Abstract
The human immunodeficiency virus (HIV) prevention continuum is a framework that illustrates the interconnectedness of each step in the spectrum of prevention services, while emphasizing that all steps are needed to decrease HIV acquisition and transmission. This continuum, similar to the HIV care continuum, begins with HIV testing followed by linkage of HIV-uninfected persons to prevention services, retention in such services, and adherence to prevention interventions with repeated HIV testing to monitor for HIV acquisition. To advance the global goal of zero new HIV infections, individuals must receive the entire continuum of prevention services, and no partial credit can be given to achievement of one step in isolation of all steps in the continuum.
Keywords: HIV, prevention, continuum of care
The human immunodeficiency virus (HIV) care continuum, or HIV care cascade, has been heralded as an important tool for measuring the performance of HIV care and treatment programs [1]. Studies have indicated that every step in the HIV care continuum is important, and that improvement in each step must be accomplished to meaningfully achieve viral load suppression [1–3]. Although this framework has gained much attention in the context of HIV care and treatment for persons living with HIV, little attention has been directed to the utility of a similar framework for HIV prevention. The conceptualization of an HIV prevention continuum illustrates the interconnectedness of each step in the spectrum of prevention services, while emphasizing that all steps are needed to decrease HIV acquisition and transmission [4].
PROPOSED STEPS IN THE HIV PREVENTION CONTINUUM
The HIV prevention continuum (Figure 1), similar to the HIV care continuum, builds on HIV testing as its foundation followed by linkage of HIV-uninfected persons to prevention services, retention in services, and adherence to services to prevent HIV acquisition and transmission. The common desired endpoint of the prevention continuum is ensuring that individuals remain HIV-uninfected.
Figure 1.
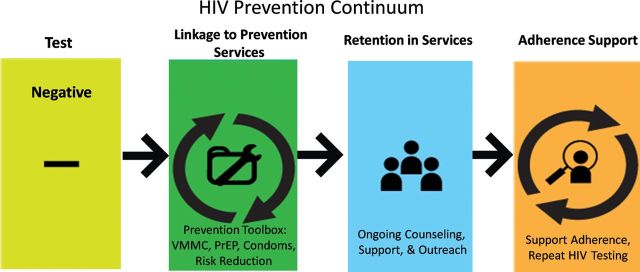
The HIV prevention continuum. Abbreviations: HIV, human immunodeficiency virus; PrEP, preexposure prophylaxis; VMMC, voluntary medical male circumcision.
In countries with high HIV prevalence, universal access to HIV testing is recommended, yet the median proportion of persons who ever received an HIV test and their results in low- and middle-income countries is 33.6% among women and 17.2% among men [5]. Annual HIV testing is recommended for people who inject drugs [6], and repeat testing, as frequent as every 3–6 months, has been recommended for men who have sex with men (MSM) [7]. Expansion of HIV testing is recommended for adolescents (aged 10–19 years) in high-prevalence settings, where access to and coverage of antiretroviral therapy (ART) is lower than for adults and children [8]. Testing efforts are critical to provide an opportunity to identify individuals at high risk such as HIV-uninfected partners in serodiscordant relationships. Whereas studies have shown disparate estimates with regard to the magnitude of contribution of transmission among discordant couples to new infections [9, 10], HIV-uninfected persons within such partnerships are at substantial risk for HIV acquisition [11, 12]. In the recent Kenya AIDS Indicator Survey from 2012, an estimated 260 000 couples were HIV discordant, highlighting the importance of testing partners of HIV-infected individuals [13].
Identification of HIV-uninfected individuals compels the need to link them to evidence-based prevention interventions—including biomedical interventions (eg, voluntary medical male circumcision [VMMC] and preexposure prophylaxis [PrEP]), behavioral interventions (eg, risk reduction counseling, condom use, syringe needle exchange programs, and opiate substitution therapy), and structural interventions that influence prevention (eg, transport reimbursement, stable housing, and policies that influence employment, migration, and gender inequality [16–18]). There is a paucity of data regarding linkage to HIV prevention services for individuals found to be HIV uninfected. Testing programs must ensure that an HIV-negative test result not be the end of the continuum, but the beginning of a path along the HIV prevention continuum through linkage of uninfected individuals into prevention services.
