochlea after kanamycin-furosemide treatment expressed CX3CR1 and not CCR2, while LPS-pretreated mice demonstrated CCR2+ monocytes in abundance (K). Scale bar in C 50 μm, applies to all figures.
Mice exposed to endotoxin and ototoxic medications, compared to mice treated with ototoxic medications alone, showed a substantial increase in CX3CR1+ cells and CCR2+ cells. CCR2+ monocytes (magenta) were observed primarily in animals that had received LPS. In general, there were more green (CX3CR1+) than magenta (CCR2+) mononuclear phagocytes in the inner ear. CCR2+ cells were not observed in the scala tympani or in the organ of Corti and were confined to the spiral ligament. Also, LPS was permissive for entry of CCR2+ monocytes into the inner ear.
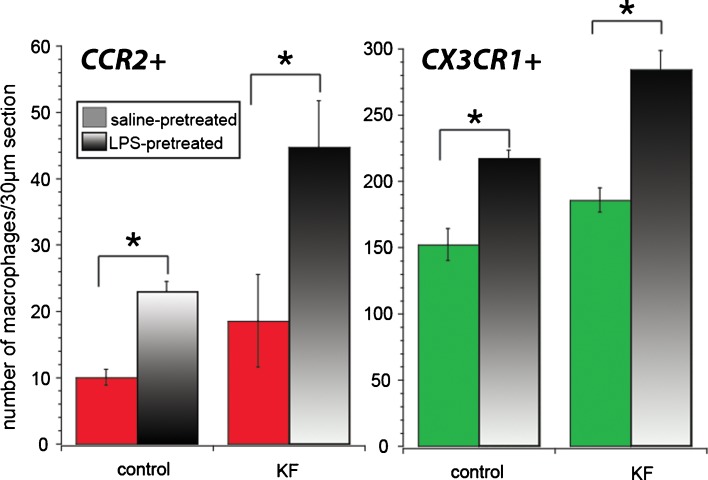
Monocytes and macrophages within the cochlea were characterized by expression of CX3CR1 (green) or CCR2 (magenta) and counted in six to seven cochlear sections from each of the following four groups: saline only, LPS only, kanamycin-furosemide, LPS-kanamycin-furosemide (see Fig. 6). These cell counts were performed on mice 5 days after exposure to ototoxic agents. Macrophages/monocytes within the membranous labyrinth were counted and averaged from six to seven sections per cochlea in 11–12 mice per experimental group. We found that with kanamycin-furosemide treatment, there was a statistically significant increase in the population of CX3CR1+ monocytes and macrophages when compared to saline controls. The number of CCR2+ cells in control animals compared with kanamycin-furosemide-treated animals was not significantly different. Mice receiving LPS alone demonstrated brisk recruitment of CCR2+ monocytes as well as a robust increase in CX3CR1+ cells compared to saline controls, without demonstrating any change in hearing thresholds. LPS-kanamycin-furosemide-treated mice harbored the largest increase of cochlear macrophages and monocytes. The number of CCR2+ monocytes was 2.5 times greater with LPS pretreatment than that with saline pretreatment in kanamycin-furosemide mice. While ototoxicity resulted in the entry of inflammatory cells into the inner ear, the effect of LPS exceeded the effect of ototoxicity in inducing monocyte and macrophage migration into the inner ear.
FIG. 6.
CCR2+ and CX3CR1+ cell counts. LPS treatment resulted in a significant increase in both CX3CR1+ and CCR2+ cells in the cochlea. In kanamycin-furosemide-treated animals, few specimens demonstrated CCR2 expression LPS was the most potent stimulus for migration of inflammatory cells into the cochlea. Statistical analysis demonstrates significantly more CCR2+ cells in LPS controls compared to saline controls and significantly more CCR2+ cells in LPS-pretreated compared to saline-pretreated mice receiving ototoxic agents. There was no significant change in CCR2 expression caused by ototoxicity alone. Both LPS and ototoxic agents independently caused increases in the CX3CR1+ monocyte/macrophage population in the inner ear.
LPS exacerbates outer hair cell loss from ototoxic injury
Figure 7 shows representative sections demonstrating histologic findings in kanamycin-furosemide exposed mice compared with LPS pretreated, kanamycin-furosemide exposed mice. In these specific examples, outer hair cell loss was noted in the apex and surprisingly, the hair cells in the base were retained. There were other cochlear specimens in which outer hair cells were absent through the entire length of the cochlear duct after kanamycin-furosemide. Other examples demonstrated hair cell loss in the apex and in the base with hair cells present in the midfrequencies. Patterns of hair cell loss were distinctly different than those observed in other studies of ototoxicity.
FIG. 7.
