Abstract
Importance
Cognitive-Behavioral Therapy for social anxiety disorder is thought to enhance cognitive reappraisal in patients with social anxiety disorder. Such improvements should be evident in cognitive reappraisal-related prefrontal cortex responses.
Objective
To determine whether Cognitive-Behavioral Therapy for social anxiety disorder modifies cognitive reappraisal-related prefrontal cortex neural signal magnitude and timing when implementing cognitive reappraisal with negative self-beliefs.
Design
Randomized controlled trial of Cognitive-Behavioral Therapy for social anxiety disorder versus waitlist control group.
Setting
Psychology department.
Participants
Seventy-five patients with generalized social anxiety disorder randomly assigned to Cognitive-Behavioral Therapy or waitlist.
Intervention
Sixteen sessions of individual-Cognitive-Behavioral Therapy for social anxiety disorder during a study that enrolled patients from 2007 to 2010.
Main Outcome Measures
Negative emotion ratings and functional magnetic resonance blood oxygen-level dependent signal when reacting to and cognitively reappraising negative self-beliefs embedded in autobiographical social anxiety situations.
Results
During reactivity trials, compared to waitlist, Cognitive-Behavioral Therapy produced (a) greater reduction in negative emotion ratings and (b) greater blood oxygen-level dependent signal magnitude in medial prefrontal cortex. During cognitive reappraisal trials, compared to waitlist, Cognitive-Behavioral Therapy produced (c) greater reduction in negative emotion ratings, (d) greater blood oxygen-level dependent signal magnitude in dorsolateral and dorsomedial prefrontal cortex, (e) earlier temporal onset of dorsomedial prefrontal cortex activity, and (f) greater dorsomedial prefrontal cortex-amygdala inverse functional connectivity.
Conclusions and Relevance
Modulation of cognitive reappraisal-related brain responses, timing and functional connectivity may be important brain changes that contribute to the effectiveness of Cognitive-Behavioral Therapy for social anxiety.
Trial Registration
ClinicalTrials.gov identifier: NCT00380731; http://www.clinicaltrials.gov/ct2/show/NCT00380731?term=social+anxiety+cognitive+behavioral+therapy+Stanford&rank=1
Keywords: social anxiety, emotion regulation, CBT, fMRI, reappraisal, brain
INTRODUCTION
Social anxiety disorder (SAD) is characterized by high prevalence (12.1%)1, early onset2, and low rates of spontaneous remission3. SAD is linked to significant impairment in social, educational, and occupational functioning4, 5, and represents a substantial problem for society6, 7. Individuals with SAD experience distressing levels of social fear, humiliation and embarrassment8, and distorted beliefs about the social self9.
COGNITIVE REAPPRAISAL IN SAD
One factor thought to lead to heightened anxiety responses in SAD is a failure to successfully employ cognitive reappraisal (henceforth “reappraisal”). Reappraisal is an emotion regulation strategy that entails reframing the meaning of an emotional stimulus in order to modify its impact. In healthy controls10, 11, neuroimaging studies of reappraisal show increased recruitment in brain networks implicated in cognitive control (dorsolateral (DL), dorsomedial (DM), and ventrolateral (VL) prefrontal cortex (PFC)) and attention (precuneus, superior parietal lobule, right DLPFC, dorsal anterior cingulate cortex)12, as well as decreases in emotion processing brain regions, specifically amygdala13.
Neuroimaging studies have found that, compared to healthy controls, patients with SAD show lesser BOLD signal responses in cognitive control (DLPFC, DMPFC) and attention (medial precuneus, posterior cingulate, bilateral dorsal parietal cortex) brain networks during reappraisal of social threat (harsh facial expressions)14. Furthermore, when reappraising self-generated negative self-beliefs (NSBs), compared to healthy controls, patients with SAD demonstrate temporally delayed PFC activation (DMPFC, bilateral DLPFC, bilateral VLPFC) and less PFC-amygdala inverse functional connectivity15. This inability to implement reappraisal in a timely manner and less successful PFC down-regulation of amygdala responses may be related to heightened levels of anxiety that inhibit recruitment of PFC and/or to deficient reappraisal implementation in the context of anxiety inducing stimuli. What is not known is whether clinical interventions impact the magnitude, timing, and functional connectivity of reappraisal-related PFC responses.
COGNITIVE-BEHAVIORAL THERAPY FOR SAD
Individual Cognitive-Behavioral Therapy (I-CBT) for SAD, as developed by Hope and Heimberg15, has been shown to be an effective treatment for SAD16, 17. One key component of CBT for SAD is cognitive restructuring, which involves reappraisal in the context of exposure to feared social situations and negative self-beliefs (NSBs)18–20. Behaviorally, increases in reappraisal self-efficacy during I-CBT have been shown to mediate the effect of I-CBT on improvement in severity of social anxiety symptoms and to predict clinical status at one-year post-treatment17. Neurally, the first neuroimaging study of group CBT and citalopram treatment for SAD showed that regional cerebral blood flow decreases in amygdala during public speaking was associated with clinical improvement 1-year later21. A recent study of group CBT demonstrated that pre-treatment increases in fMRI BOLD signal in superior and inferior portions of the occipitotemporal cortex in response to angry vs. neutral face stimuli predicted improvement in social anxiety symptoms22. The current study builds on these prior studies by investigating whether I-CBT for SAD impacts BOLD signal magnitude, timing, and functional connectivity in PFC regions implicated in reappraisal of negative self-beliefs embedded in autobiographical social anxiety situations.