HIV prevention for uninfected individuals at risk should not be perceived as a one-time event, but rather as requiring ongoing engagement, or retention, in the prevention process for as long as the risk remains in order to maintain the desired protective effect of the interventions. The importance of retention in prevention services and adherence to interventions is evident even for VMMC, a one-time biomedical intervention with 60% protective efficacy [19–21]. Protection from HIV acquisition in men who had VMMC is not complete, necessitating ongoing risk reduction counseling and consistent condom use [22]. Retention is also important to ensure repeat HIV testing and early diagnosis of HIV infection, if HIV acquisition occurs. Newly infected individuals must be promptly linked to HIV care and treatment and other prevention methods including partner testing, condom use, and antiretroviral drugs for prevention [4].
Finally, adherence to HIV prevention interventions that involve ongoing use, such as PrEP, is essential for efficacy [23]. Oral PrEP has been demonstrated to be efficacious among high-risk groups such as MSM, people who inject drugs, serodiscordant couples, and at-risk women, yet not all PrEP study results have been consistent. The iPrEX, Partners PrEP, and TDF2 studies showed that oral PrEP provided 44% protection for HIV acquisition in HIV-uninfected MSM, 73% protection for HIV-uninfected partners in discordant couples, and 63% protection for women in Botswana [12, 24, 25]. However, neither the FEM-PrEP nor the VOICE studies showed efficacy in women of oral tenofovir/emtricitabine, nor oral or vaginal tenofovir [26, 27]. Reasons for varying efficacy in the PrEP studies were contingent on adherence with the antiretroviral medication [23]. These findings have highlighted the importance of combination packages of prevention interventions to include structural and behavioral strategies to enhance both retention and adherence to the intervention, in order to achieve maximal efficacy of the prevention package [28].
INTERSECTION OF THE HIV CARE AND PREVENTION CONTINUA
The HIV care and prevention continua are not parallel trajectories, but rather should be conceptualized as complementary paths that will often intersect for individuals and couples. Individuals who are initially HIV uninfected may retest and be found to have acquired HIV infection and need to transition to the HIV care continuum for prompt assessment and initiation of ART, as appropriate, to ultimately achieve viral load suppression. Serodiscordant couples span the 2 continua, with the HIV-infected partner needing linkage to and retention in the care continuum, and the HIV-uninfected partner needing repeat testing and possibly PrEP in conjunction with adherence and risk reduction counseling. Similarly, HIV-infected pregnant women span the continua, with the HIV-infected mother requiring initiation of antiretroviral therapy and achievement of viral load suppression to prevent transmission to her infant, who will need linkage to prevention services during breastfeeding in the postpartum period.
CONCLUSIONS
At a population level, both the HIV care and the HIV prevention continua are necessary for framing a comprehensive response to the HIV epidemic. Both continua must focus on the very beginning of the trajectory—identifying those who are HIV-infected in a community but are unaware of their infection as well as those who are uninfected and should be the target of the various interventions at each step of the HIV prevention continuum. Achievement of one step in either continuum may benefit individuals and should be applauded; however, all steps must be optimized to maximize the impact of care, treatment, and prevention services and raise the bar of program performance [29]. The use of a prevention continuum may offer a new framework to measure HIV prevention program performance by linking steps and encouraging engagement across all the steps. Conceptualization and implementation of the prevention continuum for specific populations at risk will require careful analysis of the size and location of such populations, impediments to their engagement in prevention, efforts at demand generation for such services, tailoring of the prevention packages, and training of the appropriate workforce to be engaged in such efforts, as well as design of supportive activities to promote linkage, retention, and adherence in prevention services. In addition, new monitoring tools will need to be developed to measure each step of the prevention continuum and, most important, the quality and effectiveness of the overall continuum. To achieve the global goal of an AIDS-free generation, no partial credit can be given to achievement of one step in isolation of all steps [1].
Notes
Financial support. W. M. E.-S. has received funding from the National Institute of Mental Health, National Institutes of Health.