Representative sections of the mouse cochlea after kanamycin-furosemide and LPS-kanamycin-furosemide. Plastic-embedded mouse cochleas were sectioned and analyzed for histologic changes after treatment. Representative sections of the mouse basal turn (A), middle turn (C), and apical turn (E) after kanamycin-furosemide. In this specimen, the basal turn showed preserved hair cells while the apical hair cells were absent. The organ of Corti was otherwise preserved, and the inner hair cells were present. B, D, F representative sections after LPS-kanamycin-furosemide treatment. All turns showed complete loss of outer hair cells. In other specimens in this series, we observed sporadic retention of outer hair cells mostly in the basal half. Scale bar in A 50 μm (applies to A, B), C 25 μm (applies to C, D, E, and F). Arrows indicate area of outer hair cells.
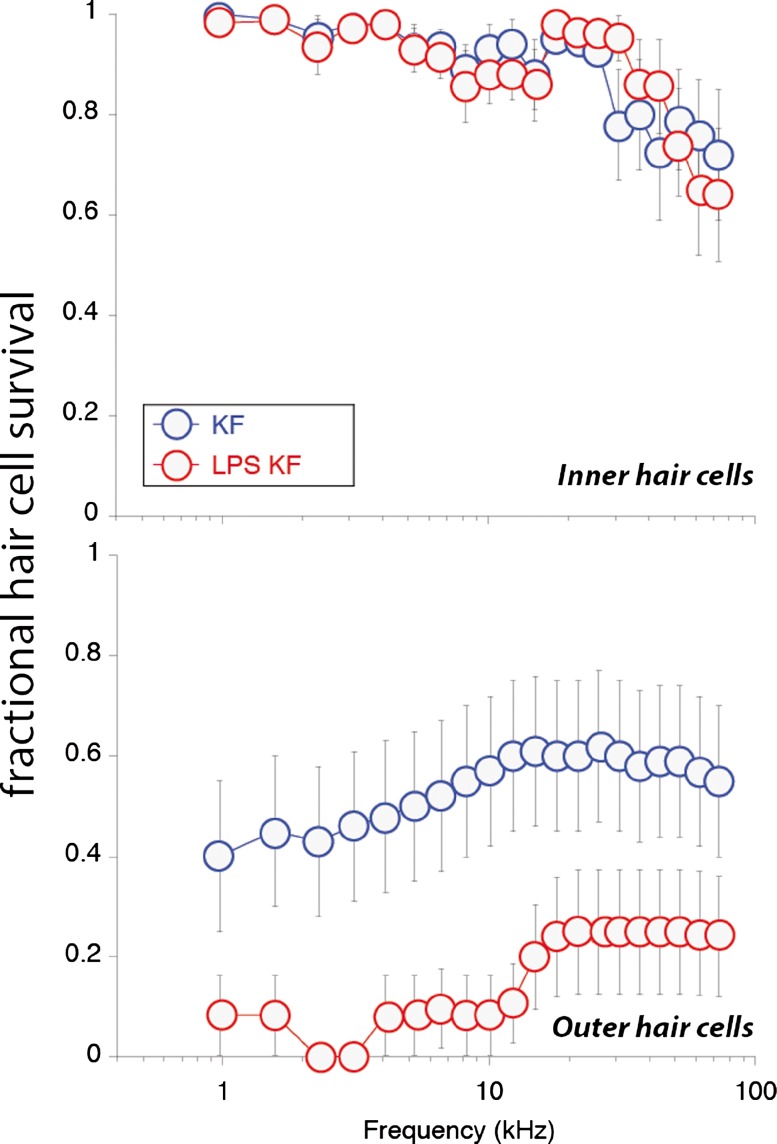
High-frequency, outer hair cells are known to be the target of aminoglycoside antibiotic ototoxicity, and in this study, we anticipated a similar lesion with respect to hair cell loss. As other laboratories have shown, murine hair cells are relatively resistant to aminoglycoside ototoxicity, and exceptionally high and frequent doses of antibiotics or a combination of ototoxic agents are required to induce hair cell ablation in mice (Oesterle et al. 2008; Taylor et al. 2008; Wu et al. 2001). In our experiments, we first observed minimal high-frequency inner hair cell loss in both saline-pretreated and LPS-pretreated mice after ototoxic injury. Both groups suffered the loss of approximately 20–30 % of high-frequency inner hair cells for 5 days after treatment (Fig. 8). There was no observable difference in inner hair cell susceptibility to ototoxicity with the addition of LPS pretreatment.
FIG. 8.