THE PRESENT STUDY
Our goal was to investigate the impact of I-CBT for SAD vs. Waitlist (WL) on BOLD signal magnitude and temporal dynamics in a priori regions of interest (ROIs) in the DMPFC, DLPFC, and VLPFC (implicated in reappraising NSBs15) and in the amygdala (implicated in emotion generation13). Additionally, we used a context-dependent functional connectivity analysis to investigate whether CBT influenced PFC-amygdala connectivity. We expected that, compared to WL, I-CBT would result in lesser negative emotion and amygdala responses to NSBs, greater and quicker responses in reappraisal-related PFC regions (DMPFC, DLPFC, VLPFC), and greater inverse functional connectivity between reappraisal-related PFC and emotion-related amygdala.
METHOD
PARTICIPANTS
Participants met DSM-IV23 criteria for a principal diagnosis of generalized SAD based on the Anxiety Disorders Interview Schedule for the DSM-IV-Lifetime (ADIS-IV-L) version24. Of 436 individuals assessed for eligibility, 110 were administered the ADIS-IV-L to determine eligibility (Figure 1). After 35 patients were excluded, the 75 patients who met DSM-IV criteria for a principal diagnosis of generalized SAD were randomly assigned to either immediate I-CBT (n=38) or a WL control group (n=37) who were subsequently offered I-CBT. After dropout from I-CBT (n=6) and WL (n=5), and incomplete neuroimaging data at post-CBT (n=1) and post-WL (n=3), the final sample for this fMRI study consisted of 31 CBT completers and 29 WL completers. Baseline data from 27 of the 60 patients with SAD examined in this study were reported in a previous paper on differential SAD vs. healthy control brain responses to negative self-beliefs15.
Figure 1.
Consolidated Standards of Reporting Trials Diagram for a Randomized Controlled Trial of Individual Cognitive-Behavioral Therapy (I-CBT) vs. Waitlist (WL) Groups.
EXCLUSION CRITERIA
Patients had to pass a MRI safety screen, be right-handed as assessed by the Edinburgh Handedness Inventory25. They were excluded for current pharmacotherapy or psychotherapy, past CBT, and history of neurological disorders. Patients were also excluded if generalized SAD was not determined to be the principal clinical diagnosis. Patients were not excluded if they meet diagnostic criteria for generalized anxiety disorder, agoraphobia without panic attacks, specific phobia, major depression or dysthymia.
PROCEDURE
Patients were recruited through referrals and web listings. They had to pass a telephone screen, the ADIS-IV-L interview conducted in person, and all baseline assessments before being randomly assigned to I-CBT or WL using Efron’s biased coin randomization procedure26, which promotes equal sample sizes throughout the clinical trial. All participants completed the same measures at baseline and again 19 weeks later after I-CBT or WL. This study was approved by the Institutional Review Board at Stanford University, and participants provided written informed consent.
CLINICAL ASSESSMENT
To measure social anxiety symptoms, we administered the Liebowitz Social Anxiety Scale-Self-Report (LSAS-SR27, 28), which has good reliability and construct validity29. Its internal consistency (Cronbach’s alpha = .91) was excellent in this study. To measure SAD-related disability in work, social, and family domains, we administered the Sheehan Disability Scale (SDS30) which has good internal consistency and validty31 and had good internal consistency (alpha = .67) in this study.
INDIVIDUAL-COGNITIVE-BEHAVIORAL THERAPY FOR SAD
I-CBT was delivered by four Ph.D.-level clinical psychologists trained by Dr. Heimberg using Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach, a manualized treatment protocol which included a therapist guide32 and a client workbook33, and consisted of 16 individual one-hour sessions (except for the first in-session exposure session which lasted 1.5 hours) administered over 4 months. I-CBT covered: (1) psychoeducation and orientation to CBT; (2) cognitive restructuring skills; (3) graduated exposure to feared social situations, within session and as homework; (4) examination and modification of core beliefs; and (5) relapse prevention and termination16, 32, 33.
All 16 sessions for each client were digitally recorded and rated using the Cognitive-Behavioral Therapy for Social Anxiety Disorder: Therapist Adherence Scale32 by research team members who had clinical training and extensive familiarity with the CBT treatment adherence. They rated each session according to various criteria, using a 5-point Likert-type scale ranging from 1 (ineffective) to 5 (extremely effective). Average adherence ratings had to be greater than 4 to be considered “in protocol.” All four study therapists achieved this standard for each therapy case (overall Mean = 4.61, SD = 0.24).
EXPERIMENTAL TASK
Patients identified four personally-salient autobiographical social situations characterized by social anxiety, humiliation, and embarrassment, and composed (a) a single paragraph to describe each situation, and (b) situation-specific NSBs that were used to probe reactivity and reappraisal.
Before scanning, patients were trained using methods developed by Gross and Ochsner34, 35 to either “REACT” by considering how the NSB reflected something true about themselves, or “REFRAME” by using reappraisal to “actively reframe the belief by thinking in a way that re-interprets the content of the belief and thereby make the belief less negative and toxic for you.” For example, if the NSB is “NO ONE LIKES ME,” reframing may be telling yourself “That is not always true,” “Some people like me,” or “This is only a thought, not a fact.”
