Summary
It has been long appreciated that during meiosis DNA replication is coordinated with the subsequent formation of the double-strand breaks (DSBs) that initiate recombination, but a mechanistic understanding of this process was elusive. We now show that in yeast the replisome-associated components Tof1 and Csm3 physically associate with the Dbf4-dependent Cdc7 kinase (DDK) and recruit it to the replisome, where it phosphorylates the DSB-promoting factor Mer2 in the wake of the replication fork, synchronizing replication with an early prerequisite for DSB formation. Recruiting regulatory kinases to replisomes may be a general mechanism to ensure spatial and temporal coordination of replication with other chromosomal processes.
Introduction
Chromosome replication, repair, and segregation are regulated to ensure their fidelity and to integrate them with one another and other cell-cycle events. Some of this control is cell-wide, e.g., via oscillation of cyclin-dependent kinases (CDKs), but spatially patterned regulation is often critical. For example, replication is coordinated locally with sister chromatid cohesion (Sherwood et al., 2010) and reconstitution of chromatin (Smith and Whitehouse, 2012). Few regulatory modules connecting replication to other processes are understood in detail.
Replication is also coordinated with initiation of meiotic recombination. Meiosis appends two rounds of chromosome segregation to one round of DNA replication to make haploid gametes. During meiosis in most organisms, homologous recombination occurs at many locations across the genome. Recombination promotes pairing and segregation of homologous chromosomes and increases genetic diversity, but there is also potential for harm if DSBs are repaired incorrectly or not at all (Hochwagen and Amon, 2006). Thus, cells tightly regulate Spo11, the protein that generates DSBs (Keeney, 2007; Murakami and Keeney, 2008).
DSBs in Saccharomyces cerevisiae usually occur ~90 min after replication and break timing is dictated by local replication timing (Borde et al., 2000). Yeast chromosome (chr) III has seven major replication origins (ARS, autonomously replicating sequences) (Newlon et al., 1993) (Figure 1A, right). In wild type (ARS+), meiotic DNA replication occurs at similar times on both arms of chr III, but left-arm replication can be delayed ~30 min by inactivating that arm’s origins (arsΔ). This delays DSB formation on the left arm by the same margin, without affecting timing elsewhere (Borde et al., 2000). In ARS+/arsΔ heterozygotes, delay of both replication and DSBs occurs only on the origin-deleted copy of chr III, so this DSB control works in cis (Murakami et al., 2003). In Schizosaccharomyces pombe, altering origin-firing patterns changes DSB frequency, thus replication-DSB coordination may be conserved (Wu and Nurse, 2014).
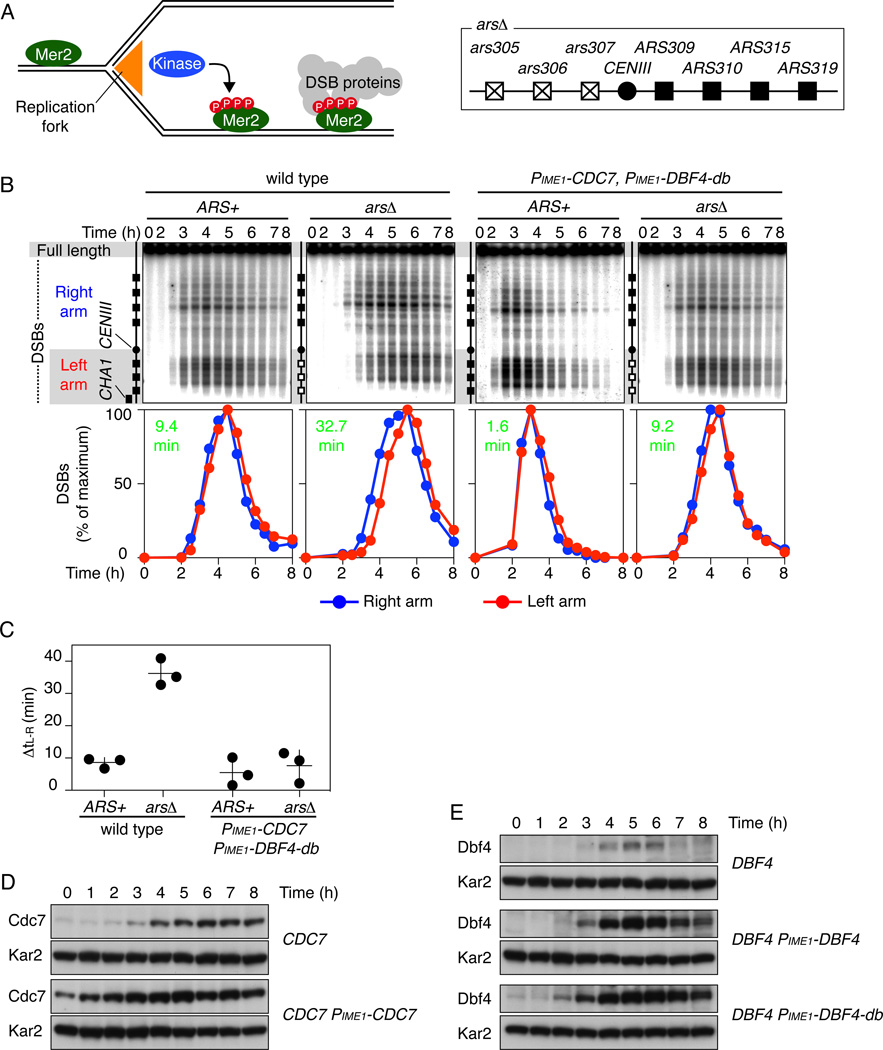
Figure 1. Replication-DSB coordination requires limiting DDK levels.
(A) Left: A kinase (DDK and/or CDK-S) phosphorylates Mer2 specifically on replicated chromatin, promoting recruitment of other proteins needed for DSB formation. Right: Chr III right-arm origins (filled boxes) and deleted left-arm origins (crossed-out boxes).
(B) DSB kinetics. Upper panels: Southern blots of genomic DNA extracted during sporulation and separated by PFGE. Chr III was detected with a CHA1 probe. Bottom panels: normalized, Poisson-corrected DSB frequencies; green numbers are between-arm time differences (ΔtL-R).
(C) DDK overproduction suppresses the DSB delay caused by delaying replication. Each point is an independent culture; bars are mean ± SD.
(D, E) Western blot analyses of wild type and DDK-overexpressing strains. Kar2 is a loading control. DBF4-db is a destruction box mutant (R62A, F65A (Ferreira et al., 2000)). Also see Figures S1 and S2.
How is temporospatial coordination achieved? It was once thought that replication is a strict prerequisite for DSBs (Borde et al., 2000; Smith et al., 2001), but Spo11 can break unduplicated chromosomes efficiently when the replication initiation factor Cdc6 is depleted in S. cerevisiae (Blitzblau et al., 2012; Hochwagen et al., 2005) or when replication is blocked in S. pombe (Murakami and Nurse, 2001; Ogino and Masai, 2006; Tonami et al., 2005). Thus, replication is dispensable for DSBs per se.
An alternative hypothesis builds on DSB control by the cell cycle regulatory kinases CDK-S (CDK plus an S-phase cyclin, Clb5 or Clb6) and DDK (Cdc7 kinase and its regulatory subunit Dbf4) (Murakami and Keeney, 2008). DSB formation requires that both of these kinases phosphorylate Mer2, one of nine proteins required along with Spo11 for DSB formation (Henderson et al., 2006; Sasanuma et al., 2008; Wan et al., 2008). Mer2 associates with chromosomes independently of phosphorylation, but phosphorylation allows it to recruit other DSB proteins (Henderson et al., 2006; Panizza et al., 2011; Sasanuma et al., 2008). In mitotic S phase, DDK controls origin firing by phosphorylating replicative helicase components (Labib, 2010). In meiosis, DDK activity is limiting early and lower levels are needed for origin firing than for DSB formation (Matos et al., 2008; Wan et al., 2008). Similar differences may apply for CDK-S (Henderson et al., 2006). Hence, when meiosis begins and CDK-S and DDK activities start rising, levels needed for replication are reached before thresholds required for DSBs. This can account for replication normally preceding DSB formation and DSBs forming in the absence of replication, but does not explain replication-DSB coordination in cis. However, if Mer2 phosphorylation occurs preferentially on replicated chromatin, this could spatially coordinate replication and DSB timing (Murakami and Keeney, 2008) (Figure 1A, left).
