Abstract
Context
Risk markers including coronary artery calcium (CAC), carotid intima-media thickness (CIMT), ankle-brachial Index (ABI), brachial flow-mediated dilation (FMD), high sensitivity C -reactive protein (hs-CRP) and family history (FH) of coronary heart disease (CHD) have been reported to improve on the Framingham risk score (FRS) for prediction of CHD. However, there are no direct comparisons of these markers for risk prediction in a single cohort.
Objective
We compared improvement in prediction of incident CHD/cardiovascular disease (CVD) of these 6 risk markers within intermediate risk participants (5 % < FRS < 20%) in the Multi-Ethnic Study of Atherosclerosis (MESA).
Design, Setting and Participants
Of 6814 MESA participants from 6 US field centers, 1330 were intermediate risk, without diabetes mellitus, and had complete data on all 6 markers. Recruitment spanned July 2000 to September 2002; follow-up extended through May 2011. Probability- weighted Cox proportional hazard models were used to estimate hazard ratios (HR). Area under the receiver operator characteristic curve (AUC) and net reclassification improvement (NRI) were used to compare incremental contributions of each marker when added to the FRS + race/ethnicity.
Main Outcome Measures
Incident CHD defined as MI, angina followed by revascularization, resuscitated cardiac arrest or CHD death. Incident CVD additionally included stroke or CVD death.
Results
After median follow-up of 7.6 years (IQR 7.3 – 7.8 years), 94 CHD and 123 CVD events occurred. CAC, ABI, hs-CRP and FH were independently associated with incident CHD in multivariable analyses [HR (95%CI: 2.60(1.94-3.50), 0.79(0.66-0.95), 1.28(1.00-1.64) and 2.18(1.38-3.42) respectively]. CIMT and FMD were not associated with incident CHD in multivariable analyses [HR (95%CI) 1.17(0.95- 1.45) and 0.95(0.78 −1.14) respectively]. Although the addition of the markers individually to the FRS +race/ethnicity improved the AUC, CAC afforded the highest increment (0.623 vs. 0.784) while FMD afforded the least [0.623 vs. 0.639]. For incident CHD, the NRI with CAC was 0.659, FMD 0.024, ABI 0.036, CIMT 0.102, FH 0.160 and hs-CRP 0.079. Similar results were obtained for incident CVD.
Conclusion
CAC, ABI, hs-CRP and FH are independent predictors of incident CHD/CVD in intermediate risk individuals. CAC provides superior discrimination and risk reclassification compared with other risk markers.
Introduction
Current trends in primary prevention of cardiovascular disease (CVD) emphasize the need to treat individuals based on their global cardiovascular risk (1, 2). Accordingly, practice guidelines recommend approaches to classify individuals as either “high”, “intermediate,” or “low” risk using the Framingham Risk Score (FRS) or other similar CVD risk prediction models(3). However, there is increasing recognition of the imprecision of these classifications such that the “intermediate risk” group actually represents a composite of higher risk individuals for whom more aggressive (i.e., drug) therapy might be indicated. The “intermediate” risk group also contains lower risk individuals who might be managed with lifestyle measures alone. This recognition has motivated research to identify markers that could offer greater discrimination of higher and lower risk patients within the intermediate risk group.
Risk markers that have shown promise in improving risk discrimination include carotid intima-media thickness (CIMT), coronary artery calcium scores (CAC), brachial flow-mediated dilation (FMD), ankle brachial index (ABI), high sensitivity C-reactive protein (hs-CRP) and family history of coronary heart disease (FH) (4-9). A recent American College of Cardiology Foundation/American Heart Association (ACCF/AHA) statement on the use of markers to improve cardiovascular risk prediction beyond the FRS gave family history a class I recommendation; CIMT, CAC, ABI and hs-CRP received class II recommendations, while the ACCF/AHA recommended against the use of brachial FMD (Class III) (10). However, these recommendations were limited by the relative paucity of published data and the fact that the published studies of individual risk markers were performed in different cohorts with different composite outcomes, analytic methodologies and inadequate statistical power to detect improvements of risk prediction beyond commonly used risk prediction algorithms. Moreover, there are no comprehensive head-to-head comparisons of these risk markers in a single population cohort similar to the US population. Determining the relative improvements in prediction afforded by various risk markers, especially when applied to FRS-intermediate risk individuals, could help determine the most efficient strategy to identify selected intermediate risk subjects for more aggressive primary prevention interventions including the use of aspirin and lower targets for drug treatments of LDL cholesterol and blood pressure.
In this report, we assess the improvements in prediction accuracy and reclassification to high and low risk categories using CIMT, CAC, FMD, ABI, hs-CRP and family history of CHD, in asymptomatic adults with intermediate Framingham risk who participated in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
Study Population and Data Collection
The study design for the MESA study has been published elsewhere (11). In brief, MESA is a prospective cohort study to investigate the prevalence, correlates, and progression of subclinical CVD in persons without known CVD at baseline.
