Abstract
Oral antiretroviral preexposure prophylaxis (PrEP) has been shown to decrease human immunodeficiency virus (HIV) incidence in studies of men who have sex with men, heterosexual men and women, and injecting drug users. One study of pericoital tenofovir gel demonstrated that it reduced HIV incidence in South African women. However, other studies of African women failed to demonstrate protection with either oral tenofovir or tenofovir-emtricitabine, or daily tenofovir gel. The magnitude of PrEP protection appears to be highly correlated with medication adherence. New studies are evaluating whether different antiretrovirals, including dapivirine, rilpivirine, maraviroc, and new integrase inhibitors. Different formulations are also being evaluated, including gels, films, vaginal rings, and injectable medication. Although PrEP efficacy has been demonstrated, and several normative bodies (eg, the US Food and Drug Administration) have approved PrEP for clinical use, uptake has been slow. Reasons may include lack of sufficient provider and consumer education, residual concerns about costs, potential long-term toxicities, and behavioral disinhibition. Additional work is under way to determine how to best educate consumers and providers about optimal adherence and to use PrEP in conjunction with risk mitigation.
Keywords: PrEP, HIV prevention, HIV risk, antiretrovirals, medication adherence
The HIV Prevention Trials Network (HPTN) 052 study was the first randomized controlled trial to demonstrate that early initiation of antiretroviral therapy decreased human immunodeficiency virus (HIV) transmission from HIV-infected individuals to their uninfected partners [1]. However, despite the 96% decrease in transmission seen in HPTN 052, some have expressed concerns that relying on treating all people living with HIV to arrest the epidemic will not yield short-term benefits [2]. The reasons for that include the fact that there were very few men who have sex with men (MSM) and injecting drug users in HPTN 052, so the full magnitude of the effect is not fully understood. Moreover, in the United Kingdom and Denmark, despite widespread access to medication, HIV incidence has not decreased [3, 4]. Moreover, only about a third of people living with HIV across the globe are currently on treatment, so full access will take years [5]. Additionally, not all individuals who are living with HIV may want to initiate treatment when they are asymptomatic and have high CD4 counts, and some individuals may equate initiation of treatment with an expectation that they have more advanced disease [6]. Globally, the majority of people living with HIV are unaware of their status; thus, initial efforts for treatment as prevention will have to focus on getting more individuals tested and linked to care, but population-level virologic suppression may take a considerable amount of time and resources. Moreover, HIV stigma continues to limit willingness to disclose [7]. The ability of preexposure prophylaxis (PrEP) to prevent new HIV infections should not be seen a substitute for wider access to treatment for people living with HIV, but may be a helpful adjunct to slowing epidemic spread if clinicians and public health officials can identify those individuals who might be most likely to benefit from PrEP [8, 9].
Current Status of PrEP
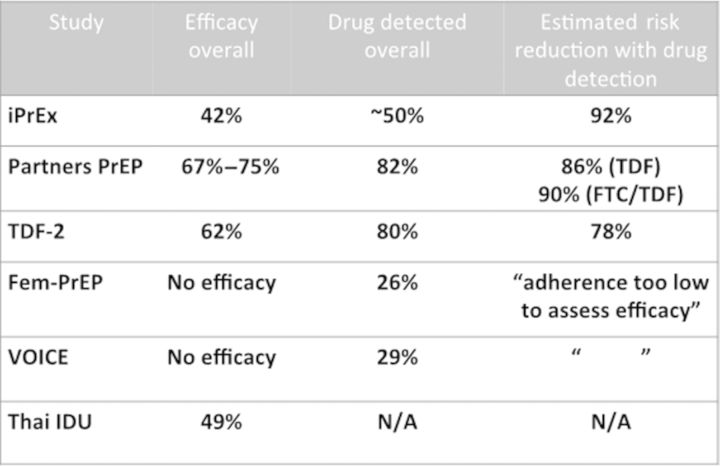
As of early 2014, there have been 6 efficacy trials of antiretroviral PrEP, with 4 demonstrating significant benefit in diverse populations. The iPrEX Study (“Iniciativa Profilaxis Pre-Exposicion” in Spanish) followed MSM and transgender women in North and South America (“Iniciativa Profilaxis Pre-Exposicion” in Spanish) as well as Thailand and South Africa and demonstrated a 42% overall efficacy [10]. The Partners PrEP [11] and the tenofovir disiproxil fumarate 2 (TDF2) [12] studies followed heterosexuals in sub-Saharan Africa, with the former focusing on discordant couples and the latter on individuals who did not enroll with infected partners. Both studies demonstrated efficacy for men and women with a 75% decrease in HIV incidence among individuals randomized to tenofovir-emtricitabine in Partners PrEP (and 67% decrease in HIV incidence among those randomized to tenofovir alone), and 62% efficacy for participants enrolled in TDF2). A more recent study among injecting drug users in Thailand demonstrated a decrease in HIV incidence of 49% percent [13]. However, 2 other studies, FEM-PrEP and the VOICE trial, did not demonstrate efficacy in any of the active intervention arms, which included tenofovir-emtricitabine in both FEM-PrEP [14] and VOICE [15], as well as oral tenofovir alone and tenofovir gel in VOICE. The VOICE gel findings were surprising, given that an earlier study of pericoital topical tenofovir gel, the CAPRISA-004 study, demonstrated a 39% decrease in HIV incidence among women randomized to use the antiretroviral gel [16].