During scanning, patients read their autobiographical social situations with NSBs embedded in the unfolding story. After each NSB, patients rated “How negative do you feel?” (1=not at all to 5=very much).
The task involved five situations. The first was an experimenter-composed neutral situation about cleaning a car that was used to obtain baseline emotion ratings and brain responses. The second through fifth situations were participant-generated autobiographical social anxiety situations with idiographic NSBs.
Three situations were presented in a first run lasting 9 minutes, 21 seconds, followed by two situations in a second run of 6 minutes, 24 seconds. The sequence of 5 situations was fixed (neutral, react NSB, reappraise NSB, react NSB, and reappraise NSB) for several reasons. First, we wanted to control for order effects at the pre- and post-treatment assessments. Second, we wanted to obtain an unbiased estimate of brain responses to neutral statements (prior to emotional reactivity). Third, we wanted to assess react NSB prior to reappraise in order to minimize the effect of reappraisal on reactivity. Negative emotion ratings during the first and second react NSBs blocks did not differ (p>.45).
Each situation consisted of an instruction to react or reframe (6 seconds), 16 sentences describing the situation (3 seconds each) in white font against a black background, 10 NSBs (9 seconds each) embedded in the unfolding story in uppercase letters that flashed 9 times to maximize attentional engagement with the text (850 ms on + 150 ms off), and a negative emotion rating (3 seconds) after each NSB (Figure 2). NSBs appeared in white font for react trials and green font for reframe trials as a visual reminder to react or reappraisal.
Figure 2.
Experimental Design. Components and structure of one autobiographical social situation trial. After each negative self-belief (NSB), participants provide a negative emotion rating from 1=not negative to 5=extremely negative.
The experimental task was administered during a 1.5 hours scanning session at baseline and again post-CBT/WL. After scanning, patients rated: (1) how successful they were at implementing reappraisal during reframe trials on a scale of 0% to 100% successful and (2) how often they used reappraisal during the react trials on a scale from 0 (not at all) to 50 (moderately) to 100 (always).
IMAGE ACQUISITION
We used a GE 3-T Signa magnet with a T2*-weighted gradient echo spiral-in/out pulse sequence36 to acquire 630 functional volumes from 22 axial slices (repetition time=1500 milliseconds, echo time=30 milliseconds, flip angle=60°, field of view=22 cm, matrix=64×64, resolution=3.438 mm2 × 4.5 mm). High-resolution anatomical scans were acquired using fast spin-echo spoiled-grass (.85942 × 1.5 mm; field of view=22 cm, frequency encoding=256).
fMRI DATA PROCESSING
We used AFNI software37 to remove outliers, register, motion correct, spatially smooth (4 mm3 isotropic kernel), high-pass filter (.011 Hz), linear detrend, and convert into percent signal change each functional run. No volumes demonstrated motion in the x, y, or z directions in excess of ±0.8 mm. There was no evidence of stimulus-correlated motion (all ps>.30).
fMRI STATISTICAL ANALYSIS
We used 3dDeconvolve to conduct a multiple-regression that included removal of mean, linear, and quadratic trends, and motion-related variance in the BOLD signal. Regressors for the neutral statements, react, and reappraise were convolved with the gamma variate model38 of the hemodynamic response function.
We converted brain maps into Talairach atlas space39 and second-level group statistical parametric maps were produced according to a random-effects model. We investigated 10 a priori PFC ROIs in the DMPFC, dorsal anterior cingulate cortex, and bilateral medial anterior PFC, DLPFC, and VLPFC that were previously identified as more actively and rapidly recruited when reappraising NSBs in non-anxious healthy adults compared to patients with SAD15. Because of its importance in anxiety disorders and emotional reactivity, we also investigated a priori Talairach atlas defined amygdala ROIs.
We created spherical masks (radius = 7mm, volume = 1,437mm3) centered on the peak xyz Talairach coordinates within each reappraisal-related ROI and amygdala masks, extracted mean BOLD responses, and conducted a MANOVA with the Sidak correction for multiple comparisons to examine between-group differences in pre-to-post BOLD signal changes for react NSB vs. neutral and reappraise NSB vs. neutral contrasts. We removed one participant from CBT and one from WL with BOLD signal more than 3 SDs from the mean in the left amygdala. We also provide results for a supplemental whole-brain analysis of differential CBT vs. WL change for react NSB vs. neutral and reappraise NSBs vs. neutral.
We investigated neural temporal dynamics in only the a priori ROIs that showed differential pre-to-post-CBT/WL change for react NSBs versus neutral (1 ROI) and reappraise NSBs versus neutral (2 ROIs). To do this, we conducted a 2 Group (CBT, WL) × 6 time points (x 1.5 seconds = 9 seconds) repeated-measures ANOVA (Huynh-Feldt corrected for autocorrelation in time series) on BOLD signal for each trial (with a 6 seconds shift to account for the temporal delay in the hemodynamic response). A follow-up paired t-test tested for differential early (0–3 seconds) vs. late (6–9 seconds) BOLD responses.