This model provided a plausible framework, but alternative scenarios were possible (e.g. Hochwagen and Amon, 2006) and it was unclear how Mer2 phosphorylation is targeted to replicated regions. Here, we show that key predictions of the model are met and provide evidence that replication is connected to DSB formation via recruitment of DDK to replisomes. Our findings suggest a paradigm for how replication can be coordinated locally and temporally with other chromosomal events, and illuminate a new aspect of the control of DDK activity.
Results
Experimental system
To measure DSB timing, genomic DNA embedded in agarose plugs is prepared from synchronous meiotic cultures then separated by pulsed-field gel electrophoresis (PFGE) and detected by Southern blotting (Figure 1B, upper panels). We quantify DSBs on right and left arms, Poisson-adjust to correct for multiple breaks on the same chromatid, and normalize to peak values (Figure 1B, lower panels; Experimental Procedures). The time difference between right and left arms (ΔtL-R) is then compared between isogenic ARS+ and arsΔ strains (Figure 1B,C).
On average, a wild-type ARS+ strain formed DSBs slightly later on the left arm (ΔtL-R = 8.6 ± 1.6 min, mean ± SD), while an origin-deleted strain showed a greater time difference (36.2 ± 4.2 min, p = 0.00044, t test) (Figure 1B, 1C). This ~30 min DSB delay from origin inactivation matches prior findings (Borde et al., 2000).
Two features allow sensitive and robust measurement of replication-DSB coordination. First, origin inactivation forces the left arm of nearly every chr III in the population to replicate later than its right arm, via a fork initiated in the right arm. This minimizes confounding effects of cell-to-cell variation in time of origin firing. Second, left- and right-arm DSBs are assessed in the same culture. Internally controlled measurement of relative DSB timing is critical, as absolute timing cannot be determined with similar precision because culture-to-culture variation can be large (Cha et al., 2000).
High DDK levels eliminate the DSB delay caused by delaying replication
For preferential phosphorylation of Mer2 on replicated chromatin, the kinase’s activity must be limiting, because excess kinase could phosphorylate Mer2 wherever it was located. DSB formation requires higher DDK activity than replication (Hollingsworth and Sclafani, 1993; Matos et al., 2008; Schild and Byers, 1978; Wan et al., 2006), so we asked if replication-DSB coordination is compromised when DDK is overproduced.
As in prior studies (Matos et al., 2008), endogenous Cdc7 and Dbf4 were low or undetectable in wild type after pre-sporulation growth, remained low for ~2–3 h in sporulation medium, and reached maxima at ~5–6 h (Figure 1D, 1E). Cdc7 then remained abundant while Dbf4 declined. To overexpress DDK, we drove CDC7 and DBF4 with the meiotically induced IME1 promoter (PIME1) in strains with intact CDC7 and DBF4. PIME1-CDC7 produced more Cdc7 at all time points: Cdc7 at 0 h was comparable to 4 h in wild type, with a peak (5 h) ~2-fold higher than wild type (6 h) (Figure 1D).
PIME1-DBF4 overproduced Dbf4 at later time points (3–8 h) but not earlier (0–2 h) (Figure 1E), so we introduced a destruction box mutation (DBF4-db) that stabilizes the protein against anaphase-promoting complex (APC) directed proteolysis in mitotic G1 (Ferreira et al., 2000). This construct elevated Dbf4 at 0–2 h to a level not reached by wild type until ~3 h (Figure 1E), so Dbf4 is controlled by APC early in meiosis. More importantly, these constructs provided graded overexpression in early prophase. Spore viabilities were high in ARS+ and arsΔ backgrounds (90% and 84%, respectively; Figure S1A).
In an ARS+ strain, the relative timing of right- and left-arm DSBs was unaffected by overexpressing both Cdc7 and Dbf4 (ΔtL-R = 5.5 ± 4.4 min), but in arsΔ the left-arm delay was completely eliminated (ΔtL-R = 7.6 ± 4.9 min; p = 0.76) (Figure 1B, 1C). Left-arm DSBs were still delayed in an arsΔ strain carrying PIME1-CDC7 plus PIME1-DBF4 lacking the stabilizing mutation (ΔtL-R = 33.3 min), so overexpressing Cdc7 alone is not sufficient to alter DSB timing (Figure S2A). However, PIME1-DBF4-db alone partially attenuated the delay (ΔtL-R = 17.7 min) (Figure S2A). Since Cdc7 was detected at early times in wild type (Figure 1D), we infer that DDK activity is partially increased by driving up Dbf4 expression. DSBs were still Spo11-dependent in DDK-overproducing strains (Figure S2B), ruling out inappropriate activation of nuclease(s) such as Nuc1.
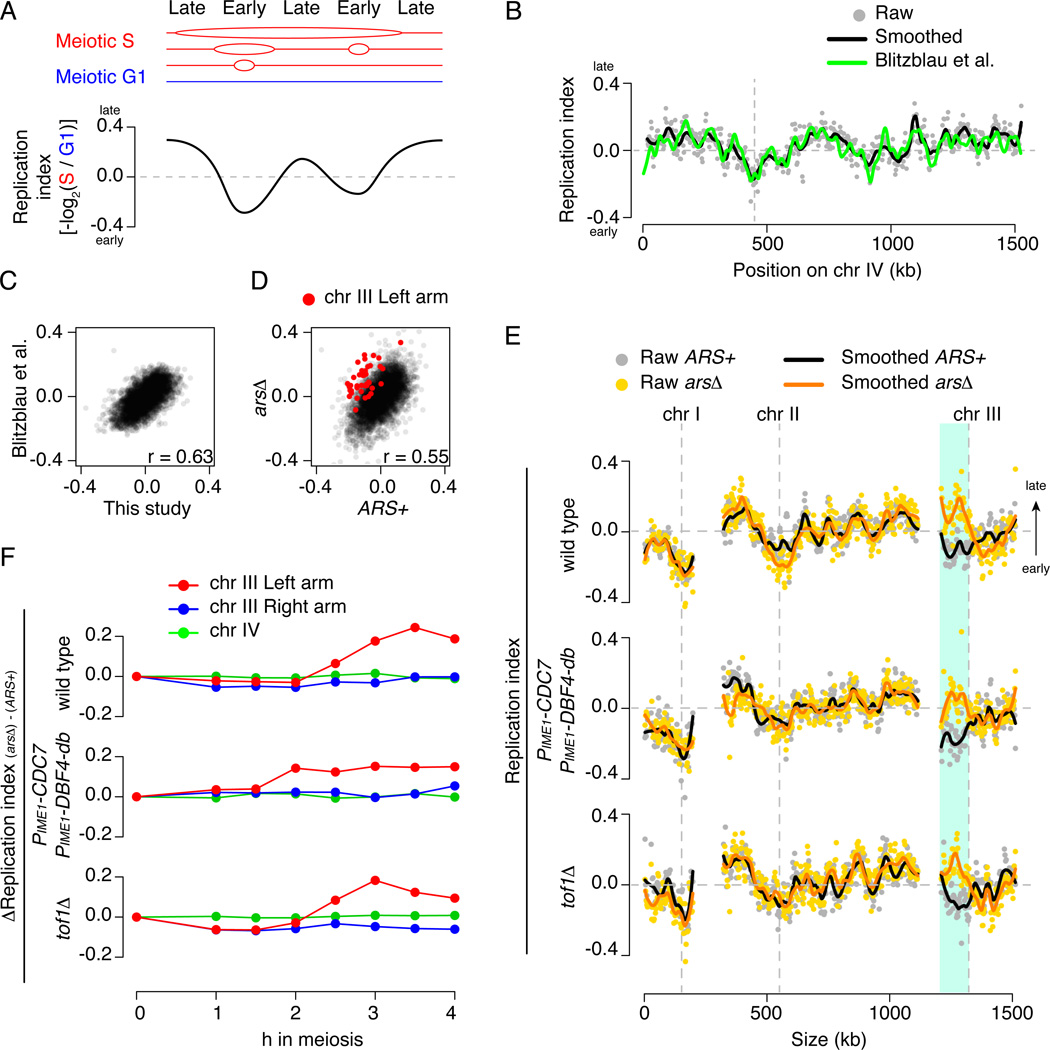
We assessed replication kinetics to exclude that DDK overproduction alters replication timing, e.g., by firing cryptic origins. For a population of S phase cells, DNA of early-replicating regions is over-represented relative to later regions (Blitzblau et al., 2012; Yabuki et al., 2002). We sequenced DNA from premeiotic cells (G1) and at 30-min intervals from 1–4 h during sporulation and normalized the read density in meiotic S phase to that in G1. Then, we defined a “replication index” as −log2(relative coverage); the sign inversion makes the replication index proportional to time, i.e., earlier replication gives a smaller value (Figure 2A). The results matched prior reports well (Blitzblau et al., 2012) (Figure 2B, 2C). As expected for delayed replication of the origin-deleted left arm, wild-type ARS+ and arsΔ strains displayed similar replication indices except for the left arm in the arsΔ strain (Figure 2D, 2E), beginning during S phase (~2.5 h, Figure 2F). The delay was retained in the arsΔ strain overproducing DDK (Figure 2E, 2F), confirmed by direct analysis of replication intermediates (Figure S3).