Cytocochleograms. Five days after ototoxic injury, cochleas were harvested and processed for light microscopy. Some inner hair cells in the basal turn were missing after kanamycin-furosemide treatment (KF). LPS pretreatment with kanamycin-furosemide (LPS-KF) did not alter the amount or pattern of inner hair cell damage. LPS pretreatment resulted in a significant reduction of outer hair cells when combined with kanamycin-furosemide. Statistical analysis demonstrated no significant difference in inner hair cell survival. Outer hair cell survival was lower in LPS-kanamycin-furosemide-treated animals compared with kanamycin-furosemide alone when comparing average OHC loss over the entire cochlear duct (KF n = 12, LPS-KF n = 12, error bars represent standard error of the means).
However, the outer hair cell counts revealed two interesting observations. First, the typical high-frequency susceptibility of outer hair cells in ototoxicity was not observed in these studies using single-dose combination of kanamycin-furosemide. Hair cell loss was more evenly distributed across frequencies. Second, the LPS-pretreated group had more loss of outer hair cells than the saline-pretreated group. Both groups of kanamycin-furosemide-treated animals showed a large degree variability in hair cell injury as shown by the error bars in Figure 8. Approximately 50–60 % of outer hair cells overall remained across the cochlear duct in mice treated with kanamycin-furosemide, whereas 10–20 % of outer hair cells overall remained in the LPS-pretreated group. Individual cochleas demonstrated either extensive loss of outer hair cells distributed evenly across the cochlear duct or a bimodal distribution, with loss of low- and high-frequency outer hair cells with preservation of midfrequency outer hair cells. The addition of furosemide to kanamycin in a single-dose protocol appears to change the high-frequency selectivity of aminoglycoside ototoxicity, and the addition of LPS exacerbates outer hair cell susceptibility across all frequencies.
DISCUSSION
LPS-induced inflammation exacerbates ototoxic injury
Low-dose LPS has been studied as a method of priming the immune system before subsequent insults, including stroke, infection, or traumatic injury where the predominant effect of LPS has been protective. Thus, we originally designed these experiments to determine if LPS priming would provide protection against ototoxicity. Instead, we found that LPS pretreatment resulted in exacerbation of hair cell injury and hearing loss. Unlike other experiments where low-dose LPS created an environment that was more resistant to injury, in this case, the inner ear appears more vulnerable as a result of low-level systemic inflammation. LPS-pretreated mice showed higher threshold shifts long term and more extensive outer hair cell loss when compared with animals receiving ototoxic agents alone. In LPS-pretreated animals, we also observed increased numbers of inflammatory cells in the damaged cochleas and the presence of CCR2-expressing monocytes, which are not frequently observed in the injured cochlea.
However, we cannot conclude that inflammatory cells are independently detrimental to the inner ear. In LPS control mice, similar mononuclear phagocytes entered the cochlea without causing hearing loss or inner ear injury, demonstrating that inflammatory cells, in the absence of ototoxic agents, are not alone capable of causing direct injury to the cochlea at least in these small numbers. In the setting of hair cell injury, immune-mediated processes initiated by LPS, including the migration of inflammatory cells into the cochlea, appear to be harmful and to augment the cytotoxic effect of these agents. Part of this effect may be mediated by the specific population of cells that gain entry into the inner ear with LPS pretreatment. The majority of cochlear macrophages and monocytes identified in the past expressed CX3CR1 and not CCR2. In mice pretreated with LPS, we observe a population of CCR2+ “inflammatory” monocytes in addition to the CX3CR1+ patrolling monocytes. The distinction between CX3CR1+- and CCR2+-expressing mononuclear cells has been elucidated in other organ systems. CX3CR1+ monocytes and macrophages are the long-term residents of the peripheral tissues, they serve a surveillance role, and their numbers turn over relatively slowly, whereas CCR2+-expressing monocytes are summoned into peripheral tissues when active inflammatory signals are present and are more rapidly returned to the circulation. Both CX3CR1- and CCR2-expressing mononuclear cells were present in LPS control ears, but both were present in lower numbers when compared with LPS-kanamycin-furosemide-treated animals. From these experiments, it appears that LPS facilitates entry of CCR2+ cells into the inner ear. Previous studies have shown that LPS compromises the blood brain barrier (Jangula and Murphy 2013; Wispelwey et al. 1988). LPS may allow entry of CCR2 monocytes into the inner ear by similarly affecting the blood labyrinth barrier and reducing barrier selectivity.