To investigate PFC-amygdala circuitry, we implemented a context-dependent functional connectivity analysis for each group at pre and post CBT/WL for only one contrast (reappraise vs. react NSBs). To reduce false positive detection, the connectivity analysis was seeded to the single reappraisal-related a priori ROI (DMPFC) that showed both BOLD signal magnitude and timing results. We then computed pre-to-post change scores and conducted a between-group t-test to identify the interaction of group by time on PFC-amygdala connectivity. We focused the connectivity analysis within the a priori Talairach-defined amygdala search region using a t-value associated with p<.025 and cluster volume ≥160 mm3.
RESULTS
PRELIMINARY ANALYSES
Patients in I-CBT and WL groups did not differ significantly in gender, age, education, ethnicity, yearly income, marital status, current or past Axis I comorbidity, past psychotherapy or pharmacotherapy, age at symptom onset, or years since symptoms onset (Supplemental Table 1). Ethnicity was self-reported by research participants in accordance with NIH requirements.
As reported elsewhere17, an intent-to-treat analysis showed that, compared to WL, I-CBT resulted in significantly greater reduction of social anxiety symptoms, ΔI-CBT=−29.7 vs. ΔWL=−8.2; F(2,73)=20.0, p<.001, ηp2=.21, and disability, ΔI-CBT=−9.3 vs. ΔWL=−1.2; F(2,73)=5.6, p<.05, ηp2=.07. Using the Jacobson and Truax40 method, we determined that 19 (61.3%) of 31 patients who completed I-CBT and the fMRI assessments demonstrated clinically significant reduction in social anxiety symptoms.
As a manipulation check, at post-MR scanning, we examined the self-reported success in implementing reappraisal of NSBs. A 2 Group (I-CBT, WL) × 2 Time (pre, post) repeated-measures ANOVA yielded a significant group by time interaction (F(2,55)=18.14, p<.001, η p2 =.25), no effect of group (F(1,56)=1.81, p>.18), but a significant effect of time (F(1,56)= 27.63, p<.001, η p2 =.33). Follow-up paired t-tests showed reappraisal success increased from pre-to-post-I-CBT (Mean±SD; Pre: 34.68±21.09 vs. Post: 69.52±17.62; t(30)=6.55, p<.001, η p2=.59) and did not change from pre-to-post-WL (Pre: 44.81±19.97 vs. Post: 48.46±23.48; t(25)=0.75, p>.46). There was no significant difference between groups in reappraisal success at baseline (t(55)=1.85, p>.07).
We also examined at post-MR scanning self-reported use of reappraisal during react NSBs trials when participants were not instructed to implement reappraisal (i.e., uncued or spontaneous use of reappraisal). A 2 Group (I-CBT, WL) × 2 Time (pre, post) repeated-measures ANOVA yielded a significant group by time interaction (F(2,52)=4.34, p<.05, η p2=.08), no effect of group (F(1,52)=0.35, p>.56), but a significant main effect of time (F(1,52)= 4.34, p<.05, η p2=.08). Follow-up t-tests showed that spontaneous reappraisal during react NSBs increased from pre-to-post-I-CBT (Pre: 23.67±27.57 vs. Post: 37.75±30.13; t(29)=2.91, p<.01) but did not change in the WL group (Pre: 26.88±25.27 vs. Post: 26.88±21.46; t(23)=0, p>.99). There was no significant difference between groups in reappraisal during react trials at baseline (t(53)=0.57, p>.57).
RESPONSES TO NEGATIVE SELF-BELIEFS
Behavioral Responses
A 2 Group (I-CBT, WL) × 2 Time (baseline, post) repeated-measures ANOVA on negative emotion ratings resulted in a group by time interaction when reacting to NSB (F(2,58)=8.63, p=.005, η p2=.13), a main effect of time (F(1,58)=16.24, p=.001, η p2=.23), and no effect of group (F(1,58)=1.37, p>.24) (Figure 3). Follow-up t-tests showed CBT-related decreases in negative emotion (t(30)=5.18, p<.001, η p2=.47) and no change in the WL group (t(27)=0.74, p>.46). There was no significant difference between groups at baseline (t(59)=0.76, p>.45).
Figure 3.

Changes in Negative Emotion Intensity Ratings for React Negative Self-Beliefs and Cognitive Reappraisal of Negative Self-Beliefs in Patients with Social Anxiety Disorder (SAD) Before and After Individual Cognitive-Behavioral Therapy (I-CBT) vs. Waitlist (WL) Groups. Negative emotion ratings after the offset of each stimulus were provided by participants in response to “How negative do you feel?” (1=not at all, 2=slightly, 3=moderately, and 4=very much, 5=extreme). * p<.001; error bars = standard error of the mean.
Brain Response Magnitude
When we examined a priori amygdala ROI responses for the contrast of react NSBs vs. neutral statements with a 2 Group (CBT, WL) × 2 Time (Baseline, Post) repeated-measures ANOVA, there were no interactions or main effects in the left (all ps>.11) or right amygdala (ps >.06).
For the 10 reappraisal-related a priori PFC ROIs, a MANOVA with the Sidak correction for multiple comparisons on pre-to-post BOLD signal change for react NSBs vs. neutral statements yielded only between-group difference in the MPFC region (F(2,58)=4.29, p<.05, η p2=.07) consisting of CBT increases (t(30)=2.20, p<.05, η p2=.14) but no change in WL (t(27)=1.26, p>.21).
Brain Response Temporal Dynamics
The examination of BOLD signal temporal dynamics in the MPFC across the six 1.5 second time points for react NSBs versus neutral yielded no interaction (p>.80) or main effects of group (p>.39) or time (p>.35).