The full cohort includes 6,814 women and men ages 45 to 84 years without known CVD, recruited from 6 U.S. communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan, New York; and St. Paul, Minnesota). Self-reported race/ethnicity was collected to explore the possible racial differences in the development and progression of atherosclerosis. MESA included 38% white, 28% African American, 22% Hispanic, and 12% Chinese adults. Participants with diabetes were excluded because it is considered a CHD risk-equivalent. Diabetes was defined as self-reported history of diabetes mellitus, diabetes medication use or fasting glucose ≥126mg/dl. Demographics, medical history, and anthropometric and laboratory data for the present study were taken from the first examination (July 2000 to August 2002). Current smoking was defined as having smoked a cigarette in the last 30 days. Use of antihypertensive and other medications was based on review of prescribed medication containers. Resting blood pressure was measured three times in the seated position, and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, or use of medication prescribed for hypertension. Body mass index was calculated as weight (kg) divided by height (m2). Total cholesterol and high density lipoprotein (HDL) cholesterol were measured from blood samples obtained after a 12-h fast. Low-density lipoprotein cholesterol was estimated by the Friedewald equation (12). High sensitivity CRP was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, Illinois) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, Vermont). Analytical intra-assay coefficient of variations ranged from 2.3% to 4.4%, and inter-assay coefficient of variation ranged from 2.1% to 5.7% with a detection level of 0.18 mg/L. Family history of CHD was obtained by asking participants whether any member in their immediate family (first-degree relatives: parents, siblings and children) experienced fatal or nonfatal myocardial infarction. The MESA study was approved by the institutional review boards of each study site, and written informed consent was obtained from all participants.
Measurement of Ankle-Brachial Index
Details of the MESA ankle-brachial index measurement protocol has been published by Criqui et al (9). Briefly, SBP measurements in the bilateral brachial, dorsalis pedis, and posterior tibial arteries were obtained in the supine position using a hand-held Doppler instrument with a 5-mHz probe. To avoid potential bias from subclavian stenosis, the higher of the brachial artery pressures was used as the denominator. For each lower extremity, the ABI numerator used was the highest pressure (dorsalis pedis or posterior tibial) from that leg. Reproducibility of ABI was evaluated using measurements of 43 participants by two technicians. The inter- and intra-reader correlation coefficients were 0.845 and 0.937 respectively with an intra- and inter-reader coefficient of variation of 5.14% and 3.27% respectively.
Measurement of Coronary Artery Calcium (CAC) Score
Details of the MESA CT scanning and interpretation methods have been reported by Carr et al (13). Scanning centers assessed CAC by chest computed tomography (CT) with either a cardiac-gated electron-beam CT scanner (Chicago, Illinois; Los Angeles, California; and New York, New York field centers) or a multidetector CT system (Baltimore, Maryland; Forsyth County, North Carolina; and St Paul, Minnesota field centers). Certified technologists scanned all participants twice over phantoms of known physical calcium concentration. A radiologist or cardiologist read all CT scans at a central reading center (Los Angeles Biomedical Research Institute at Harbor–UCLA, Torrance, California). We used the mean Agatston score for the 2 scans in all analyses (14) Intraobserver and interobserver agreements were excellent (κ = 0.93 and κ = 0.90, respectively).
Measurement of Brachial Flow Mediated Dilation
Methods for the MESA brachial FMD measurement and interpretation have been reported by Yeboah et al (8). Intrareader reproducibility for baseline diameter, maximum diameter, and %FMD was evaluated by comparing an original and a blinded quality control reread of ultrasounds from 40 MESA participants. The intraclass correlation coefficients (ICC) were 0.99, 0.99, and 0.93, respectively. Intrasubject variability was evaluated by comparing results from repeated examinations of 19 subjects on 2 days a week apart. The ICC for baseline diameter, maximum diameter, and %FMD were 0.90, 0.90, and 0.54, respectively. Percent technical error of measurement was 1.39% for baseline diameter measurement, 1.47% for maximum diameter measurement, and 28.4% for %FMD measurement.
Measurement of Carotid Intima-Media Thickness
The details for CIMT measurement and interpretation have been reported by Polak et al (15).The mean of maximum intima-media thickness of the common carotid artery was used. Reproducibility was assessed by blinded replicate readings of CIMT performed by 2 readers. One reader re-read 66 studies, for a between-reader correlation coefficient of 0.84 (n=66), and the other re-read 48 studies, for a correlation coefficient of 0.86. The re -scan and the re-read coefficient of variation were 7.07% and 3.48% respectively.