When LPS has been used in isolation and applied to the inner ear, the effects were uniformly detrimental. In a prior study of experimental otitis media, LPS was injected through the eardrum against the round window membrane in experimental animals, and hearing loss was observed (Kim and Kim 1995). In these experiments, LPS was used at a higher dose (1.0 mg/kg) and applied directly to the round window membrane. Another study in guinea pigs demonstrates that LPS injected directly into the perilymph caused injury to the stria vascularis and induced elevation in hearing thresholds, not observed in saline-injected controls (Guo et al. 1994). Together, the studies that apply LPS to the middle ear space to model otitis media and those that place LPS in the perilymph suggest that LPS is damaging. These prior studies have not investigated how LPS, administered systemically, affects inner ear function by accessing the cochlea through the circulation. The likelihood of LPS being circulated through the vasculature due to a remote infection in the lungs, the kidney, or the abdominal cavity is considerably higher than the chance of LPS entering the perilymph directly; hence, the effects of LPS in circulation on inner ear structure and function are highly relevant. Furthermore, the finding that systemic LPS causes migration of leukocytes into the inner ear without a specific insult to the inner ear is surprising and potentially important in understanding processes that affect hearing when there is an infection present at a distant site.
With regard to effects of endotoxin more broadly, LPS is a specific type of endotoxin, derived from the cell walls of gram-negative bacteria and a member of a class of molecules described as pathogen-associated molecular patterns (PAMPs). PAMPs contain conserved motifs and activate the immune system by binding to toll-like receptors (TLRs). TLR4 binds LPS and is the most studied of all the TLRs. TLR4 is expressed on monocytes, T cells, B cells, and dendritic cells and is inducible in nonhematopoietic cells (Chakravarty and Herkenham 2005). Thus, LPS, through TLR4, communicates the presence of bacteria to the first responders of the immune system, by binding to these receptors. Intraperitoneal injection of moderate doses of LPS in experimental animals provides a model for sepsis. LPS at lower doses can be used to stimulate innate immunity short of causing sepsis and emulates the presence of bacterial elements in the blood stream. Most effects of intraperitoneal LPS are mediated by NF-kappaB and type I interferons. Downstream events include migration of monocytes and macrophages into the peritoneal cavity, increase in capillary permeability, activation of IL-1R-associated kinases (IRAKs) and production of inflammatory cytokines through activation of the transcription factor, NF-kappaB (Nahid et al. 2011; Bosmann et al. 2012; Wang et al. 2011; De Arras et al. 2013; Deng et al. 2006; Bauerfeld et al. 2012; Figueroa et al. 2012; Xiong and Medvedev 2011). The effects of systemic LPS are extensive and are critically important in the study of infection and immunity. There is a high likelihood that individuals receiving antibiotic therapy with aminoglycosides are subject to many of these effects of LPS as a result of bacterial infection. Therefore, the interaction between the immune system’s response to infection with its effects on inner ear injury is relevant to understanding the overall effect of ototoxic antibiotic agents.
LPS has been shown to be detrimental in cisplatin ototoxicity
A recently published study indicates a similar exacerbation of hearing loss when systemic LPS was administered in combination with cisplatin in mice (Oh et al. 2011). Considerably, higher doses of LPS were given in this study with cisplatin (1.25, 2.5, or 5.0 mg/kg compared to 0.5 mg/kg in our study) at 6 and 24 h after cisplatin (5 mg/kg). Higher doses of LPS in the range of 5–10 mg/kg are models for true bacterial sepsis. The experiments presented in our paper employ a priming dose (0.5 mg/kg × 2 days), which is twofold to tenfold lower than the doses of LPS used in the cisplatin study. In preliminary experiments, we tested LPS pretreatment at 0.1, 0.5, and 1.0 mg/kg administered via intraperitoneal route (IP) in control mice and in ototoxic-injured mice. LPS pretreatment at 1.0 mg/kg showed mild cochlear damage with LPS alone, thereby introducing a third ototoxic agent in addition to kanamycin-furosemide, which was not the goal of our study. We selected the dose of 0.5 mg/kg after preliminary experiments showed no effect at 0.1 mg/kg and exacerbation of hearing thresholds after ototoxic injury with 0.5 mg/kg and no evidence of ototoxic effects given with LPS 0.5 mg/kg given independently.
In the study of Oh et al. cisplatin alone resulted in mild hearing impairment, while cisplatin given with LPS (at doses of 2.5 mg/kg or higher) resulted in 10–15-dB exacerbation of this hearing impairment. TLR4 null mice were protected against the effects of LPS-induced exacerbation of ototoxic injury. Surprisingly, the TLR4 mice were also completely protected against cisplatin ototoxicity even in the absence of LPS. The mechanism of protection from cisplatin ototoxicity by TLR4 deletion was not elucidated. Whether innate immunity plays a role in cisplatin otoxicity was not investigated in this paper.