COGNITIVE REAPPRAISAL OF NEGATIVE SELF-BELIEFS
Behavioral Responses
A 2 Group (I-CBT, WL) × 2 Time (baseline, post) repeated-measures ANOVA on negative emotion ratings during reappraise NSBs resulted in a group by time interaction (F(2,58)=21.59, p<.001, η p2=.28), no effect of group (F(1,58)=1.66, p>.20), but a significant main effect of time (F(1,58)=41.29, p<.001, η p2=.42) (Figure 3). Follow-up paired t-tests showed CBT-related decreases in negative emotion (16.1%; t(30)=8.93, p<.001, η p2=.73) and no change in the WL group (1.7%; t(27)=1.11, p>.27). There was no difference between groups at baseline (t(59)=1.59, p>.12).
Brain Response Magnitude
For the a priori amygdala ROIs, 2 Group (CBT, WL) × 2 Time (Baseline, Post) repeated-measures ANOVA on BOLD responses for reappraise NSB vs. neutral statements yielded no main or interaction effects in the left (all ps>.15) and right amygdala (all ps>.06).
For the 10 reappraisal-related ROIs, a MANOVA with the Sidak correction for multiple comparisons on pre-to-post change for reappraise NSBs vs. neutral statements yielded overall significance between-groups (F(10,47)=3.22, p=.003, η p2=.41). For the corrected model, there was evidence of between-group difference in only two regions: DMPFC (Δ=.10, 95% CI [.01–.19]; F(2,58)=4.70, p=.035, η p2=.08), consisting of CBT increases (Δ=.11; t(30)=3.36, p<.002, η p2=.27), but no change for WL (Δ=.01; t(27)=0.23, p>.82), and left DLPFC (Δ=.08, 95% CI [.01–.15]; F(2,58)=5.20, p<.026, ηp2=.09), consisting of CBT increases (Δ=.06; t(30)=2.37, p<.05, ηp2=.16) and no change for WL (Δ=−.02; t(27)=0.86, p>.39) (Figure 4). The supplemental whole-brain analysis confirmed the ROI-based observation of increased BOLD responses in DMPFC and left DLPFC (Supplemental Table 2).
Figure 4.
Changes in Brain Responses in Dorsomedial Prefrontal Cortex (DMPFC) and Left Dorsolateral Prefrontal Cortex (DLPFC) A Priori Cognitive Reappraisal-related Prefrontal Cortical Brain Regions of Interest in Patients with Social Anxiety Disorder (SAD) Before and After Individual Cognitive-Behavioral Therapy (I-CBT) vs. Waitlist (WL) Groups. The red dot represents the spherical region of interest mask used in the analysis. BOLD signal represents pre-to-post change scores in BOLD signal responses.
Brain Response Temporal Dynamics
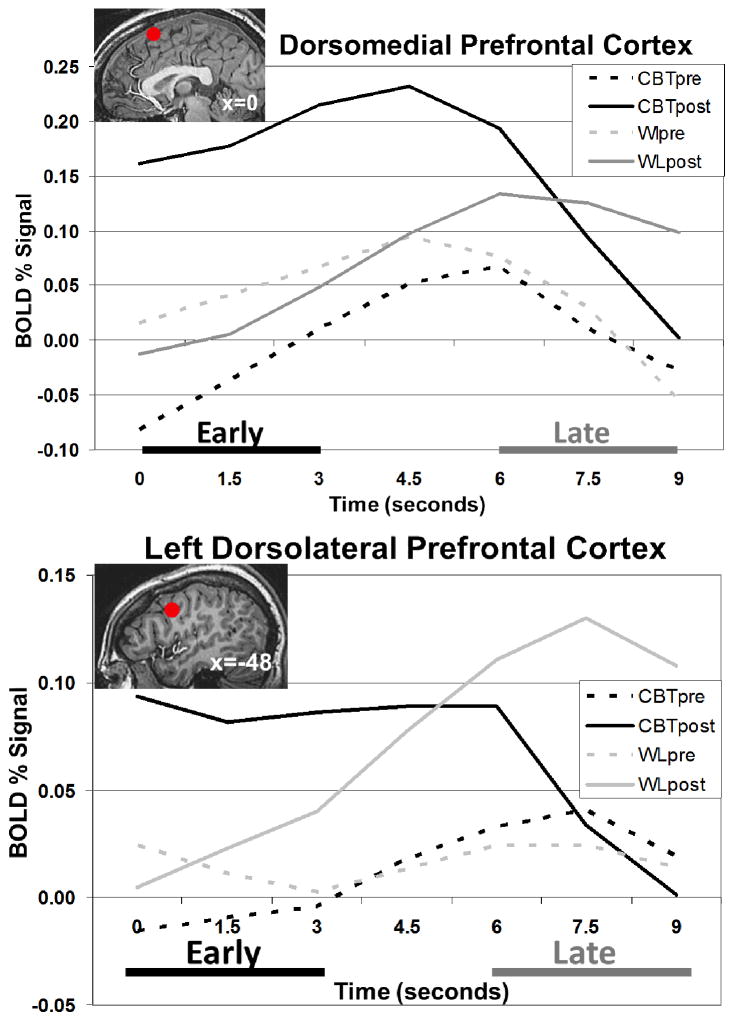
For the DMPFC, there was a significant interaction of group by time (F(8,52)=2.42, p<.05, ηp2=.04), and no main effects of group (p>.34) or time (p>.43) (Figure 5). Follow-up pre-to-post within-group paired t-tests showed that the interaction was driven by linear changes (t=2.79, p=.007, η p2=.12) characterized by significantly increased early (0–3s) vs. late (6–9s) responses in the I-CBT group (early: .23% vs. late: .05%; t(30)=2.22, p<.05) and decreased early vs. late responses in the WL group (early: −.15% vs. late: .01%; t(27)=2.05, p<.05). For the left DLPFC, the same analysis yielded no main or interaction effects (ps>.30).