Ascertainment of Incident CHD and CVD
Follow –up was through May 2011. CVD events were adjudicated by a MESA study committee that included cardiologists, physician epidemiologists, and neurologists. A detailed description of the adjudication process has been published (8). For the purposes of this study, we defineincident CHD as myocardial infarction (MI), CHD death, resuscitated cardiac arrest, definite or probable angina if followed by coronary revascularization. Incident CVD additionally included stroke or CVD death as defined by the MESA protocol (www.mesa.nhlbi.org). Thus CHD is a subset of CVD.
Statistical Analysis
The study population was limited to MESA participants classified as intermediate-risk (estimated 10-yr CHD risk of > 5 and <20%) based on the Framingham Risk Equation (3). The intermediate risk range was chosen to make our results comparable to other studies that have reported data on intermediate risk participants using some of the risk markers under consideration (4-6). Descriptive data are presented as mean ± SD for continuous variables or frequencies of participants for categorical variables. CAC scores were expressed as In(CAC+1). FH was entered into models as a categorical variable (yes/no), hs-CRP had a highly skewed distribution and was log transformed; all other variables were expressed as continuous variables. Weighted analyses were done to reflect the sampling from the overall MESA cohort. Probability-weighted Cox proportional hazard analysis with robust variance estimates was used to assess the association between each of the markers (CAC, FMD, CIMT, ABI, hs-CRP and family history of CHD) and incident CHD or CVD in univariable and in multivariable models adjusting for age, sex, race/ethnicity, total and HDL cholesterol, cigarette smoking status, BMI, blood pressure medication use and HMG CoA reductase inhibitor use. These confounders were chosen based on their association with the outcomes of interest (incident CHD/CVD) in the current analysis and prior published data.
We assessed the improvement in discrimination by comparing the area under the receiver operator characteristic curves (AUC) in models with and without each novel risk marker, using the method of DeLong et al. The Framingham risk score (derived using age, gender, total cholesterol, HDL cholesterol, smoking status, SBP, blood pressure medication use), a general clinical practice tool plus race/ethnicity served as the baseline model. ROC curves were developed using a probability-weighted Cox model. We assessed the classification of risk using the net reclassification improvement (NRI), defined as:
NRI= [Prob (being correctly reclassified to a higher risk category∣event) – Prob (being incorrectly reclassified to a lower risk category∣event)] + [Prob (being correctly reclassified to a lower risk category∣non-event) – Prob (being incorrectly classified to a higher risk category∣non-event)] (17)
The NRI captures the relative improvement in classification associated with the additional predictive variable, while explicitly balancing tradeoff between changes in sensitivity and specificity. NRI is the sum of two percentages with different denominators and hence is reported as a proportion (possible range is from −2.0 to 2.0). At the time of these analyses the mean observed follow-up in MESA was 7.5 years (maximum follow up of 9 years). To account for the fact that actual follow-up was less than 10 years we redefined the risk in terms of 7.5 year-risk when calculating the NRI, using a logistic regression model with probability weighting to reflect the sampling from the overall cohort. Based on the new model, intermediate 7.5 year risks categories for CHD and CVD were defined as 2.0%-15.4% and 3.4%-21.1% respectively. With the addition of each novel risk marker to the base model, participants were considered to be reclassified to high risk if their estimated risks for CHD and CVD were greater than 15.4% and 21.1% respectively, and reclassified to low risk if their estimated risks were lower than 2.0% and 3.4% for CHD and CVD respectively. As a sensitivity analysis, we repeated our evaluation of ABI and the imaging markers using the Reynolds score calculated separately for men and women (6, 18) instead of the FRS to define these risk groups. This score incorporates family history and log-transformed hs-CRP in addition to other risk factors. A 2-tailed value of P<0.05 was considered significant. All statistical analyses were performed with the use of SAS version 9.2 (SAS Institute, Cary, NC).
Results
Study Cohort
The final study population included 1330 participants without diabetes mellitus, had a Framingham Risk Score >5% and < 20% and complete data on all six of the novel risk markers. The median (IQR) for the Framingham risk score of the cohort at baseline was 8.8 %( 6.5% – 12.2%). The baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline Characteristics of Participants in the Multi-Ethnic Study of Atherosclerosis at Intermediate Framingham Risk (N=1330).