Figure 2. Neither DDK overproduction nor tof1 deletion alleviates the replication delay caused by origin deletion.
(A) Replication profiling. Red lines: partially replicated molecules in S-phase; blue line: unreplicated DNA in G1. If S-phase coverage is normalized to G1 and −log2 transformed, the resulting “replication index” is negative for early replicating regions and positive for late regions.
(B,C,D) Reproducibility. (B) Replication index maps from this study (wild-type ARS+, 3.5 h) and a prior study (Blitzblau et al., 2012). (C) Whole-genome comparison. (D) Comparing replication indices of ARS+ (3.5 h) and arsΔ (3 h) cultures. Points are 5-kb overlapping bins; black line in (B) is a smoothed fit (loess). In (D), chr III left-arm bins (red) have higher replication indices (later replication) in the arsΔ strain.
(E) In all three backgrounds, replication indices on chr III left arm (cyan region) are higher in arsΔ strains. Chr I and II are internal controls. Time points best correlated with data of Blitzblau et al. (2012) were chosen (3.5 h for wild-type ARS+ and DDK-overproducing arsΔ strains, 3 h for the rest). Vertical dashed lines indicate centromeres.
(F) Time course of replication difference between ARS+ and arsΔ. Each point shows the difference (arsΔ – ARS+) of replication indices for the indicated arm or chromosome. Delayed replication yields larger values for the left arm of chr III in arsΔ strains, beginning in S phase. Also see Figure S3.
Thus, when DDK is overexpressed, inactivation of left-arm origins still delays replication of that arm but no longer delays DSB formation. We conclude that excess DDK — and by inference its kinase activity — compromises temporospatial coordination of replication and DSBs in a dose-dependent manner.
Components of the fork protection complex interact with Dbf4 and coordinate replication with DSB timing
How might DDK activity be preferentially targeted to replicated regions? One possibility was suggested by interaction of the S. pombe ortholog of DDK (Hsk1-Dfp1) in vegetative cells with the Swi1-Swi3-Mrc1 complex (Tof1-Csm3-Mrc1 in S. cerevisiae, and Timeless-Tipin-Claspin in vertebrates) (Matsumoto et al., 2005; Shimmoto et al., 2009). This “replication fork protection complex” (FPC) associates with replisomes (Bando et al., 2009; Katou et al., 2003), where it has poorly understood functions in transducing intra-S phase checkpoint signals, stabilizing replisomes under stress, pausing forks, and establishing sister chromatid cohesion (McFarlane et al., 2010). We hypothesized that DDK might be recruited to forks via DDK-FPC interaction, perhaps transiently, thus phosphorylating Mer2 in the wake of fork passage.
To determine if DDK interacts with the FPC during meiotic S phase in S. cerevisiae, we tested for co-immunoprecipitation (co-IP) in DNase-treated extracts prepared at 2 h in meiosis. IPs in both orientations specifically co-precipitated HA-tagged Tof1 with overexpressed Myc-tagged Dbf4-db (Figure 3A, lanes 4). However, only a small fraction of each protein was co-precipitated, and we did not detect co-IP with low Dbf4 expression, i.e., if Dbf4 contained an intact destruction box (Figure 3A, lanes 3). Thus, DDK and FPC can interact physically, but this interaction (whether direct or indirect) is likely transient in vivo and/or unstable under IP conditions.
Figure 3. Role of Tof1 in replication-DSB coordination.
(A) Co-IP of Tof1 and Dbf4 from whole cell extract (WCE) at 2 h in meiosis.
(B) Representative DSB timing analyses (layout as in Figure 1B; additional data in Figure S2C).
(C) The FPC but not its checkpoint function are required for replication-DSB coordination. Dotted blue lines are mean ΔtL-R for ARS+ and arsΔ in wild-type background (from Figure 1C).
(D) Artificially tethering DDK to replication forks by fusing Dbf4-Myc to Cdc45.
(E) Delayed DSB formation in tof1Δ. (Left) Phosphorylated Orc6 and left-arm DSB signals from a wild-type ARS+ strain. Lines are least-squares fits. Dotted lines are times when signals reached 50% of maximum. (Right) Each dot represents a single culture. Lines are mean and SD: 18.4 ± 11.3 min for wild type; 10.3 ± 18.7 min for the DDK-overproducing strain; and 37.9 ± 17.9 min for tof1Δ. Data from wild-type ARS+ and arsΔ strains were pooled. Asterisk, p < 0.05 (one tailed t-test). Additional data are in Figure S4.
Importantly, both Tof1 and Csm3 are essential for replication-DSB coordination: in the absence of Tof1, origin inactivation no longer delayed left-arm DSBs (ΔtL-R = 3.3 ± 7.2 min in ARS+, vs. 0.3 ± 3.5 min in arsΔ; Figure 3B, 3C), despite retention of replication delay (Figure 2E, 2F, S3). Deleting CSM3 had a similar effect (ΔtL-R = 6.8 ± 8.2 min in ARS+, vs. 11.2 ± 7.1 min in arsΔ), but mutating MRC1 did not (1.3 ± 10.0 min vs. 33.6 ± 21.3 min) (Figure 3C, S2C). Spore viability was decreased in tof1Δ and csm3Δ mutants, and was made worse by origin deletion (~80% in ARS+ vs. ~60% in arsΔ; Figure S1A). Even lower spore viability was seen in mrc1Δ, but origin deletion did not alter this (66–73%; Figure S1A). Decreased spore viability in mrc1Δ may be due to random spore death rather than defects in chromosome segregation (LeClere et al., 2012) (see Discussion for tof1Δ and csm3Δ).
Tof1 and Csm3 are mutually dependent for replisome association and are required for Mrc1 binding to replisomes (Bando et al., 2009). In contrast, Mrc1 is required for FPC checkpoint functions (Alcasabas et al., 2001), but Tof1-Csm3 still interact with replisomes in its absence (Bando et al., 2009). Therefore, we conclude that Tof1-Csm3 — but not the intra-S-phase checkpoint — is required for replication-DSB coordination, possibly via DDK recruitment.
Tethering Dbf4 to replisomes bypasses the FPC requirement
If the principal function of Tof1-Csm3 in replication-DSB coordination is to link DDK to the replisome, we reasoned that the Tof1-Csm3 requirement should be bypassed by artificially tethering DDK to the replisome by fusing it to a core component of the replication machinery (Figure 3D). To test this prediction, we integrated DBF4 in-frame at the 3′ end of the CDC45 locus. Cdc45 is a subunit of the CMG complex (Cdc45-MCM-GINS) thought to be the replicative helicase (Labib and Gambus, 2007). Cells grew normally with the fusion as the only source of Cdc45 (Figure S1B), and CDC45-DBF4 complemented dbf4Δ, albeit with a mild slow-growth defect (Figure S1C). Thus, both Cdc45 and Dbf4 moieties are functional. The CDC45-DBF4 tof1Δ double mutant was also viable, but the fusion exacerbated a tof1Δ growth defect (Figure S1D) and spore viability was reduced (26–35%; Figure S1A), indicating the fusion may cause deleterious replication stress in tof1Δ.
The Cdc45-Dbf4 fusion greatly rescued replication-DSB coordination in the absence of Tof1. In a CDC45-DBF4 tof1Δ background, inactivation of left-arm origins went back to causing a delay in DSB formation (ΔtL-R = 5.9 ± 4.0 min in ARS+, vs. 19.9 ± 3.4 min in arsΔ, p = 0.023; Figure 3B, 3C), although ΔtL-R was not restored to the value in an otherwise wild-type background (i.e., arsΔ TOF1+: 36.2 ± 4.2 min, Figure 1C). Incomplete bypass may reflect the fusion protein being only partially functional as a substitute for normal DDK recruitment, roles for Tof1 in addition to DDK recruitment, and/or compensatory attenuation of replication-DSB coordination if the fusion yields modest Dbf4 overexpression. Nonetheless, the ability of this artificial system to substantially bypass the Tof1 requirement argues strongly that recruitment of DDK to replisomes is a key component of replication-DSB coordination.