Supplement sponsorship. This article is published as part of a supplement entitled “Controlling the HIV Epidemic With Antiretrovirals,” sponsored by the International Association of Providers of AIDS Care.
Potential conflicts of interest. Both authors report no potential conflicts.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McNairy ML, El-Sadr WM. The HIV care continuum: no partial credit given. AIDS. 2012;26:1735–8. doi: 10.1097/QAD.0b013e328355d67b. [DOI] [PubMed] [Google Scholar]
- 2.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems’ performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;56:e45. doi: 10.1097/QAI.0b013e3181fdbf20. [DOI] [PubMed] [Google Scholar]
- 4.McNairy ML, Howard AA, El-Sadr WM. Antiretroviral therapy for prevention of HIV and tuberculosis: a promising intervention but not a panacea. J Acquir Immune Defic Syndr. 2013;63(suppl 2):S200–7. doi: 10.1097/QAI.0b013e3182986fc6. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization/United Nations Children's Fund/United Nations Joint Programme on HIV/AIDS. Geneva, Switzerland,: 2010. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. [Google Scholar]
- 6.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach, 2010 revision. Geneva, Swizterland: 2010. [PubMed] [Google Scholar]
- 7.Finlayson TJ, Le B, Smith A, et al. HIV risk, prevention, and testing behaviors among men who have sex with men--National HIV Behavioral Surveillance System, 21 U.S. cities, United States, 2008. MMWR Surveill Summ. 2011;60:1–34. [PubMed] [Google Scholar]
- 8.World Health Organization. HIV and adolescents: guidance for HIV testing and counselling and care for adolescents living with HIV. Geneva, Swizterland: 2013. [Google Scholar]
- 9.Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371:2183–91. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 10.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287:650–4. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 11.Gray R, Ssempiija V, Shelton J, et al. The contribution of HIV-discordant relationships to new HIV infections in Rakai, Uganda. AIDS. 2011;25:863–5. doi: 10.1097/QAD.0b013e3283448790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National AIDS and STI Control Programme, Ministry of Health, Kenya. Nairobi, Kenya: Ministry of Health; 2013. Kenya AIDS indicator survey 2012 preliminary report. In. [Google Scholar]
- 16.Sumartojo E, Doll L, Holtgrave D, Gayle H, Merson M. Enriching the mix: incorporating structural factors into HIV prevention. AIDS. 2000;14(suppl 1):S1–2. doi: 10.1097/00002030-200006001-00001. [DOI] [PubMed] [Google Scholar]
- 17.Parker RG, Easton D, Klein CH. Structural barriers and facilitators in HIV prevention: a review of international research. AIDS. 2000;14(suppl 1):S22–32. doi: 10.1097/00002030-200006001-00004. [DOI] [PubMed] [Google Scholar]
- 18.Sumartojo E. Structural factors in HIV prevention: concepts, examples, and implications for research. AIDS. 2000;14(suppl 1):S3–10. doi: 10.1097/00002030-200006001-00002. [DOI] [PubMed] [Google Scholar]
- 19.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 20.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 21.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes R, Ayles H, Beyers N, et al. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment—a study protocol for a cluster randomised trial. Trials. 2014;15:57. doi: 10.1186/1745-6215-15-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baeten JM, Haberer JE, Liu AY, Sista N. Preexposure prophylaxis for HIV prevention: where have we been and where are we going? J Acquir Immune Defic Syndr. 2013;63(suppl 2):S122–9. doi: 10.1097/QAI.0b013e3182986f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 26.Marrazzo J, Ramjee G, Nair G, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE study (MTN 003). 20th Conference on Retroviruses and Opportunistic Infections,; Atlanta, GA. 2013. [Google Scholar]
- 27.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNairy ML, Cohen M, El-Sadr WM. Antiretroviral therapy for prevention is a combination strategy. Curr HIV/AIDS Rep. 2013;10:152–8. doi: 10.1007/s11904-013-0152-1. [DOI] [PubMed] [Google Scholar]
- 29.Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. JAMA. 2006;295:1168–70. doi: 10.1001/jama.295.10.1168. [DOI] [PubMed] [Google Scholar]