| Variables | (Mean ± SD) |
|---|---|
| Age (years) | 63.8 ±9.5 |
|
| |
| Females (%) | 443(33.3) |
|
| |
| Race/ Ethnicity (%) | |
| Caucasian | 475(35.7) |
| Chinese | 225(16.9) |
| African American | 292(22.0) |
| Hispanic | 338(25.4) |
|
| |
| Body mass index (Kg/m2) | 27.9 ± 4.7 |
|
| |
| Cigarette smoking status (%) | |
| Never | 616(46.3) |
| Former | 494 (37.1) |
| Current | 220(16.5) |
|
| |
| Cholesterol (C) (mg/dl) | |
| Total | 196.9 ± 34.6 |
| Low Density Lipoprotein-C | 122.4 ± 30.3 |
| High Density Lipoprotein-C | 46.5 ± 11.9 |
| Triglycerides | 140.7 ± 77.0 |
|
| |
| Blood Pressure (mmHg) | |
| Systolic | 129.9 ± 19.8 |
| Diastolic | 74.4 ± 9.8 |
|
| |
| Heart rate (bpm) | 61.9 ± 9.2 |
|
| |
| HMG CoA reductase use (%) | 187(14.1) |
|
| |
| Blood Pressure medication use (%) | 508(38.2) |
|
| |
| Coronary Calcium Score (Agatston) | Median(IQR)= 7.0(0 −111.7) |
|
| |
| Brachial Flow mediated dilation (%) | Median(IQR)= 3.60(2.1 – 5.4) |
|
| |
| Carotid Intima-media Thickness(mm) | Median(IQR)= 0.86(0.76 – 0.98) |
|
| |
| Ankle Brachial Index | Median(IQR) = 1.14(1.07 – 1.20) |
|
| |
| High sensitivity C-reactive Protein (mg/dl) |
Median(IQR)= 1.62(0.79 −3.68) |
|
| |
| Family History of Premature CHD | 567 (42.6) |
|
| |
| Framingham Risk Score (%) | Median(IQR)= 8.8(6.5 – 12.2) |
IQR- interquartile range
After a median follow-up of 7.6 years (maximum nine years, IQR 7.3 −7.8 years), 94 participants (7.1 %) experienced a CHD event and 123 (9.2%) experienced a CVD event. 43 had MI, 3 resuscitated cardiac arrest, 14 had CHD death, 44 had angina followed by revascularization and 31 participants had stroke.
Association of Risk Markers (CAC, FMD, CIMT, ABI, hs-CRP and family history of CHD) with Incident CHD and CVD
In the univariable probability-weighted Cox proportional hazard analyses, each of the novel risk markers was associated with incident CHD; however, after adjusting for confounders, the associations with CIMT and FMD were no longer significant (Table 2). Among all of the risk markers, CAC had the strongest association. Similarly, for incident CVD, in univariable analyses, each of the novel risk markers was associated with events except hs-CRP. However, after adjusting for confounders, the associations between CIMT and FMD were no longer significant (Supplement Table 1). CAC also had the strongest association in the multivariable models for CVD.
Table 2.
Association of several novel risk markers with incident coronary heart disease (# events = 94)
| Univariable | Multivariable** | |||
|---|---|---|---|---|
| Marker | Hazard Ratio*(95% CI) | P value | Hazard Ratio*(95%CI) | P value |
| CAC | 2.72(2.09 −3.55) | <0.0001 | 2.60(1.94- 3.50) | <0.0001 |
| Brachial FMD | 0.82(0.66 – 1.03) | 0.09 | 0.93(0.74 – 1.16) | 0.52 |
| Carotid IMT | 1.33(1.12-1.59) | 0.001 | 1.17(0.95 -1.45) | 0.13 |
| ABI | 0.78(0.66 – 0.93) | 0.005 | 0.79(0.66 – 0.95) | 0.01 |
| hs-CRP | 1.26(1.01 – 1.57) | 0.045 | 1.28(1.00 – 1.64) | 0.047 |
| Family History | 2.39(1.54 -3.70) | <0.001 | 2.18(1.38 – 3.42) | 0.001 |
For continuous risk markers, HRs are standardized per unit standard deviation change in the marker.
Multivariable models adjusted for age, gender, race/ethnicity, systolic blood pressure, total cholesterol, HDL, smoking status, BMI, blood pressure medication use and HMG CoA reductase inhibitor use. CACcoronary calcium score, FMD- flow mediated dilation, IMT- intima-media thickness, ABI- ankle brachial index, hs-CRP- high sensitivity C - reactive protein. CAC and hs-CRP were log transformed.