Changes in the absolute time of DSB formation
In principle, compromising replication-DSB coordination should change absolute DSB timing, not just relative to local replication. One prediction is that global DSB formation should be delayed in tof1Δ because, without the targeting system, DDK might need to reach a higher level than normal. Conversely, when DDK is overproduced its targets are presumably phosphorylated prematurely, so DSB formation should be accelerated locally on the origin-deleted left arm, and might even occur earlier than normal genome-wide.
As it is difficult to assess absolute timing because of high culture-to-culture variability, we measured the time between DSB formation and an early meiotic event in the same cultures (Figure S4A, S4B). We used CDK phosphorylation of the origin-binding factor Orc6, which marks S-phase onset (Weinreich et al., 2001) (Figure S4B). We analyzed DSBs on the right arm of chr III, as these should be unaffected by origin deletion on the left. To smooth noise and improve precision, we fitted logistic (Orc6) or log-normal (DSB) functions to data points (Figures 3E, S4C).
We observed longer times between Orc6 phosphorylation and DSB formation in tof1Δ (p = 0.04, one-sided t test) (Figures 3E, S4C, S4D), so DSB formation is indeed delayed in the absence of Tof1, as predicted. In contrast, DSB timing was not significantly affected by DDK overproduction (p = 0.22) (Figures 3E, S4C, S4D). Our method may not be sensitive enough to detect small differences, but it is also possible that the earliest time when DSBs can form is constrained by other factors aside from DDK, such as the intra-S-phase checkpoint (Blitzblau and Hochwagen, 2013) or the need to accumulate Spo11 and other proteins. Importantly, however, combining this result with those in Figure 1C shows that DSB acceleration on the left arm is how DDK overproduction eliminates right-vs-left arm timing difference in origin-deleted strains, as predicted.
Delaying replication delays Rec114 accumulation in cis
Mer2 is a strong candidate for replication-associated phosphorylation because it is a direct DDK target (Sasanuma et al., 2008; Wan et al., 2008) and binds chromatin prior to and independent of phosphorylation (Henderson et al., 2006). We thus predicted that Mer2 phosphorylation is locally coordinated with replication timing. As suitable anti-phospho-Mer2 antibodies are unavailable, we used Rec114 chromatin association as an indirect readout of Mer2 phosphorylation. Rec114 is meiosis-specific, is essential for DSB formation, and interacts physically with Mer2 (Keeney, 2007). Mer2 phosphorylation modulates Mer2-Rec114 interaction (Henderson et al., 2006) and promotes Rec114 binding to chromatin (Panizza et al., 2011; Sasanuma et al., 2008). We therefore performed chromatin IP (ChIP) to assess when and where binding of myc-tagged Rec114 occurs relative to replication.
We measured Rec114 ChIP efficiency (IP as percent of input) using quantitative PCR (qPCR) with primer pairs for two loci on the left arm of chr III, three loci on the right arm (two major Rec114 sites and one minor), and two loci near strong DSB hotspots on chr VI (GAT1) and chr VII (ERG1) (Figure 4A). GAT1 and ERG1 are internal controls whose replication and DSB timing should be unaffected by changes on chr III. Rec114 ChIP increased as meiosis progressed, was maximal at ~2.5–4 h, then decreased (Figure 4B). ChIP signal was highly specific: anti-myc ChIP was indistinguishable from negative controls (mock IPs) in premeiotic cells (0 h) when Rec114 is not expressed, and mock IPs gave little signal (Figure 4B).
Figure 4. Rec114 chromatin association is temporally coordinated with replication.
(A) Primer pairs (PP) used for Rec114 ChIP-qPCR. Not to scale. PP6 is a site with low Rec114 signal used to scale ChIP-seq data (Figure S5A).
(B) Transient Rec114 chromatin association, color coded as in (A). Lines are least-squares fits (see panel C); tRec114 values are indicated. Mock controls are in gray. Note that control loci (GAT1 and ERG1) had a mean difference of 16.5 min between ARS+ and arsΔ strains, indicating fluctuation between cultures rather than genotypic difference.
(C) Illustration of two-step curve fitting. A log-normal curve (dashed line) is fitted to all points to define the peak (black circle), then a logistic curve (solid line) is fitted to early points; tRec114 is where the logistic curve reaches 50% of the peak level.
(D) Origin deletion delays Rec114 chromatin association. Fitted curves from (B) were normalized by peak height and averaged separately for left-arm (red), right-arm (blue), and control (green) loci, then overall left-arm vs. right-arm time differences were determined.
We fitted a log-normal distribution to all time points to determine the peak in each Rec114 ChIP profile, then used this peak to fit a saturating exponential growth (logistic) curve to early time points to smooth the data and facilitate comparisons between loci and between time courses. We defined “tRec114” as the time of 50% of maximum Rec114 ChIP (Figure 4C). Similar results were obtained if we used other fitting methods or alternative definitions of tRec114 (Extended Experimental Procedures and data not shown).
In the ARS+ strain, tRec114 was consistently earlier at the left-arm sites than at the major right-arm sites or GAT1 and ERG1 (Figure 4B). In contrast, the arsΔ strain displayed later tRec114 on the left arm vs. the other sites (Figure 4B). When the curves from left-arm primer pairs and major right-arm primer pairs were separately merged, the ARS+ strain showed an overall AtL-R for Rec114 ChIP of −12.0 min, compared with +14.4 min in arsΔ (Figure 4D). This change (26.4 min) closely matched that for DSBs (27.6 min; Figure 1C). The shift in timing of left-arm vs. right-arm sites is clearly seen from inspection of the individual ChIP data points (compare red and blue points from 2–3 h in Figure 4D), so the overall conclusion is independent of the smoothing exercise.
We extended the analysis by deep-sequencing the ChIP samples. Sequence coverage was normalized to coverage from input samples, then scaled using qPCR data (Figure S5A) for a genome-wide measure of absolute ChIP signal (Figure 5A). The 3-h ChIP-seq map agreed well with a prior ChIP-microarray map obtained at 4 h (Panizza et al., 2011) (Figure S5B, S5C), showing pronounced alternating peaks and valleys (Figure 5A, S5C). At 3 h, the ARS+ and arsΔ strains had globally similar Rec114 distributions (Figure 5A, S5D). Moreover, Rec114 accumulated on all but chr III with similar kinetics in ARS+ and arsΔ, except that the arsΔ strain began to accumulate Rec114 slightly earlier, exemplifying culture-to-culture variability in absolute timing (Figure 5A right panel, and data not shown). Importantly, however, the entire left arm of chr III in the origin-deleted strain showed a distinct delay in accumulating Rec114 (Figure 5A left panel, Figure S5D). Since Rec114 binding to chromatin requires Mer2 phosphorylation, we infer that delaying replication delays Mer2 phosphorylation in cis.
Figure 5. Genome-wide correlation between replication and Rec114 accumulation.
(A) Delaying replication delays Rec114 accumulation on the entire left arm of chr III. Rec114 ChIP and input DNA were sequenced and read coverage (ChIP/input) was scaled with qPCR data (Figure S5A). Chr VI is an internal control. Dashed lines are centromeres.
(B) Reproducibility between ARS+ and arsΔ cultures for timing of Rec114 ChIP (left) and replication (right). Left: each point is the tRec114 for a Rec114 ChIP peak (Figure S5E). Right: replication indices (from ChIP input DNA) in non-overlapping 5-kb windows.
(C) Correlation between replication indices (non-overlapping 5-kb windows) from total genomic DNA or ChIP inputs.
(D) Correlation between timing of replication and Rec114 ChIP. Points are Rec114 peaks.
(E) Spatial patterns for timing of replication and Rec114 ChIP on representative chromosomes. Points are Rec114 peaks. Dashed gray lines, centromeres; dashed blue lines, qPCR primer pairs.
Rec114 accumulation on chromatin is coordinated with DNA replication genome-wide
If coupling of Mer2 phosphorylation to replication is a general feature, i.e. not limited to the artificial situation of origin-deletion mutants, then we expect a global correlation between the timing of DNA replication and Rec114 chromatin association. To test this prediction, we measured tRec114 for >1000 peaks of Rec114 binding across the genome (Figure S5E). These measurements agreed well between the ARS+ and arsΔ time courses, except for the expected late accumulation specifically in the left arm of the origin-deleted strain (Figure 5B, left panel). We then generated replication indices for the same cultures using the sequence coverage from the ChIP input samples for the zero-hour (G1) and two-hour (meiotic S phase) time points. Again, the ARS+ and arsΔ cultures matched one another well except for the left arm of chr III (Figure 5B, right panel), and these replication indices agreed with those from sequencing of total DNA from independent cultures (Figure 5C).