Other studies have examined the outcomes of LPS priming in the central nervous system and have shown an inhibition of leukocyte migration and suppression of local inflammation with low-dose LPS pretreatment (Rosenzweig et al. 2004; Marsh et al. 2009). Nevertheless, we observed an enhancement of cochlear leukocyte entry with LPS priming. In experiments that our group has conducted using cisplatin, we have observed enhanced entry of cochlear macrophages into the inner ear associated with high-frequency hearing loss and loss of hair cells in the cochlear base due to cisplatin ototoxicity (unpublished). We speculate that LPS administered with cisplatin may result in increased mononuclear phagocyte entry into the inner ear, as we observed with combination of kanamycin-furosemide. The mechanisms that render the inner ear of LPS-pretreated animals more vulnerable to aminoglycoside treatment may be applicable to cisplatin ototoxicity. The effect of LPS in cisplatin and aminoglycoside ototoxicity is comparable: Hearing thresholds are made worse by augmenting the inflammatory response at the time of injury.
CCR2+ inflammatory monocytes enter the cochlea with LPS treatment, which may play role in determining outcome
After LPS treatment, we observed a population of inflammatory monocytes that is not commonly observed in the inner ear after damage. These monocytes are characterized by expression of CCR2. Recent work has divided monocytes into two distinct categories: one that expresses CCR2 and migrates rapidly out of the circulation, homing to sites of inflammation and reentering the circulation, and a second that expresses CX3CR1 and serves the role of the patrolling or resident inflammatory cell (Schulz et al. 2012; Geissmann et al. 2003). We have observed that most monocytes and macrophages in the inner ear express CX3CR1 and not CCR2 in control and injured cochleas in previous studies of acoustic injury or ototoxicity (Hirose et al. 2005; Sato et al. 2008). However, after LPS treatment, CCR2+ inflammatory monocytes are observed in abundance alongside a large population of CX3CR1-positive resident monocytes and macrophages.
We have previously explored the role of CX3CR1 in ototoxic injury through the use of CX3CR1 knockout mice and found that CX3CR1 is protective in ototoxicity. We found CX3CR1 null mice to be more vulnerable to ototoxic injury than CX3CR1 wild-type mice. Also, in the absence of CX3CR1 signaling, the number of macrophages recruited to the inner ear was increased. In addition, CX3CR1 null macrophages and monocytes exacerbated kanamycin ototoxicity in mice where CX3CR1 was knocked out exclusively in the cells of the bone marrow (Sato et al. 2010). We surmised that CX3CR1 plays a role in controlling the trafficking of monocytes in the cochlea and in modulating their cytotoxic effects. It is difficult to summarize the overall contributions of innate immunity in cochlear injury because certain leukocytes may be associated with a worse outcome while others may provide a protective effect. We have found that the expression of chemokine receptors on mononuclear phagocytes entering the inner ear affects the final outcome in ototoxicity. We propose that CX3CR1+ monocytes and macrophages play a protective role while CCR2+ monocytes are detrimental in ototoxicity (Sato et al. 2010). However, LPS initiates many steps that could play an important role in ototoxicity, independent of inducing inflammatory cell migration. For example, the direct effects of LPS on inner ear vascular permeability may result in increased concentrations of aminoglycosides entering the inner ear. Similarly, inflammatory cytokines present in circulation may gain access to inner ear fluids, independent of inflammatory cell recruitment into the cochlea, and may participate in pathways mediated by TLR4 and MyD88 as previously described, leading to hair cell demise.
LPS does not systematically affect endocochlear potential after ototoxic injury
Loss of the EP was considered as a potential source of LPS-induced changes in hearing threshold, particularly because inflammatory cells are typically aggregated in the cochlear lateral wall, where perturbations of vascular permeability or ion transport could be induced by inflammatory signals and could cause compromise of the EP. We discovered that the EP was not reduced any more in LPS-pretreated mice when compared to animals after kanamycin-furosemide treatment alone. Thus, exacerbation of hearing thresholds could not be attributed to changes in EP in LPS-pretreated mice. Other investigators have explored changes in EP after furosemide, but none of them shed light on the paradigm of inflammatory preconditioning where LPS causes further worsening of hearing thresholds (Rybak 1993; Rybak et al. 1991; Naito and Watanabe 1997; Ding et al. 2002; Mathog et al. 1970). Histologic changes in the stria vascularis have also been described in previous studies, but these studies do not address what could be causing threshold elevation that we observe with LPS pretreatment when added to these known ototoxins (Azuma et al. 2002; Arnold et al. 1981).
Changes in vascular permeability within the inner ear could play a critical role in LPS-induced exacerbation of ototoxicity. Sepsis models using higher doses of LPS have been shown to compromise endothelial barriers, which result in increased entry of proteins and cells into target organ (Maus et al. 2002; O'Dea et al. 2009; Tauseef et al. 2012). Compromise of the blood-perilymph barrier mediated by LPS could be associated with an increase in ototoxic drug uptake from the vasculature into the inner ear. LPS has been shown to be capable of increasing vascular permeability in other tissues; thus, it would appear feasible that LPS acts by a similar mechanism in the inner ear and increased drug entry into cochlear fluids may be a sequela. Further studies are necessary to explore this possibility. Others have postulated a similar effect of bacterial sepsis in aminoglycoside entry (Quintanilla-Dieck et al. 2013).