Improvement of Discrimination by Addition of Novel Risk Markers to the Framingham Risk Score
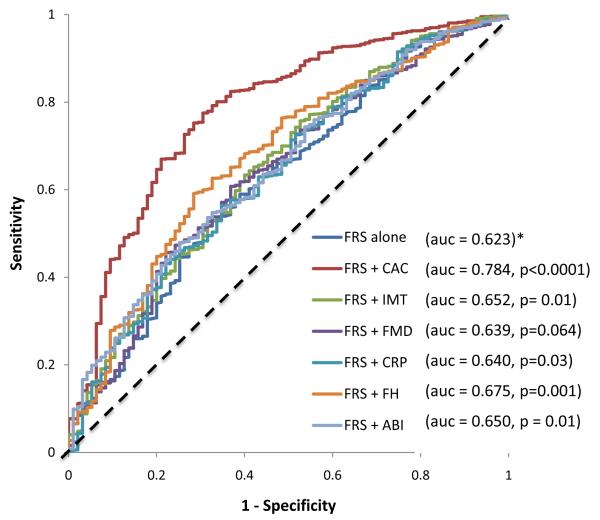
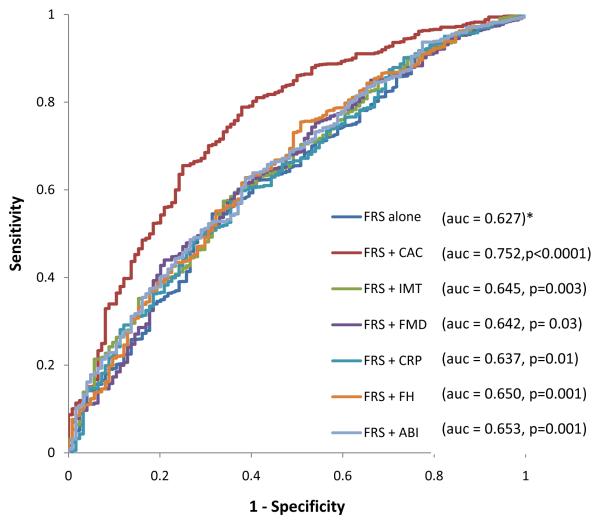
For CHD/CVD events, the addition of each of the 6 risk markers to the baseline model improved the AUC. CAC showed the highest increment while FMD showed the least increment (Figure 1A) for incident CHD. CAC also showed the highest increment while hs- CRP showed the least increment for incident CVD (Figure 1B).
Figure 1.
A: Receiver operator characteristic curves showing the area under the curve (AUC)for FRS, FRS + CAC , FRS + FMD , FRS +ABI, FRS + IMT, FRS + FH and FRS + CRP for incident CHD in MESA Intermediate Risk Participants . * denotes reference. CAC-coronary calcium score, FMD- flow mediated dilation, IMT- intima-media thickness, ABI- ankle brachial index, hs-CRP- high sensitivity C - reactive protein.
Figure 2.
B: Receiver operator characteristic curves showing the area under the curve for FRS, FRS + CAC, FRS + FMD, FRS +ABI, FRS + IMT, FRS + FH and FRS + CRP for incident CVD in MESA Intermediate Risk Participants . * denotes reference. CAC- coronary calcium score, FMD- flow mediated dilation, IMT- intima-media thickness, ABI- ankle brachial index, hs-CRP- high sensitivity C - reactive protein.
Classification of risk
Using Table 3 row number 4 as an example; 51.1% of participants who had CHD (events) and 54.9% of those who did not have CHD (non-events) during follow up period were reclassified either to low or high risk by the addition of CAC to the FRS(+ race/ethnicity). Applying the NRI formula, a net 25.5% of the events group were reclassified to high risk appropriately while a net 40.4% of the non-events group were appropriately reclassified into the low risk group by the addition of CAC to FRS(+race/ethnicity). The NRI for the addition of CAC to FRS (+race/ethnicity) is therefore calculated by adding 0.255 to 0.405 (0.255 + 0.405 =0.659).
Table 3.
Net Reclassification Improvement for Incident coronary Heart disease Events with Addition of novel risk markers to the Framingham Risk Score in MESA Participants with Intermediate Risk (n=1330).
| Variable | Percent Reclassified |
Low | Risk Category Intermediate |
High | Net Correct Reclassification (%) |
NRI | |
|---|---|---|---|---|---|---|---|
| FRS | Events | - | - | 94 | - | - | - |
| Non Events | - | - | 1236 | - | - | ||
|
| |||||||
| FRS + IMT | Events | 7.4 | 0 | 87 | 7 | 7.4 | 0.102 |
| Non Events | 5.3 | 50 | 1170 | 16 | 2.8 | ||
|
| |||||||
| FRS + CAC | Events | 51.1 | 12 | 46 | 36 | 25.5 | 0.659 |
| Non Events | 54.9 | 589 | 557 | 90 | 40.4 | ||
|
| |||||||
| FRS + FMD | Events | 0.0 | 0 | 94 | 0 | 0.0 | 0.024 |
| Non Events | 3.2 | 35 | 1196 | 5 | 2.4 | ||
|
| |||||||
| FRS + ABI | Events | 4.3 | 1 | 90 | 3 | 2.1 | 0.036 |
| Non Events | 4 | 34 | 1186 | 16 | 1.5 | ||
|
| |||||||
| FRS + CRP | Events | 4.3 | 0 | 90 | 4 | 4.3 | 0.079 |
| Non Events | 5.2 | 54 | 1172 | 10 | 3.6 | ||
|
| |||||||
| FRS + FH | Events | 8.5 | 0 | 86 | 8 | 8.5 | 0.160 |
| Non Events | 11.2 | 116 | 1097 | 23 | 7.5 | ||
FRS – Framingham Risk Score, IMT – carotid intima- media thickness ,CAC – coronary calcium score, FMD- brachial flow mediated dilation, ABI – ankle brachial index, FH- Family history of coronary heart disease, CRP – high sensitivity c-reactive protein.