As predicted, we found a strong correlation between timing of replication and Rec114 ChIP, i.e., early replicating regions tended to accumulate Rec114 early (Figure 5D). Likewise at the level of individual chromosomes, the spatial distribution of tRec114 values resembled the replication profile (Figure 5E and data not shown). These findings argue strongly that Rec114 accumulation and, by inference, Mer2 phosphorylation are coupled spatially and temporally to replication throughout the genome.
Tof1 and the dynamics of Rec114 accumulation on chromatin
We further examined the effect of deleting TOF1 on Rec114 chromatin association. The results support the hypothesis that Tof1 helps preferentially target Mer2 in replicated regions for phosphorylation, but unexpected complexities also emerged.
In tof1Δ, Rec114 ChIP profiles resolved into two distinct peaks or a peak plus a prominent shoulder (Figure 6A). Merging two log-normal curves gave better fits than single curves (Figure 6A and data not shown). This pattern was reproducible in two biological replicates (data not shown) and occurred at all loci analyzed, indicating that biphasic Rec114 accumulation is an intrinsic property of tof1Δ mutants. This pattern is not from two populations of cells proceeding at different paces, because other landmark events were not biphasic (Orc6 phosphorylation, DNA replication, DSB formation, DSB disappearance, and meiotic divisions; Figures 2F, 3B, S4C). For TOF1+, we cannot exclude occurrence of two waves with near coincident timing, but fits were not improved with two curves (data not shown).
Figure 6. Altered Rec114 accumulation in the absence of Tof1.
(A) Biphasic accumulation of Rec114 (ChIP-qPCR data as in Figure 4B). Dashed lines merge two fitted log-normal curves (magenta lines). In each plot, tRec114 is on the left, and times of the first and second peaks are upper right.
(B) Summary of event timing in tof1Δ cultures. The number below DSB peak (2.0 ± 0.5 hr) indicates mean ± SD.
(C) The delay in initial Rec114 accumulation caused by origin deletion is attenuated in tof1Δ cells. Merged logistic curves from (A) were set to the same height then averaged separately for left-arm (red), right-arm (blue), and control (green) loci. Red and blue arrows indicate tRec114 for left and right arms. The difference between arms (ΔtR-L) and total difference between ARS+ and arsΔ (Δt+/-origin) are shown.
(D) Reproducibility of tof1Δ ARS+ and tof1Δ arsΔ cultures for timing of Rec114 ChIP (left) and replication (right) (as in Figure 5B).
(E) Correlation of replication indices from total genomic DNA or ChIP input samples (as in Figure 5C).
(F) Correlation between timing of replication and Rec114 ChIP (as in Figure 5D).
We compared DSB presence with timing of the fitted Rec114 maxima, using as an internal timestamp the point when half of each culture had passed MI. The early Rec114 maxima clearly preceded DSB formation, whereas later maxima did not occur until after steady-state DSB levels began to decline (Figure 6B). Since the late Rec114 accumulation is too late to contribute to formation of most DSBs, and since our principal goal was to ask if initial Rec114 chromatin association is temporally coordinated with replication, we focused on timing estimated from logistic regression of the early time points. When the fitted curves were merged separately for left-arm and major right-arm primer pairs, the ARS+ strain showed an overall AtL-R of −3.6 min, versus +6.3 min in arsΔ (Figure 6C). The net change in AtL-R is thus smaller in a tof1Δ background (9.9 min) than in TOF1+ (26.4 min). The residual apparent effect of origin deletion on tRec114 may reflect imprecision in estimating timing, or a small Tof1-independent component of replication-Rec114 coordination. Nevertheless, tof1 mutation clearly attenuates the delay in Rec114 accumulation caused by delaying replication, so we infer that Tof1 temporally couples replication and Mer2 phosphorylation, just as it couples replication timing to DSB timing.
Interestingly, deleting left-arm origins caused a decrease in Rec114 ChIP signal at left-arm loci in tof1Δ, primarily during the first wave of Rec114 accumulation (Figure 6A). A similar but less pronounced decrease also occurred in TOF1+ (Figure 4B). We also note that the later Rec114 ChIP maxima (in both ARS+ and arsΔ) coincided with maximal Dbf4 (and hence DDK) protein accumulation in wild type (compare with Figure 1E). Multiple factors regulate removal of Rec114 and other proteins from chromosomes, including negative regulatory circuits triggered by DSBs and by interactions between homologous chromosomes (Carballo et al., 2013; Thacker et al., 2014). It is thus plausible that chromosomal regions that have not experienced a DSB and/or have not yet engaged in homologous synapsis may remain permissive for Rec114 accumulation driven by sharply rising DDK levels late in prophase. If so, this later wave appears to contribute little to total DSB formation since there was no evidence of a second peak in DSBs (Figure 3B). We infer that uncoupling Mer2 phosphorylation from replication causes complex changes in the balance between factors controlling association and dissociation of pro-DSB proteins from chromosomes.
Tof1 coordinates replication and Rec114 accumulation genome-wide
To examine global patterns, we sequenced tof1Δ Rec114 ChIP samples and scaled sequence coverage with the qPCR data (Figure S6A). The tof1Δ ChIP-seq maps agreed with maps from TOF1 strains, so Tof1 absence does not grossly alter Rec114 distribution (Figure S6B, S6C). As expected, the tof1Δ arsΔ strain had globally similar Rec114 distributions as tof1Δ ARS+, except for reduced signal on the left arm of chr III (Figure S6D).
When we measured tRec114 for > 900 Rec114 peaks, the ARS+ and arsΔ time courses agreed well genome-wide (Figure 6D, left panel). As expected, loci on the left arm of chr III now showed similar behavior in arsΔ and ARS+ strains, unlike in TOF1 (compare Figures 6D and 5B). Also as expected, replication indices matched well between tof1Δ ARS+ and tof1Δ arsΔ cultures except for the left arm of chr III (Figure 6D, right panel). These replication indices also agreed with those from total DNA from tof1Δ cultures (Figure 6E).
Importantly, deleting TOF1 greatly attenuated the genome-wide correlation between replication timing and Rec114 ChIP timing. Whereas replication timing explained ~20% of the variation in tRec114 values in TOF1 cultures (R2 = 0.194; Figure 5D), only 4% of the variation was explained in tof1Δ cultures (R2 = 0.036; Figure 6F). Likewise, at the level of individual chromosomes, the spatial distribution of tRec114 values lost its strong resemblance to the replication index maps, exhibiting rather flat patterns across chromosomes (Figure 6G and data not shown; compare with Figure 5E). These findings support the hypothesis that Tof1 has a critical function in temporospatial coupling of Mer2 phosphorylation with replication throughout the genome.
Discussion
We provide evidence that a key prerequisite for DSB formation — phosphorylation of Mer2 by DDK — is linked to replication via association of the kinase with replisome components. The molecular events we envision are in Figure 7. A) When cells enter meiosis, Mer2 (which is up-regulated in meiosis but constitutively expressed at low levels) is already chromatin-bound independently of phosphorylation. CDK-S and DDK levels begin to rise, triggering replication origin firing. B) CDK-S phosphorylates Mer2. We infer this is at least partially independent of replication and/or the FPC because simply overproducing DDK or tethering it to the replisome was sufficient to disrupt replication-DSB coordination, even though Mer2 phosphorylation by DDK is partially dependent on prior phosphorylation by CDK-S. C) DDK recruited to forks by the FPC preferentially phosphorylates Mer2 in replicating regions, whereas Mer2 bound to unreplicated regions remains unphosphorylated until replication occurs there or enough DDK accumulates to obviate fork-associated targeting. Sequestration of DDK by binding to the FPC may also effectively reduce the free pool of DDK, further enhancing preferential phosphorylation of Mer2 in replicated regions. The model is indifferent to whether DDK is constitutively bound to replication forks or is recruited transiently, thereby increasing local DDK concentration in actively replicating regions.
Figure 7. Temporospatial coordination of DNA replication with DSB formation.
Events linking DSBs to replication. (A) Mer2 binds chromatin independently of phosphorylation; CDK-S and DDK levels begin to rise, firing replication origins. (B) CDK-S phosphorylates Mer2, at least partially independent of replication. (C) DDK recruited to replisomes by the FPC phosphorylates chromatin-bound Mer2 encountered by the fork. (D–G) Phosphorylated Mer2 recruits Rec114 and other proteins; higher order chromosome structure changes form axis-loop interactions that lead to DSB formation.