Figure 5.
Blood Oxygen Level Dependent (BOLD) Signal Time Series in Dorsomedial Prefrontal Cortex (DMPFC) and Left Dorsolateral Prefrontal Cortex (DLPFC) During Reappraise Negative Self-Beliefs (NSBs) vs. Read Neutral Statements.
Context-Dependent Functional Connectivity
The DMPFC-seeded functional connectivity analysis for reappraise vs. react negative self-beliefs showed that, compared to WL, CBT produced greater inverse connectivity between the DMPFC and left amygdala and the right hippocampus and positive connectivity in the medial PFC and two dorsolateral PFC regions (Table 1, Figure 6).
Table 1.
Differential Between-Group Pre-to-Post Change in Dorsomedial Prefrontal Cortex-Seeded Context-Dependent Functional Connectivity of Cognitive Reappraisal vs. React Negative Self-Beliefs
| Brain Regions | Pre-to-Post Change | xyz | Vol (mm3) | t-value |
|---|---|---|---|---|
| Positive FC | ||||
| Medial PFC | CBT>WL | 0 25 53 | 266 | 3.74 |
| R DLPFC | CBT>WL | 38 45 29 | 583 | 3.71 |
| R DLPFC | CBT>WL | 41 56 15 | 848 | 3.79 |
| Inverse FC | ||||
| R Posterior Hippocampus | CBT<WL | 21 −44 −2 | 583 | 3.50 |
| L Amygdala a | CBT<WL | −20 −9 −18 | 160 | 2.95 |
Note. xyz = Talairach coordinates, t-value ≥2.91, voxel p<.005, minimum cluster volume threshold ≥266 mm3 (5 voxels × 3.44 mm2 × 4.5 mm), cluster-wise p<.01. BA=Brodmann Area, BOLD=blood oxygen dependent, FC= functional connectivity, L=left, PFC=prefrontal cortex, DLPFC=dorsolateral prefrontal cortex, R=right, Vol=volume.
For this cluster within the a priori Talairach-defined amygdala search region, functional connectivity was observed at a more lenient threshold consisting of t-value≥2.30, voxel p<.025 and minimum cluster volume threshold ≥160 mm3.
Figure 6.
Differential Between-Group Pre-to-Post Change in Dorsomedial Prefrontal Cortex-Seeded Context-Dependent Functional Connectivity of Cognitive Reappraisal vs. React Negative Self-Beliefs
1=MPFC, 2=right DLPFC, 3=right MFG, 4=DMPFC seed, 5=left hippocampus, 6=left amygdala. For the whole-brain, thresholding consisted of per voxel p<.005 and cluster volume threshold ≥263 mm3. Within the amygdala search region, thresholding consisted of per voxel p<.025 and cluster volume threshold ≥160 mm3.
DISCUSSION
This study found that, compared to WL, I-CBT for SAD resulted in reduction of negative emotions when reacting and reappraising NSBs, no change in amygdala responses, and changes in BOLD signal magnitude, temporal dynamics, and functional connectivity during cognitive reappraisal of NSBs in patients with SAD.
RESPONSES TO NEGATIVE SELF-BELIEFS
When reacting to NSBs, compared to WL, I-CBT resulted in a significant reduction of self-reported negative emotion. The absence of a change in negative emotion from pre-to-post WL suggests that there was no habituation to NSBs from time 1 to time 2 assessments. Reduction in emotional reactivity to NSBs post-CBT may be associated with extensive practice in cognitive restructuring during exposures during CBT, which likely leads to greater skill in implementing reappraisal strategies. The decreases in negative emotion from pre-to-post-CBT may reflect increased spontaneous (uncued) reappraisal during react trials, an interpretation that is consistent with CBT participants’ greater reports of reappraisal during react trials.
Neurally, there were no interactions or main effects of group and time in the left or right amygdalae, suggesting that amygdala reactivity to NSBs remained consistent across time and across groups. While some studies have observed CBT-related reductions in amygdala responses using PET during public speaking21, the ability to detect changes in the amygdala using fMRI depends very much on task design, timing, and speed of habituation. Our baseline study showed rapid habituation of amygdala responses during both react and reappraise NSBs in both patients with SAD and healthy controls 15. There was, however, a single interaction of group by time in the medial PFC. This region is involved in reappraisal in general41, 42 and is specifically implicated in reappraisal of negative emotion15. Like the negative emotion ratings, this may reflect spontaneous implementation of reappraisal even when uninstructed.