The addition of CAC to the FRS (+ race/ethnicity) resulted in the highest NRI (0.659) and the greatest absolute number of correctly reclassified subjects (n=625) (Table 3) while the addition of FMD resulted in the lowest NRI (0.024) and the fewest total number of correctly reclassified individuals. Carotid IMT, ABI, CRP and FH afforded modest NRIs for CHD events (Table 3). CAC also provided the greatest NRI and total number of correctly reclassified participants for CVD events (Figure 1B and Table 4). Among the non-CAC risk markers, FH performed the best for CHD risk reclassification (NRI =0.160) while ABI performed the best for CVD risk re-classification (NRI = 0.068).
Table 4.
Net Reclassification Improvements for Incident cardiovascular disease Events with Addition of IMT,CAC, FMD, ABI, family history of CHD and Hs CRP over Framingham Risk Score in individuals with intermediate risk (n=1330) in MESA.
| Variable | Percent Reclassified |
Low | Risk Category Intermediate |
High | Net Correct Reclassification (%) |
NRI | |
|---|---|---|---|---|---|---|---|
| FRS | Events | - | - | 123 | - | - | - |
| Non Events | - | 1207 | - | - | |||
|
| |||||||
| FRS + IMT | Events | 3.3 | 0 | 119 | 4 | 3.3 | 0.060 |
| Non Events | 3.8 | 39 | 1161 | 7 | 2.7 | ||
|
| |||||||
| FRS + CAC | Events | 36.6 | 16 | 78 | 29 | 10.6 | 0.466 |
| Non Events | 45.7 | 493 | 655 | 59 | 36.0 | ||
|
| |||||||
| FRS + FMD | Events | 2.4 | 3 | 120 | 0 | -2.4 | 0.023 |
| Non Events | 5.6 | 62 | 1140 | 5 | 4.7 | ||
|
| |||||||
| FRS + ABI | Events | 4.1 | 0 | 118 | 5 | 4.1 | 0.068 |
| Non Events | 4.6 | 44 | 1151 | 12 | 2.7 | ||
|
| |||||||
| FRS + CRP | Events | 1.6 | 0 | 121 | 2 | 1.6 | 0.037 |
| Non Events | 3.2 | 32 | 1168 | 7 | 2.1 | ||
|
| |||||||
| FRS + FH | Events | 2.4 | 1 | 120 | 2 | 0.8 | 0.040 |
| Non Events | 4.9 | 49 | 1148 | 10 | 3.2 | ||
FRS – Framingham Risk Score, IMT – carotid intima- media thickness, CAC – coronary calcium score, FMD- brachial flow mediated dilation, ABI – ankle brachial index, FH- Family history of coronary heart disease, CRP – high sensitivity c-reactive protein.
The respective AUCs for the RS, RS + CAC, RS + CIMT, RS + ABI and RS + FMD were 0.642, 0.766, 0.643, 0.648 and 0.642 respectively for incident CHD in the present cohort. The NRI for RS +CAC, RS + CIMT, RS + ABI, and RS + FMD over RS for incident CHD were 0.528, 0.003, 0.002 and zero respectively. Similarly the AUC for RS, RS + CAC, RS + CIMT, RS + ABI, and RS + FMD were 0.645, 0.742, 0.645, 0.656 and 0.646 respectively for incident CVD. The NRI for RS +CAC, RS + CIMT, RS + ABI, and RS + FMD over RS for incident CVD were 0.415, 0, 0.008 and 0.007 respectively.
Discussion
The current study shows that among six of the most promising novel risk markers CAC provides the highest improvement in discrimination over the FRS and RS in individuals classified as intermediate risk. The present study provides additional support for the use of CAC as a tool for refining cardiovascular risk prediction in individuals classified as intermediate risk by the Framingham Risk Score or the Reynolds Risk Score. To our knowledge this is the first study to compare directly the improvement in risk prediction by several different novel markers in a multi ethnic cohort with intermediate Framingham or Reynolds risk.