The immune system is relevant in aminoglycoside ototoxicity
The primary function of the immune system is to patrol host tissues for foreign invaders and to kill infecting microorganisms while limiting damage to normal structures. Of patients who are treated with aminoglycoside antibiotics and are vulnerable to ototoxicity, virtually all are being treated for bacterial infections, resulting in an activated host immune response involving many effects that are mediated by LPS. Aminoglycoside antibiotics are given consistently in an environment with an infecting organism and bacterial elements in circulation, an environment that is modeled in these experiments. Here, we demonstrate that LPS pretreatment in ototoxicity is associated with a worse outcome and that activation of innate immunity by bacterial sepsis makes matters worse when combined with exposure to ototoxic medications. Further studies are needed to elucidate the specific mechanisms associated with this worse outcome. We offer the possibility that the entry of CCR2+ monocytes and compromise of the blood-labyrinth barrier may be important in this effect.
Acknowledgments
Many thanks to Drs. Mark Warchol and Alec Salt for careful feedback on the manuscript and to Dorina Kallojieri for assistance with statistical analysis. Funding sources supporting this work include NIH DC011315 and a research grant from the American Otological Society.
Conflict of Interest
None of the authors who have authored or provided materials for this work have a financial, personal, or other conflicting interest in the results of this research or publication of this work.
Author Contributions
All authors had full access to the data in this study and take responsibility for the integrity and accuracy of the data analysis. Study concept and design: KH, KKO, RMR. Acquisition of data: SZL, KKO, KH. Analysis and interpretations of the data: KH, KKO, RMR. Writing of the manuscript: KH. Statistical analysis: KH. Funding awarded to: KH. Study supervision: KH.
Contributor Information
Keiko Hirose, Phone: +1-314-4544033, FAX: +1-314-4542174, Email: keiko_hirose@post.harvard.edu.
Song-Zhe Li, Email: lis@ent.wustl.edu.
Kevin K. Ohlemiller, Email: ohlemillerk@ent.wustl.edu
Richard M. Ransohoff, Email: ransohr@ccf.org
References
- Arnold W, Nadol JB, Jr, Weidauer H. Ultrastructural histopathology in a case of human ototoxicity due to loop diuretics. Acta Otolaryngol. 1981;91(5–6):399–414. doi: 10.3109/00016488109138521. [DOI] [PubMed] [Google Scholar]
- Asakuma S, Snow JB., Jr Effects of furosemide and ethacrynic acid on the endocochlear direct current potential in normal and kanamycin sulfate-treated guinea pigs. Otolaryngol Head Neck Surg. 1980;88(2):188–193. [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Azuma H, Takeuchi S, Higashiyama K, Ando M, Kakigi A, Nakahira M, Yamakawa K, Takeda T. Bumetanide-induced enlargement of the intercellular space in the stria vascularis requires an active Na + -K + -ATPase. Acta Otolaryngol. 2002;122(8):816–821. doi: 10.1080/003655402/000028051. [DOI] [PubMed] [Google Scholar]
- Bauerfeld CP, Rastogi R, Pirockinaite G, Lee I, Huttemann M, Monks B, Birnbaum MJ, Franchi L, Nunez G, Samavati L. TLR4-mediated AKT activation is MyD88/TRIF dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages. J Immunol. 2012;188(6):2847–2857. doi: 10.4049/jimmunol.1102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann M, Russkamp NF, Ward PA. Fingerprinting of the TLR4-induced acute inflammatory response. Exp Mol Pathol. 2012;93(3):319–323. doi: 10.1016/j.yexmp.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci Off J Soc Neurosci. 2005;25(7):1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arras L, Seng A, Lackford B, Keikhaee MR, Bowerman B, Freedman JH, Schwartz DA, Alper S. An evolutionarily conserved innate immunity protein interaction network. J Biol Chem. 2013;288(3):1967–1978. doi: 10.1074/jbc.M112.407205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116(9):2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, McFadden SL, Woo JM, Salvi RJ. Ethacrynic acid rapidly and selectively abolishes blood flow in vessels supplying the lateral wall of the cochlea. Hear Res. 2002;173(1–2):1–9. doi: 10.1016/S0378-5955(02)00585-3. [DOI] [PubMed] [Google Scholar]
- Ehret G. Peripheral anatomy and physiology II. In: Willott J, editor. The auditory psychobiology of the mouse. Springfield: Charles C. Thomas; 1983. pp. 169–200. [Google Scholar]