COGNITIVE REAPPRAISAL OF NEGATIVE SELF-BELIEFS
Behaviorally, during reappraisal of NSBs, compared to WL, I-CBT resulted in greater reduction of self-reported negative emotion experience. The effect size (η p2) of this reduction was larger for reappraisal (0.73) than for react NSB (0.47), which may represent the differential impact of I-CBT on cued reappraisal versus spontaneous reappraisal. These behavioral results are consistent with CBT participants’ reports of greater success at using reappraisal. The absence of change in reappraisal-related reduction of negative emotion from pre-to-post-WL highlights the stability of reappraisal deficits in patients with SAD when untreated.
Neurally, group by time interactions occurred in a priori DMPFC and left DLPFC ROIs, which were characterized by increases following I-CBT and no change following WL. These regions are implicated in the cognitive control of emotion and usually co-activate when down-regulating negative emotion42. A recent meta-analysis of emotion regulation processes has shown that, in contrast to fear extinction and placebo control, reappraisal uniquely involves left dorsolateral and DMPFC41.
Prior investigations of neural temporal dynamics when implementing reappraisal of NSBs15 have demonstrated differential timing in reappraisal-related PFC regions consisting of quicker recruitment in healthy controls and delayed recruitment in patients with SAD. In the present study, at post-WL, patients with SAD continued to show delayed recruitment of left dorsolateral and DMPFC. Post-CBT, however, there was a shift to the normative earlier recruitment of DMPFC, which may be related to enhanced ability to access and implement reappraisal strategies and/or due to reduced emotional reactivity to NSBs (which might interfere with implementing reappraisal effectively). Figure 5 shows a post-CBT pattern of greater earlier recruitment of DLPFC and later non-recruitment similar to DMPFC. However, the BOLD signal magnitude was too small to yield significance. This differential pattern raises the question of whether DLPFC and DMPFC serve different cognitive functions at different time points during reappraisal.
The present findings – as well of those of other studies – suggest that the DMPFC plays a key role in emotion regulation. Studies have demonstrated that greater DMPFC activity is related to greater reappraisal success in both healthy controls and patients with SAD14, 43. In the present study, in addition to BOLD signal magnitude increases in DMPFC during reappraisal following I-CBT, there was evidence that CBT altered the link between DMPFC activity and amygdala response.
The DMPFC-seeded functional connectivity analysis showed a group by time DMPFC-left amygdala interaction driven by inverse DMPFC-amygdala connectivity at post-CBT only and not at baseline in either group or after WL. Interestingly, while there was no overall CBT-related BOLD signal magnitude change in amygdala responses, these results highlight that distributed rather than absolute changes in limbic-PFC patterns may be more reflective of underlying abnormalities in SAD and also in understanding one neurobiological mechanism of change during CBT. The DMPFC positive connectivity with three more PFC regions converges with prior evidence that DMPFC is important for effective top-down cognitive reappraisal of negative emotion in SAD14, 15, 42. The inverse DMPFC-hippocampus connectivity may be related to lesser activation of memories associated with the autobiographical situation and NSBs.
CLINICAL IMPLICATIONS
Findings from this study highlight CBT-related enhancement of reappraisal, specifically, decreased negative emotion experience along with greater and quicker recruitment of reappraisal-related DMPFC neural responses which are related to lesser amygdala responses to NSBs. This reinforces the clinical insight that emotion regulation and clinical symptoms are modified by CBT.
Modifiable temporal dynamics of reappraisal suggests that it might be clinically valuable to have clients record how quickly they can volitionally implement reappraisal following emotional triggers in social situations. This could be experimentally controlled during therapy sessions and measured during in vivo exposures outside of therapy sessions. Bringing attention to the timing of reappraisal and what factors enhance or interfere with more rapid implementation of reappraisal strategies could itself be a specific clinical insight that can be incorporated into the cognitive restructuring and exposure components of CBT protocols for treating anxiety disorders. Specifically, research needs to determine whether different forms of attention training (e.g., that reduce self-focused attention) might impact the timing of reappraisal in SAD.
Equally intriguing is the finding indicating that CBT enhances not only cued reappraisal, but also spontaneous reappraisal. This may be due to the generalization of the skills learned during CBT and may reflect a shift from initially effortful to subsequently spontaneous or automatic implementation of reappraisal. This raises intriguing questions about the extent to which the association of cued and spontaneous reappraisal (and their neural timing) is related to (a) clinical symptoms in patients with SAD and (b) immediate and longer-term treatment outcome of CBT.
LIMITATIONS AND FUTURE DIRECTIONS
The current study is limited to inferences about reappraisal of participant-generated NSBs during autobiographical recall of personally meaningful events. Although this represents a key target of psychotherapy, it will be useful to investigate the impact of CBT on reappraisal of other-focused negative beliefs, and on reappraisal to enhance positive experiences (i.e., up-regulate positive emotions), which may also be important skill trained in CBT. Neurally, CBT for SAD yielded significant interactions in three of the 10 normative reappraisal-related a priori PFC ROIs. The functional connectivity analysis provided evidence for the co-activation in three more PFC regions implicated in cognitive reappraisal. Further research is necessary to identify which PFC regions contribute to longer-term treatment responses in SAD.
Our focus on one type of therapy for one type of disorder was dictated both by theory and by the available literature. Future research could investigate how different forms of CBT (e.g., individual vs. group) and other clinical interventions for SAD influence the temporal dynamics of brain networks involved in different emotion regulation strategies. It will be important to examine how these therapeutic effects generalize to other disorders.