Previous studies showed that CIMT, CAC, brachial FMD, ABI, hs-CRP and family history of CHD improve the classification of risk over the FRS, but to varying degrees. Direct comparisons between studies should be made with caution, because they were conducted in different cohorts, did not have uniform definitions of the primary outcome, and had varying duration of follow-up. Nambi et al showed in the Atherosclerosis Risk In Communities (ARIC) study that CIMT is an improved the AUC from 0.742 to 0.750 and had a net clinical NRI of 0.167(4). Polonsky et al showed in a larger subset of the MESA study that CAC is an independent predictor of CHD, improved the AUC from 0.76 to 0.81 and has a net clinical NRI of 0.55 in the intermediate risk stratum(5). Yeboah et al showed that brachial FMD is an independent predictor of incident CVD, did not improve the AUC of the FRS ( 0.74) but has a net clinical NRI ,defined using the 10-20% Framingham risk, of 0.28 (8). Fowles et al showed in a meta-analysis that ABI is an independent predictor of incident CVD, improves the AUC from 0.646 to 0.655, and reclassification of the risk category and modification of treatment recommendations in approximately 19% of men and 36% of women (19). Wilson et al showed in the Framingham Heart Study that hs-CRP is an independent predictor of incident CHD/CVD, improved AUC from 0.795 to 0.865 and 0.799 respectively and had an NRI of 0.118 and 0.056 respectively(20). Sivapalaratnam et al showed in the EPIC-Norfolk study that family history of CHD is an independent predictor of incident CHD with a net clinical NRI of 0.021(5).
Recently, investigators from the Rotterdam study also performed a direct comparison of several novel risk markers, and also found that CAC provided the most robust improvement in risk prediction. Kavousi et al compared the N-terminal fragment of prohormone B-type natriuretic peptide, Von Willebrand factor antigen, fibrinogen levels, homocysteine levels, uric acid levels, hs-CRP, leukocyte count, chronic kidney disease, CAC, CIMT, peripheral artery disease and pulse wave velocity to the FRS using a similar definition of CHD and statistical approach to the current study. The authors found that CAC provided the highest increment in AUC and NRI over the FRS (21). It is noteworthy that the Rotterdam investigators found similar results to the current study, despite differences in the two study populations. The Rotterdam Study participants were all Caucasians and about 13 percent of the cohort had diabetes mellitus.
Even though our study indicates considerable superiority of CAC over several risk markers for risk prediction of CHD and CVD, several other factors should be considered before making broad recommendations about incorporation of CAC into primary prevention screening strategies. One notable concern is that measurement of CAC exposes individuals to a small but non-trivial amount of ionizing radiation (approximately 0.9-1.1 mSv). Recent efforts have been made to standardize equipment and imaging protocols to reduce radiation exposure during CAC imaging (22); however, the extent to which these recommendations have been implemented in general medical practice is not known. Previous studies suggest wide variations in radiation dose during CAC imaging by region/type of institution/protocol (23). Even with the lowest possible radiation dose there remains uncertainty about the magnitude of long-term cancer risks (24). A small risk associated with the lowest possible radiation dose during CAC imaging could translate into a large number of avoidable cancers if CAC were to be uniformly applied to the estimated 23 million people in the US currently classified as intermediate risk by the FRS (25). Similarly, the benefits and risks associated with incidental findings detected during CAC imaging remain unclear. These indirect costs, in addition to the direct financial costs of CAC imaging, need to be weighed against the presumed benefits from better discrimination of subjects at high risk for CHD and CVD events to best determine the role of CAC screening of patients with an intermediate risk for a CHD/CVD event. Thus, the ultimate decision regarding the optimum test to order should not be based solely on improvement in risk prediction afforded by a test but also cost effectiveness, acceptability to patients and the potential risk and benefits associated with the test (26).
The current study has limitations. We limited our analysis to the subset of MESA participants with complete data on all six risk markers, which decreased our sample size. Nevertheless, there were sufficient numbers of events to demonstrate clearly the superiority of CAC over the other measures in head-to-head ROC and NRI analyses. Finally, in MESA we did not specifically define family history of CHD as premature (i.e. before the age of 55 for men and 65 for women). This may have influenced the association of family history with CHD and CVD.
Conclusion
CAC, ABI, hs-CRP and family history are independent predictors of incident CHD/CVD beyond traditional risk factors but have varying degrees of improvement in discrimination and classification of risk within intermediate risk individuals. CAC has the highest improvement in both AUC and NRI when added to the FRS/RS. Additional research is warranted to explore further both the costs and benefits of CAC screening in intermediate risk individuals.
Supplementary Material
Acknowledgement
The Authors would like to thank the investigators, the staff, and the participants of MESA study for their valuable contributions. This research was supported by contracts N01-HC-95159 through N01-HC-95167 and Diversity Supplement R01HL098445 (PI: Jeffery Carr). A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
All authors have nothing to disclose. Joseph Yeboah and Robyn McClelland had complete access to the data.
Joseph Yeboah: writing of MESA proposal, acting as the principal investigator, statistical analysis and manuscript preparation
Robyn McClelland: Statistical analysis and manuscript preparation
Tamar Polonsky: manuscript preparation, editing and critical scientific additions
Gregory L Burke: manuscript preparation, editing and critical scientific contents
Christopher T Sibley: Editing and critical scientific information
Daniel O’Leary: critical expertise, data gathering, editing
J Jeffrey Carr: data gathering, critical expertise, scientific content and editing.