- Figueroa L, Xiong Y, Song C, Piao W, Vogel SN, Medvedev AE. The Asp299Gly polymorphism alters TLR4 signaling by interfering with recruitment of MyD88 and TRIF. J Immunol. 2012;188(9):4506–4515. doi: 10.4049/jimmunol.1200202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredelius L, Rask-Andersen H. The role of macrophages in the disposal of degeneration products within the organ of corti after acoustic overstimulation. Acta Otolaryngol. 1990;109(1–2):76–82. doi: 10.3109/00016489009107417. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wu Y, Chen W, Lin J. Endotoxic damage to the stria vascularis: the pathogenesis of sensorineural hearing loss secondary to otitis media? J Laryngol Otol. 1994;108(4):310–313. doi: 10.1017/S0022215100126623. [DOI] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J Assoc Res Otolaryngol: JARO. 2001;2(2):118–129. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Sato E. Comparative analysis of combination kanamycin-furosemide versus kanamycin alone in the mouse cochlea. Hear Res. 2011;272(1–2):108–116. doi: 10.1016/j.heares.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol. 2005;489(2):180–194. doi: 10.1002/cne.20619. [DOI] [PubMed] [Google Scholar]
- Jangula A, Murphy EJ. Lipopolysaccharide-induced blood brain barrier permeability is enhanced by alpha-synuclein expression. Neurosci Lett. 2013;551:23–27. doi: 10.1016/j.neulet.2013.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–4114. doi: 10.1128/MCB.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Rivest S. MyD88-deficient bone marrow cells accelerate onset and reduce survival in a mouse model of amyotrophic lateral sclerosis. J Cell Biol. 2007;179(6):1219–1230. doi: 10.1083/jcb.200705046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Kim HJ. Auditory brain stem response changes after application of endotoxin to the round window membrane in experimental otitis media. Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 1995;112(4):557–565. doi: 10.1177/019459989511200409. [DOI] [PubMed] [Google Scholar]
- Komune S, Snow JB., Jr Nature of the endocochlear dc potential in kanamycin-poisoned guinea pigs. Arch Otolaryngol. 1982;108(6):334–338. doi: 10.1001/archotol.1982.00790540006002. [DOI] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Effects of chronic furosemide treatment and age on cell division in the adult gerbil inner ear. J Assoc Res Otolaryngol: JARO. 2003;4(2):164–175. doi: 10.1007/s10162-002-2056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig S, Danenberg HD, Kafri Y, Kobiler D, Ben-Nathan D. Viral neuroinvasion and encephalitis induced by lipopolysaccharide and its mediators. J Exp Med. 1992;176(3):707–712. doi: 10.1084/jem.176.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci Off J Soc Neurosci. 2009;29(31):9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathog RH, Thomas WG, Hudson WR. Ototoxicity of new and potent diuretics. A preliminary study. Arch Otolaryngol. 1970;92(1):7–13. doi: 10.1001/archotol.1970.04310010033002. [DOI] [PubMed] [Google Scholar]
- Maus UA, Koay MA, Delbeck T, Mack M, Ermert M, Ermert L, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. Am J Physiol Lung Cell Mol Physiol. 2002;282(6):L1245–L1252. doi: 10.1152/ajplung.00453.2001. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Endotoxemia prevents the cerebral inflammatory wave induced by intraparenchymal lipopolysaccharide injection: role of glucocorticoids and CD14. J Immunol. 2002;169(6):3370–3381. doi: 10.4049/jimmunol.169.6.3370. [DOI] [PubMed] [Google Scholar]
- Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol. 2011;8(5):388–403. doi: 10.1038/cmi.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito H, Watanabe K. Alteration in capillary permeability of horseradish peroxidase in the stria vascularis and movement of leaked horseradish peroxidase after administration of furosemide. ORL J Otorhinolaryngol Relat Spec. 1997;59(5):248–257. doi: 10.1159/000276948. [DOI] [PubMed] [Google Scholar]
- Nishioku T, Dohgu S, Takata F, Eto T, Ishikawa N, Kodama KB, Nakagawa S, Yamauchi A, Kataoka Y. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol. 2009;29(3):309–316. doi: 10.1007/s10571-008-9322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dea KP, Wilson MR, Dokpesi JO, Wakabayashi K, Tatton L, van Rooijen N, Takata M. Mobilization and margination of bone marrow Gr-1high monocytes during subclinical endotoxemia predisposes the lungs toward acute injury. J Immunol. 2009;182(2):1155–1166. doi: 10.4049/jimmunol.182.