Supplementary Material
Acknowledgments
This research was supported by an NIMH Grant R01 MH076074, awarded to James Gross, Ph.D. Richard Heimberg, Ph.D. is the author of the commercially available CBT protocol which was utilized in this study. We wish to thank Gary Glover, Ph.D. for his technical assistance with magnetic resonance imaging. Philippe Goldin, who is independent of any commercial funder had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
None of the authors of this manuscript have any biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Otto MW, Pollack MH, Maki KM, et al. Childhood history of anxiety disorders among adults with social phobia: Rates, correlates, and comparisons with patients with panic disorder. Depression and Anxiety. 2001;14:209–213. doi: 10.1002/da.1068. [DOI] [PubMed] [Google Scholar]
- 3.Keller MB. The lifelong course of social anxiety disorder: A clinical perspective. Acta Psychiatrica Scandinavica, Supplement. 2003;108(417):85–94. doi: 10.1034/j.1600-0447.108.s417.6.x. [DOI] [PubMed] [Google Scholar]
- 4.Stein MB, Kean YM. Disability and quality of life in social phobia: Epidemiologic findings. American Journal of Psychiatry. 2000;157:1606–1613. doi: 10.1176/appi.ajp.157.10.1606. [DOI] [PubMed] [Google Scholar]
- 5.Schneier FR, Heckelman LR, Garfinkel R, et al. Functional impairment in social phobia. Journal of Clinical Psychiatry. 1994;55:322–331. [PubMed] [Google Scholar]
- 6.Acarturk C, Smit F, de Graaf R, van Straten A, ten Have M, Cuijpers P. Economic costs of social phobia: A population-based study. Journal of Affective Disorders. 2009;115(3):421–429. doi: 10.1016/j.jad.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Patel A, Knapp M, Henderson J, Baldwin D. The economic consequences of social phobia. J Affect Disord. 2002;68(2–3):221–233. doi: 10.1016/s0165-0327(00)00323-2. [DOI] [PubMed] [Google Scholar]
- 8.Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371(9618):1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 9.Goldin PR, Ramel W, Gross JJ. Mindfulness meditation training and self-referential processing in social anxiety disorder: Behavioral and neural effects. Journal of Cognitive Psychotherapy. 2009;23(3):242–256. doi: 10.1891/0889-8391.23.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 11.Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.LeDoux J. The amygdala. Curr Biol. 2007;17(20):R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66(2):170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological Psychiatry. 2009;66(12):1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledley DR, Heimberg RG, Hope DA, et al. Efficacy of a Manualized and Workbook-Driven Individual Treatment for Social Anxiety Disorder. Behavior Therapy. 2009;40(4):414–424. doi: 10.1016/j.beth.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Goldin P, Werner K, Ziv M, et al. Cognitive reappraisal self-efficacy mediates the effects of individual cognitive-behavioral therapy for social anxiety disorder. Journal of Consulting and Clinical Psychology. doi: 10.1037/a0028555. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social phobia: Diagnosis, assessment, and treatment. New York: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- 19.Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 20.Heimberg RG, Brozovich FA, Rapee RM. A cognitive-behavioral model of social anxiety disorder: Update and extension. In: Hofmann SG, DiBartolo PM, editors. Social anxiety: Clinical, developmental, and social perspectives. 2. New York: Academic Press; 2010. pp. 395–422. [Google Scholar]
- 21.Furmark T, Tillfors M, Marteinsdottir I, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archives of General Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- 22.Doehrmann O, Ghosh SS, Polli FE, et al. Predicting Treatment Response in Social Anxiety Disorder From Functional Magnetic Resonance Imaging. Arch Gen Psychiatry. 2012:1–11. doi: 10.1001/2013.jamapsychiatry.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author: American Psychiatric Association; 1994. [Google Scholar]
- 24.DiNardo PA, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: Lifetime version (ADIS-IV-L) New York, NY: Oxford University Press; 1994. [Google Scholar]
- 25.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58(3):403–417. [Google Scholar]
- 27.Liebowitz MR. Social phobia. Modern Problems of Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- 28.Fresco DM, Coles ME, Heimberg RG, et al. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine. 2001;31(6):1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- 29.Rytwinski NK, Fresco DM, Heimberg RG, et al. Screening for social anxiety disorder with the self-report version of the Liebowitz Social Anxiety Scale. Depression and Anxiety. 2009;26:34–38. doi: 10.1002/da.20503. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan DV. The anxiety disease. New York: Scribner; 1983. [Google Scholar]
- 31.Hambrick JP, Turk CL, Heimberg RG, Schneier FR, Liebowitz MR. Psychometric properties of disability measures among patients with social anxiety disorder. Journal of Anxiety Disorders. 2004;18(6):825. doi: 10.1016/j.janxdis.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Hope DA, Heimberg RG, Turk CL. Therapist guide for managing social anxiety: A cognitive-behavioral therapy approach. New York: Oxford University Press; 2006. [Google Scholar]
- 33.Hope DA, Heimberg RG, Juster HR, Turk CL. Managing social anxiety: A cognitive-behavioral approach. San Antonio, TX: The Psychological Corp; 2000. [Google Scholar]
- 34.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 35.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 36.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 37.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 38.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 39.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 40.Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59(1):12. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 41.Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58(1):275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 42.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.