David Goff: manuscript preparation, editing and scientific content
Philip Greenland: manuscript preparation, editing and scientific content.
David Herrington: data gathering, statistical analysis, manuscript preparation and scientific content.
Role of the Sponsors: The NHLBI participated in the design and conduct of MESA. A member of the NHLBI staff served as a coauthor and had input into the collection, management, analysis, and interpretation of the data and in preparation of the manuscript, as did the other coauthors. Although additional members of the NHLBI staff were able to view the manuscript prior to submission, they did not participate in the decision to submit the manuscript or approve it prior to publication. The National Center for Research Resources had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Reference
- 1.Hayward RA, Krumholz HA, Zulman DM, Timbie JW, Vigan S. Optimizing statin treatment for primary prevention of coronary artery disease. Ann Intern Med. 2010;152:69–77. doi: 10.7326/0003-4819-152-2-201001190-00004. [DOI] [PubMed] [Google Scholar]
- 2.Collins GS, Altman DG. An independent external validation and evaluation of QRISK cardiovascular risk prediction: a prospective open chart study. BMJ. 2009;339:b2584. doi: 10.1136/bmj.b2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cholesterol Education Program Expert Panel on Detection. Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballntyne CM. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC study. J. Am. Coll. Cardiol. 2010;55:1600–7. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010 Apr 28;303(16):1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM, Paynter NP, Rafai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–51. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivapalaratnam S, Boekholdt SM, Trip MD, Sandhu MS, Luben R, Kastelein JJ, Wareham NJ, Khaw KT. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. Heart. 2010 Dec;96(24):1985–9. doi: 10.1136/hrt.2010.210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009 Aug 11;120(6):502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010 Oct 26;56(18):1506–12. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenland P, Alpert JS, et al. American College of Cardiology Foundation American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M., Jr. Detrano R Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Polak JF, Pencina MJ, O’Leary DH, D’Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke. 2011 Jul;42(11):3017–21. doi: 10.1161/STROKEAHA.111.625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delong ER, Delong CM, Clarke-Pearson DL. Comparing the area under two or more correlated receiver operating characteristic curves. a non-parametric approach. Biometric. 1998;44:845–857. [PubMed] [Google Scholar]
- 17.Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin. Chem. Lab. Med. 2010;48:1703–11. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women. the Reynolds risk score. JAMA. 2007;297:611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 19.Fowkes FG, Murray GD, Butcher I, et al. Ankle Brachial Index Combined With Framingham Risk Score to Predict Cardiovascular Events and Mortality. A Meta-analysis. JAMA. 2008;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson PW, Pencina M, Jacques P, Selhub J, D’Agostino R, Sr, O’Donnell CJ. C - reactive protein and Reclassification of Cardiovascular Risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008 Nov;1(2):92–7. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavousi M, Elias-Smale S, Rutten JH, Leening MJ, Vliegenthart R, Verwoert GC, Krestin GP, Oudkerk M, de Maat MP, Leebeek FW, Mattace-Raso FU, Lindemans J, Hofman A, Steyerberg EW, van der Lugt A, van den Meiracker AH, Witteman JC. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med. 2012 Mar 20;156(6):438–44. doi: 10.7326/0003-4819-156-6-201203200-00006. [DOI] [PubMed] [Google Scholar]
- 22.Voros S, Rivera JJ, Berman DS, Blankstein R, Budoff MJ, Cury RC, Desai MY, Dey D, Halliburton SS, Hecht HS, Nasir K, Santos RD, Shapiro MD, Taylor AJ, Valeti US, Young PM, Weissman G, Society for Atherosclerosis Imaging and Prevention Tomographic Imaging and Prevention Councils. Society of Cardiovascular Computed Tomography Guideline for minimizing radiation exposure during acquisition of coronary artery calcium scans with the use of multidetector computed tomography: a report by the Society for Atherosclerosis Imaging and Prevention Tomographic Imaging and Prevention Councils in collaboration with the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2011 Mar-Apr;5(2):75–83. doi: 10.1016/j.jcct.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Kim KP, Einstein AJ, Berrington de Gonzalez A. Coronary artery calcification screening: estimated radiation dose and cancer risk. Arch intern med. 2009;169:1188–94. doi: 10.1001/archinternmed.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berrington de González A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F. Land C Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009 Dec 14;169(22):2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford ES, Giles WH, Mokdad AH. The distribution of 10 year risk for coronary heart disease among US adults. J. Am. Coll. Cardiol. 2004;43:1791–6. doi: 10.1016/j.jacc.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 26.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE, Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O’Donnell CJ, Smith SC, Jr, Wilson PW, American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009 May 5;119(17):2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.