Key findings in this study are that DDK levels must be limiting; that the FPC interacts with DDK and is required for replication-DSB coordination, but becomes dispensable if DDK is tethered artificially to replisomes; and an event downstream of DDK phosphorylation of Mer2 (Rec114 recruitment) is itself coordinated with replication. Our results do not directly demonstrate the predicted recruitment of DDK to replication forks. An alternative possibility is that, instead of or in addition to traveling with the fork, DDK is recruited to replicated chromatin by something else that is FPC-dependent, such as sister chromatid cohesion. We have also not directly proven that Mer2 phosphorylation occurs preferentially in the wake of the replication fork. Further experiments are required to test these aspects of the model.
When DDK is inhibited in meiosis using an ATP-analog-sensitive mutant version of Cdc7, replication proceeds but Mer2 phosphorylation and DSB formation are blocked (Wan et al., 2008; Wan et al., 2006). If the Cdc7 inhibitor is washed out after replication, Mer2 phosphorylation occurs rapidly but DSB formation does not ensue for another ~80–100 min (Wan et al., 2008), comparable to the time between replication and DSBs in normal meiosis (on average ~90–120 min after fork passage (Borde et al., 2000)). We propose the following: First, Mer2 phosphorylation is not normally an instantaneous trigger for DSB formation, but instead sets in motion a series of events that leads ultimately to DSBs. Second, in normal meiosis, much of the delay between replication and DSB onset is due to events after Mer2 phosphorylation. Third, even though DSBs are not made until much later, synchronizing Mer2 phosphorylation with replication imposes temporal order on DSBs because early replicating regions gain a head start in executing the chain of events that follows Mer2 phosphorylation. These events may include recruitment of Rec114 and other DSB proteins (Figure 7D, E) and assembly of higher-order chromosome loop-axis structures tied to DSB formation (Panizza et al., 2011) (Figure 7F, G).
Interestingly, when CDC7 transcription was placed under the control of the NDT80 promoter, DSBs appeared roughly concurrently with detectable Cdc7 protein, at the end of prophase (Matos et al., 2008). This observation could indicate that DDK can provoke rapid DSB formation, in contrast to the proposals above. One possibility is that the time between DDK action and ensuing DSB formation is context dependent: perhaps events downstream of DDK proceed rapidly when cells have accumulated the higher levels of Rec114, Mer2 and other proteins characteristic of later prophase, whereas the lower levels of DSB-promoting proteins present during S phase and early prophase may yield a longer delay. It is also possible that DDK plays more complex roles. For example, at low levels it might set up the replication-DSB coordination we document here, but higher levels achieved later might be needed to promote additional events important for DSB formation. Finally, we note that Matos et al. (2008) did not evaluate Mer2 phosphorylation directly. Since this normally occurs when DDK levels are very low, it is possible that Mer2 may have already begun to be phosphorylated before high-level Cdc7 expression was detected in their system. Further experiments are needed to fully define events between Mer2 phosphorylation and DSB formation.
Even with a Mer2 mutant that mimics phosphorylation of DDK and CDK-S targets (Asp substitutions for Thr-28, Ser-29, and Ser-30), DDK and CDK-S are still required for DSB formation (Wan et al., 2008), so there must be other targets. Additional residues in the Mer2 N-terminus are phosphorylated in vivo and DSB defects result from mutating two or more serines in this region matching the DDK target consensus (Sasanuma et al., 2008), so replication-DSB coordination may involve multiple phosphorylation events on Mer2 itself. Another potential target is histone H3, which is phosphorylated by DDK on Thr-45 during S phase (Baker et al., 2010). The function of this histone modification is not known, and whether it is important for DSB formation and/or replication-DSB coordination in meiosis has not been tested. Another attractive target is the cohesin subunit Rec8, which is required for normal DSB formation in some genomic regions but not others (Klein et al., 1999; Kugou et al., 2009). Phosphorylation of Rec8 by DDK is important for the metaphase-to-anaphase transition in Meiosis I (Katis et al., 2010). Whether Rec8 phosphorylation is also important earlier for DSB formation remains to be seen, but if so, this could provide another means for replication-DSB coordination via targeted DDK activity.
Robust regulation of DSB timing from overlapping control mechanisms
Developmentally programmed increases in CDK-S and DDK levels as cells progress through meiosis provide a means to control DSB timing, but globally (nucleus-wide) rather than spatially patterned relative to replication (Henderson et al., 2006; Murakami and Keeney, 2008). It is likely that other pathways also contribute. For example, many proteins important for DSB formation are developmentally controlled via transcription and/or splicing (Spo11, Mei4, Mer2, Rec102, Rec104, Rec114, Hop1, Red1) (Keeney, 2007). Regulated expression may help establish proper DSB timing by setting an early limit on when cells become competent to form DSBs. Such control, working globally, is unlikely to enforce regional control or to direct coupling of replication to DSBs in cis.
Recent studies in budding and fission yeasts uncovered mechanisms regulating DSB formation in the face of replication problems. In S. pombe meiosis, inhibiting replication with hydroxyurea triggers a Rad3- and Cds1-dependent checkpoint that prevents expression of Mde2, a protein essential for DSBs (Miyoshi et al., 2012; Ogino and Masai, 2006). Hydroxyurea treatment also blocks DSB formation in S. cerevisiae via Mec1 and Rad53 (orthologs of S. pombe Rad3 and Cds1, respectively), but in addition to controlling expression of a protein required for DSB formation (Spo11 in this case), this replication checkpoint also inhibits DSB formation by down-regulating DDK activity (Blitzblau and Hochwagen, 2013).
In principle, this checkpoint could establish temporospatial replication-DSB coordination. For example, replication could impose a nucleus-wide block to DSB formation that is removed locally by the moving replication fork (Hochwagen and Amon, 2006). However, it is not yet clear if this checkpoint pathway regulates DSB formation in unperturbed meiosis, i.e., absent replication stress. Moreover, we find that Mrc1 is dispensable for replication-DSB coordination. Mrc1 is essential for the intra-S-phase checkpoint in vegetative cells (Osborn and Elledge, 2003), so if also true in meiosis, it would imply that the checkpoint is dispensable for coordination. Finally, the role of Tof1 is not consistent with this model for replication-DSB coordination, in which a factor necessary for coordination must either set up the global DSB block or mediate local relief of the block. But if Tof1 sets up the block (independently of Mrc1), then DSBs should occur earlier than normal in tof1Δ mutants — the opposite of what we observed — and the Cdc45-Dbf4 fusion is not predicted to bypass the Tof1 requirement. Conversely, if Tof1 relieves the block, then DSB formation genome-wide should be greatly reduced in tof1Δ mutants, which is not observed. We therefore favor the interpretation that the intra-S-phase checkpoint regulates DSB formation globally and contributes little if any to the replication-DSB coordination that proceeds via the FPC recruiting DDK.
The net effect of this array of distinct but overlapping processes is that DSB timing and the integration of DSB formation with other aspects of meiotic progression are made robust despite cell-to-cell variation or transient problems caused by environmental perturbations or other defects. Moreover, this robustness plus the time elapsed between Mer2 phosphorylation and DSB formation make it unlikely that DSBs frequently occur ahead of the replication fork under the conditions in this study. Since origin deletion delays replication by only ~30 min, for DSBs to form on unreplicated DNA it would be necessary for Mer2 phosphorylation not just to be uncoupled from replication, but also to occur at least 50 min earlier than normal on the left arm of chr III.
General applicability of replication-associated phosphorylation
In subcellular fractionation experiments in vegetative cells, DDK associates with chromatin beginning around the G1/S-phase boundary, after chromatin binding of the minichromosome maintenance (MCM) proteins (Weinreich and Stillman, 1999). It is likely that DDK can be recruited to origins by interaction with the Mcm2–7 complex to phosphorylate origin-bound targets and initiate replication (Labib, 2010), but it was not clear whether DDK can also associate with moving replication forks in vegetative cells.
Our findings suggest that DDK recruitment to meiotic replication forks is both necessary and sufficient to establish replication-DSB coordination. This mechanistic linkage not only ensures that two distinct chromosomal processes (replication and DSB formation) occur in the right order, but it also provides a whole-genome scanning mechanism (via replication fork traversal) which gives an opportunity to inspect whether a given region is appropriate for subsequent formation of DSBs. Given that the FPC and DDK are highly conserved, that they interact in vegetatively growing fission yeast (Matsumoto et al., 2005; Shimmoto et al., 2009), that DDK in S. cerevisiae remains on chromatin throughout S phase (Weinreich and Stillman, 1999), and that the FPC plays important roles in processes tied to fork passage such as cohesion establishment (Xu et al., 2004), it is attractive to consider that FPC recruitment of DDK to replisomes might be a general and evolutionarily conserved mechanism to spatially pattern other chromosomal processes that need to be coordinated with replication.