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S. Supporting cell characteristics in long-deafened aged mouse ears. J Assoc Res Otolaryngol: JARO. 2009;10(4):525–544. doi: 10.1007/s10162-009-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol: JARO. 2008;9(1):65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh GS, Kim HJ, Choi JH, Shen A, Kim CH, Kim SJ, Shin SR, Hong SH, Kim Y, Park C, Lee SJ, Akira S, Park R, So HS. Activation of lipopolysaccharide-TLR4 signaling accelerates the ototoxic potential of cisplatin in mice. J Immunol. 2011;186(2):1140–1150. doi: 10.4049/jimmunol.1002183. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–83. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla-Dieck L, Larrain B, Trune D, Steyger PS. Effect of systemic lipopolysaccharide-induced inflammation on cytokine levels in the murine cochlea: a pilot study. Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 2013;149(2):301–303. doi: 10.1177/0194599813491712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35(11):2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- Rybak LP. Ototoxicity of loop diuretics. Otolaryngol Clin N Am. 1993;26(5):829–844. [PubMed] [Google Scholar]
- Rybak LP, Whitworth C, Scott V. Comparative acute ototoxicity of loop diuretic compounds. Eur Arch Otorhinolaryngol. 1991;248(6):353–357. doi: 10.1007/BF00169028. [DOI] [PubMed] [Google Scholar]
- Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5(10):e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P, Geissmann F. Toward a functional characterization of blood monocytes. Immunol Cell Biol. 2011;89(1):2–4. doi: 10.1038/icb.2010.130. [DOI] [PubMed] [Google Scholar]
- Sato E, Shick HE, Ransohoff RM, Hirose K. Repopulation of cochlear macrophages in murine hematopoietic progenitor cell chimeras: the role of CX3CR1. J Comp Neurol. 2008;506(6):930–942. doi: 10.1002/cne.21583. [DOI] [PubMed] [Google Scholar]
- Sato E, Shick HE, Ransohoff RM, Hirose K. Expression of fractalkine receptor CX3CR1 on cochlear macrophages influences survival of hair cells following ototoxic injury. J Assoc Res Otolaryngol: JARO. 2010;11(2):223–234. doi: 10.1007/s10162-009-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Sewell WF. Furosemide selectively reduces one component in rate-level functions from auditory-nerve fibers. Hear Res. 1984;15(1):69–72. doi: 10.1016/0378-5955(84)90226-0. [DOI] [PubMed] [Google Scholar]
- Shi J, Johansson J, Woodling NS, Wang Q, Montine TJ, Andreasson K. The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol. 2010;184(12):7207–7218. doi: 10.4049/jimmunol.0903487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Neuroprotective effects of resident microglia following acute brain injury. J Comp Neurol. 2007;504(6):716–729. doi: 10.1002/cne.21469. [DOI] [PubMed] [Google Scholar]
- Tan BT, Lee MM, Ruan R. Bone-marrow-derived cells that home to acoustic deafened cochlea preserved their hematopoietic identity. J Comp Neurol. 2008;509(2):167–179. doi: 10.1002/cne.21729. [DOI] [PubMed] [Google Scholar]
- Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, Birnbaumer L, Malik AB, Mehta D. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J Exp Med. 2012;209(11):1953–1968. doi: 10.1084/jem.20111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RR, Nevill G, Forge A. Rapid hair cell loss: a mouse model for cochlear lesions. J Assoc Res Otolaryngol: JARO. 2008;9(1):44–64. doi: 10.1007/s10162-007-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene SV, Sato K, Pham L, Billings P, Keithley EM. Immune cell recruitment following acoustic trauma. Hear Res. 2006;222(1-2):115–124. doi: 10.1016/j.heares.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol: JARO. 2002;3(3):248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Deng M, Liu X, Ai W, Tang Q, Hu J. TLR4 activation induces nontolerant inflammatory response in endothelial cells. Inflammation. 2011;34(6):509–518. doi: 10.1007/s10753-010-9258-4. [DOI] [PubMed] [Google Scholar]
- Warchol ME. Macrophage activity in organ cultures of the avian cochlea: demonstration of a resident population and recruitment to sites of hair cell lesions. J Neurobiol. 1997;33(6):724–734. doi: 10.1002/(SICI)1097-4695(19971120)33:6<724::AID-NEU2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Wispelwey B, Lesse AJ, Hansen EJ, Scheld WM. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J Clin Invest. 1988;82(4):1339–1346. doi: 10.1172/JCI113736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158(1–2):165–178. doi: 10.1016/S0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Medvedev AE. Induction of endotoxin tolerance in vivo inhibits activation of IRAK4 and increases negative regulators IRAK-M, SHIP-1, and A20. J Leukoc Biol. 2011;90(6):1141–1148. doi: 10.1189/jlb.0611273. [DOI] [PMC free article] [PubMed] [Google Scholar]