Experimental Procedures
Detailed methods are in Extended Experimental Procedures. Yeast strains are of the SK1 background (Table S1). DDK overproduction cassettes containing a ca. 1.4-kb fragment from the IME1 promoter were integrated at URA3. The dbf4-db mutant (R62A, F65A) was constructed by site-directed mutagenesis of the pIME1-DBF4 construct. A 5-glycine linker followed by DBF4-13Myc-KanMX4 was integrated in-frame at the 3′ end of CDC45 to create the Cdc45-Dbf4 fusion.
Synchronous meiotic cultures were prepared in 2% potassium acetate with amino acids and 0.001% polypropylene glycol, using the SPS pre-growth method (Murakami et al., 2009). For DSB analysis, genomic DNA purified in agarose plugs was separated by PFGE and DSBs on chr III were detected by Southern blotting with a CHA1 probe. DSB frequencies were corrected for multiple DSBs on the same chromatid by assuming a Poisson distribution among chromatids: P(n) = (µne–µ)/n! where µ is mean DSBs per chromatid and P(n) is the probability that n DSBs occur per chromatid. Parental signal (Uobs, for “unbroken”) approximates unbroken fraction (i.e., P(0) ≈ Uobs), so the mean total DSB number (DSBtotal) is estimated as -ln(Uobs). The fraction of chromatids that are not broken in the left arm equals the fraction of unbroken chromatids (Uobs) plus the fraction of chromatids with an observable DSB on the right arm (DRobs). Thus, DSBleft = -ln(Uobs+DRobs). DSB number on the right arm (DSBright) is then estimated as DSBtotal – DSBleft. Poisson correction minimizes underestimation caused by inability of indirect end-labeling to distinguish singly from multiply cut chromatids (see Extended Experimental Procedures for further discussion). The difference in timing between right- and left-arm DSB formation (ΔtL-R) was defined as the displacement between the accumulation (upward sloping) portions of the normalized steady-state DSB curves, similar to previous studies (Borde et al., 2000; Murakami et al., 2003).
Protein expression was assessed by western blotting of extracts prepared with trichloroacetic acid followed by solubilization in SDS. For co-IP experiments, non-denaturing extracts prepared by agitating cells with glass beads were treated with benzonase. Anti-HA or anti-myc conjugated beads were used for IPs. For Rec114 ChIP, sporulating cells were crosslinked with 1% formaldehyde for 30 min at room temperature, then chromatin extracts were prepared by lysis with glass beads, sheared by sonication to an average of less than 500 bp, and subjected to IP with anti-Myc or control antibodies (mouse IgG). ChIP efficiencies (percent of IP input) were measured by qPCR. ChIP and input samples were sequenced (Illumina 50 bp or 75 bp paired-end reads), reads were mapped using BWA, then coverage maps were scaled proportional to the absolute ChIP efficiencies from qPCR. Rec114 accumulation time was measured by two-step curve fitting (log-normal followed by logistic fitting). DNA replication timing was assessed by measuring relative copy number by sequencing total genomic DNA or ChIP input samples collected at various times in meiosis, and by two-dimensional gel electrophoresis of replication intermediates. Bioinformatic analysis and curve fitting were performed in R (http://www.r-project.org/). Sequencing data were deposited at GEO (accession GSE52970 and GSE52987).
Supplementary Material
Highlights.
Local replication timing dictates meiotic DSB timing (replication-DSB coordination)
Tof1/Csm3 recruits DDK to moving replication forks to promote Mer2 phosphorylation
Mer2 phosphorylation in the fork’s wake triggers recruitment of other DSB proteins
Replication-associated phosphorylation establishes replication-DSB coordination
Acknowledgments
We thank Agnès Viale (MSKCC Genomics Core Laboratory) for DNA sequencing; Nicholas Socci (MSKCC Bioinformatics Core) for mapping sequence reads and advice on data analysis; Nicolas Nocetti, Adolfo Cuesta, Hyejin Choi and Zhicheng Qiu for experimental assistance; Bruce Stillman for anti-Orc6 antibody; Michael Lichten and Valérie Borde for strains and plasmids; Franz Klein and Hannah Blitzblau for sharing unpublished data; Dirk Remus and members of the Keeney lab, especially Corentin Claeys Bouuaert, Sarah Kim, Isabel Lam, Neeman Mohibullah, Megan van Overbeek, and Xuan Zhu, for discussion and comments on the manuscript; Andrea Farina and Satoko Murakami for experimental support to H.M. This work was supported by NIH grant R01 GM058673.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcasabas AA, Osborn AJ, Bachant J, Hu F, Werler PJ, Bousset K, Furuya K, Diffley JF, Carr AM, Elledge SJ. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat Cell Biol. 2001;3:958–965. doi: 10.1038/ncb1101-958. [DOI] [PubMed] [Google Scholar]
- Baker SP, Phillips J, Anderson S, Qiu Q, Shabanowitz J, Smith MM, Yates JR, 3rd, Hunt DF, Grant PA. Histone H3 Thr 45 phosphorylation is a replication-associated post-translational modification in S. cerevisiae. Nat Cell Biol. 2010;12:294–298. doi: 10.1038/ncb2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando M, Katou Y, Komata M, Tanaka H, Itoh T, Sutani T, Shirahige K. Csm3, Tof1, and Mrc1 form a heterotrimeric mediator complex that associates with DNA replication forks. J Biol Chem. 2009;284:34355–34365. doi: 10.1074/jbc.M109.065730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzblau HG, Chan CS, Hochwagen A, Bell SP. Separation of DNA replication from the assembly of break-competent meiotic chromosomes. PLoS Genet. 2012;8:e1002643. doi: 10.1371/journal.pgen.1002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzblau HG, Hochwagen A. ATR/Mec1 prevents lethal meiotic recombination initiation on partially replicated chromosomes in budding yeast. Elife. 2013;2:e00844. doi: 10.7554/eLife.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borde V, Goldman AS, Lichten M. Direct coupling between meiotic DNA replication and recombination initiation. Science. 2000;290:806–809. doi: 10.1126/science.290.5492.806. [DOI] [PubMed] [Google Scholar]
- Carballo JA, Panizza S, Serrentino ME, Johnson AL, Geymonat M, Borde V, Klein F, Cha RS. Budding yeast ATM/ATR control meiotic double-strand break (DSB) levels by down-regulating Rec114, an essential component of the DSB-machinery. PLoS Genet. 2013;9:e1003545. doi: 10.1371/journal.pgen.1003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha RS, Weiner BM, Keeney S, Dekker J, Kleckner N. Progression of meiotic DNA replication is modulated by interchromosomal interaction proteins, negatively by Spo11p and positively by Rec8p. Genes Dev. 2000;14:493–503. [PMC free article] [PubMed] [Google Scholar]
- Ferreira MF, Santocanale C, Drury LS, Diffley JF. Dbf4p, an essential S phase-promoting factor, is targeted for degradation by the anaphase-promoting complex. Mol Cell Biol. 2000;20:242–248. doi: 10.1128/mcb.20.1.242-248.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson KA, Kee K, Maleki S, Santini PA, Keeney S. Cyclin-dependent kinase directly regulates initiation of meiotic recombination. Cell. 2006;125:1321–1332. doi: 10.1016/j.cell.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochwagen A, Amon A. Checking your breaks: surveillance mechanisms of meiotic recombination. Curr Biol. 2006;16:R217–228. doi: 10.1016/j.cub.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Hochwagen A, Tham WH, Brar GA, Amon A. The FK506 binding protein Fpr3 counteracts protein phosphatase 1 to maintain meiotic recombination checkpoint activity. Cell. 2005;122:861–873. doi: 10.1016/j.cell.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Hollingsworth RE, Jr, Sclafani RA. Yeast pre-meiotic DNA replication utilizes mitotic origin ARS1 independently of CDC7 function. Chromosoma. 1993;102:415–420. doi: 10.1007/BF00360406. [DOI] [PubMed] [Google Scholar]
- Katis VL, Lipp JJ, Imre R, Bogdanova A, Okaz E, Habermann B, Mechtler K, Nasmyth K, Zachariae W. Rec8 phosphorylation by casein kinase 1 and Cdc7-Dbf4 kinase regulates cohesin cleavage by separase during meiosis. Dev Cell. 2010;18:397–409. doi: 10.1016/j.devcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katou Y, Kanoh Y, Bando M, Noguchi H, Tanaka H, Ashikari T, Sugimoto K, Shirahige K. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature. 2003;424:1078–1083. doi: 10.1038/nature01900. [DOI] [PubMed] [Google Scholar]
- Keeney S. Spo11 and the Formation of DNA Double-Strand Breaks in Meiosis. In: Lankenau DH, editor. Recombination and Meiosis. Heidelberg, Germany: Springer-Verlag; 2007. pp. 81–123. [Google Scholar]
- Klein F, Mahr P, Galova M, Buonomo SB, Michaelis C, Nairz K, Nasmyth K. A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell. 1999;98:91–103. doi: 10.1016/S0092-8674(00)80609-1. [DOI] [PubMed] [Google Scholar]
- Kugou K, Fukuda T, Yamada S, Ito M, Sasanuma H, Mori S, Katou Y, Itoh T, Matsumoto K, Shibata T, et al. Rec8 guides canonical Spo11 distribution along yeast meiotic chromosomes. Mol Biol Cell. 2009;20:3064–3076. doi: 10.1091/mbc.E08-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev. 2010;24:1208–1219. doi: 10.1101/gad.1933010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib K, Gambus A. A key role for the GINS complex at DNA replication forks. Trends Cell Biol. 2007;17:271–278. doi: 10.1016/j.tcb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- LeClere AR, Yang JK, Kirkpatrick DT. The role of CSM3, MRC1, and TOF1 in minisatellite stability and large loop DNA repair during meiosis in yeast. Fungal Genet Biol. 2012;50:33–43. doi: 10.1016/j.fgb.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos J, Lipp JJ, Bogdanova A, Guillot S, Okaz E, Junqueira M, Shevchenko A, Zachariae W. Dbf4-dependent CDC7 kinase links DNA replication to the segregation of homologous chromosomes in meiosis I. Cell. 2008;135:662–678. doi: 10.1016/j.cell.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Ogino K, Noguchi E, Russell P, Masai H. Hsk1-Dfp1/Him1, the Cdc7-Dbf4 kinase in Schizosaccharomyces pombe, associates with Swi1, a component of the replication fork protection complex. J Biol Chem. 2005;280:42536–42542. doi: 10.1074/jbc.M510575200. [DOI] [PubMed] [Google Scholar]
- McFarlane RJ, Mian S, Dalgaard JZ. The many facets of the Tim-Tipin protein families' roles in chromosome biology. Cell Cycle. 2010;9:700–705. doi: 10.4161/cc.9.4.10676. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Ito M, Kugou K, Yamada S, Furuichi M, Oda A, Yamada T, Hirota K, Masai H, Ohta K. A central coupler for recombination initiation linking chromosome architecture to S phase checkpoint. Mol Cell. 2012;47:722–733. doi: 10.1016/j.molcel.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Murakami H, Borde V, Nicolas A, Keeney S. Gel electrophoresis assays for analyzing DNA double-strand breaks in Saccharomyces cerevisiae at various spatial resolutions. Methods Mol Biol. 2009;557:117–142. doi: 10.1007/978-1-59745-527-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Borde V, Shibata T, Lichten M, Ohta K. Correlation between premeiotic DNA replication and chromatin transition at yeast recombination initiation sites. Nucleic Acids Res. 2003;31:4085–4090. doi: 10.1093/nar/gkg441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Keeney S. Regulating the formation of DNA double-strand breaks in meiosis. Genes Dev. 2008;22:286–292. doi: 10.1101/gad.1642308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Nurse P. Regulation of premeiotic S phase and recombination-related double-strand DNA breaks during meiosis in fission yeast. Nat Genet. 2001;28:290–293. doi: 10.1038/90142. [DOI] [PubMed] [Google Scholar]
- Newlon CS, Collins I, Dershowitz A, Deshpande AM, Greenfeder SA, Ong LY, Theis JF. Analysis of replication origin function on chromosome III of Saccharomyces cerevisiae. Cold Spring Harb Symp Quant Biol. 1993;58:415–423. doi: 10.1101/sqb.1993.058.01.048. [DOI] [PubMed] [Google Scholar]
- Ogino K, Masai H. Rad3-Cds1 mediates coupling of initiation of meiotic recombination with DNA replication. Mei4-dependent transcription as a potential target of meiotic checkpoint. J Biol Chem. 2006;281:1338–1344. doi: 10.1074/jbc.M505767200. [DOI] [PubMed] [Google Scholar]
- Osborn AJ, Elledge SJ. Mrc1 is a replication fork component whose phosphorylation in response to DNA replication stress activates Rad53. Genes Dev. 2003;17:1755–1767. doi: 10.1101/gad.1098303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizza S, Mendoza MA, Berlinger M, Huang L, Nicolas A, Shirahige K, Klein F. Spo11-accessory proteins link double-strand break sites to the chromosome axis in early meiotic recombination. Cell. 2011;146:372–383. doi: 10.1016/j.cell.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Sasanuma H, Hirota K, Fukuda T, Kakusho N, Kugou K, Kawasaki Y, Shibata T, Masai H, Ohta K. Cdc7-dependent phosphorylation of Mer2 facilitates initiation of yeast meiotic recombination. Genes Dev. 2008;22:398–410. doi: 10.1101/gad.1626608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild D, Byers B. Meiotic effects of DNA-defective cell division cycle mutations of Saccharomyces cerevisiae. Chromosoma. 1978;70:109–130. doi: 10.1007/BF00292220. [DOI] [PubMed] [Google Scholar]
- Sherwood R, Takahashi TS, Jallepalli PV. Sister acts: coordinating DNA replication and cohesion establishment. Genes Dev. 2010;24:2723–2731. doi: 10.1101/gad.1976710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmoto M, Matsumoto S, Odagiri Y, Noguchi E, Russell P, Masai H. Interactions between Swi1-Swi3, Mrc1 and S phase kinase, Hsk1 may regulate cellular responses to stalled replication forks in fission yeast. Genes Cells. 2009;14:669–682. doi: 10.1111/j.1365-2443.2009.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Whitehouse I. Intrinsic coupling of lagging-strand synthesis to chromatin assembly. Nature. 2012;483:434–438. doi: 10.1038/nature10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KN, Penkner A, Ohta K, Klein F, Nicolas A. B-type cyclins CLB5 and CLB6 control the initiation of recombination and synaptonemal complex formation in yeast meiosis. Curr Biol. 2001;11:88–97. doi: 10.1016/s0960-9822(01)00026-4. [DOI] [PubMed] [Google Scholar]
- Thacker D, Mohibullah N, Zhu X, Keeney S. Homolog engagement controls meiotic DNA break number and distribution. Nature. 2014 doi: 10.1038/nature13120. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonami Y, Murakami H, Shirahige K, Nakanishi M. A checkpoint control linking meiotic S phase and recombination initiation in fission yeast. Proc Natl Acad Sci U S A. 2005;102:5797–5801. doi: 10.1073/pnas.0407236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Niu H, Futcher B, Zhang C, Shokat KM, Boulton SJ, Hollingsworth NM. Cdc28-Clb5 (CDK-S) and Cdc7-Dbf4 (DDK) collaborate to initiate meiotic recombination in yeast. Genes Dev. 2008;22:386–397. doi: 10.1101/gad.1626408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Zhang C, Shokat KM, Hollingsworth NM. Chemical inactivation of cdc7 kinase in budding yeast results in a reversible arrest that allows efficient cell synchronization prior to meiotic recombination. Genetics. 2006;174:1767–1774. doi: 10.1534/genetics.106.064303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich M, Liang C, Chen HH, Stillman B. Binding of cyclin-dependent kinases to ORC and Cdc6p regulates the chromosome replication cycle. Proc Natl Acad Sci U S A. 2001;98:11211–11217. doi: 10.1073/pnas.201387198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich M, Stillman B. Cdc7p–Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J. 1999;18:5334–5346. doi: 10.1093/emboj/18.19.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PY, Nurse P. Replication origin selection regulates the distribution of meiotic recombination. Mol Cell. 2014;53:655–662. doi: 10.1016/j.molcel.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Boone C, Klein HL. Mrc1 is required for sister chromatid cohesion to aid in recombination repair of spontaneous damage. Mol Cell Biol. 2004;24:7082–7090. doi: 10.1128/MCB.24.16.7082-7090.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuki N, Terashima H, Kitada K. Mapping of early firing origins on a replication profile of budding yeast. Genes Cells. 2002;7:781–789. doi: 10.1046/j.1365-2443.2002.00559.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.