The common thread in all the studies was that overall adherence, as measured by drug detectable in the blood, ranged from 82% in Partners PrEP to <30% in FEM-PrEP and VOICE, and that the efficacy in each of the trials was a direct function of the overall level of medication adherence in the study populations. Subsequent calculations in iPrEX found that individuals who had drug detected in their blood had a 92% decrease in HIV incidence compared with a demographically matched group of those who seroconverted [17]. Similar findings were noted in Partners PrEP and TDF2, whereas in FEM-PrEP and VOICE the levels of drug detection were considered too low to assess efficacy.
Prior to these PrEP studies, concerns were raised about behavioral risk compensation (ie, individuals engaging in riskier behavior if they thought they were protected); however, this was not noted in these initial PrEP studies. Tenofovir has been known to be associated with renal insufficiency, but creatinine elevations were uncommon and were reversible when medication was discontinued. However, it should be noted that individuals had to have normal renal function to participate in the studies; therefore, the effects of chemoprophylaxis on those who do not have normal renal function are not yet understood. The only clinical finding that was statistically significant, albeit not clinically significant, at 18 months, was a trend toward decreased bone density as measured by dual-energy X-ray absorptiometry scans. Studies are ongoing to assess whether this <1% overall decrease in bone in those randomized to tenofovir-emtricitabine continued to progress or tended to plateau, as has been the case among HIV-infected individuals on chronic tenofovir for treatment. Concerns have also been raised about the selection for, and transmission of, HIV drug-resistant strains. This was extremely uncommon in all of the thousands of person-years of follow-up in the initial chemoprophylaxis studies, but it is important to note that individuals were screened for HIV prior to starting the trials and were monitored quite frequently, generally on a monthly basis. Of individuals who developed resistant virus in the course of the study, almost all were in the process of seroconversion when they initially enrolled in the study—that is, because they were viremic with high concentrations of HIV, resistance was selected. However, the resistant strains in most cases had a 184V mutation, which would make the virus resistant to emtricitabine or lamivudine, but at the same time the virus would be less fit and less able to readily replicate, and possibly less likely to be transmitted to other partners. However, it is important to note that all these studies were efficacy trials that involved very careful follow-up and assessment of highly motivated participants. Thus, the open-label demonstration projects that have recently gotten under way are necessary to assess the full impact of PrEP in community settings outside of the controlled conditions of clinical trials.
Because of the lack of efficacy of chemoprophylaxis seen in the VOICE and the FEM-PrEP studies, some have raised the question about whether chemoprophylaxis for at-risk people is a tenable strategy; that is, will people who might benefit be sufficiently adherent to justify wide deployment of a relatively expensive prevention strategy? It should be noted that there are subsets of individuals who have demonstrated high levels of adherence and that modest interventions may be able to have a particularly salutary effect on subsequent adherence. For example, in the Partners PrEP study, there was an adherence substudy intervention among >1000 couples in Uganda that included enhanced contact if pill use dropped below 80%, including unannounced home visits and pill-use measurements by the Medication Event Monitoring System, which could record the times that the pill bottle was opened. Among the individuals in the substudy, no one randomized to tenofovir-emtricitabine became infected with HIV [18]. It may be feasible to train clinicians in optimal ways to identify individuals who may be more likely to be nonadherent. Individuals who were younger were less adherent in several of the trials, so that future interventions may be needed to be tailored for at-risk youth [19]. Additionally, individuals who had not engaged in recent unprotected anal intercourse were less likely to be adherent, suggesting that high-risk individuals may titrate their adherence to their ongoing HIV risk-taking behavior.
PrEP in the Real World
Now that PrEP has been shown to be effective in several different clinical trials, multiple real-world considerations are important to optimize its implementation. At the individual level, some people may perceive certain advantages, particularly if they do not like to use condoms, whereas there may be other challenges. Key questions remain: How to prioritize the populations who might benefit from PrEP [20, 21]? What are the key messages and how to best disseminate them? How to optimize adherence? How to decrease sexual risk taking while individuals are on PrEP? There are also operational issues related to long-term safety, optimal testing strategies, how to best deliver PrEP, and how to optimize cost-effectiveness [22]. At the present time, there are >20 000 individuals slated for enrollment in PrEP demonstration projects that involve provision of open-label medication and monitoring individuals for potential clinical toxicities, risk compensation, and acquisition of resistant virus. Several of these demonstration projects have been quite successful in enrolling clients to date and have demonstrated that there is some interest in PrEP that is increasing among some at-risk persons, particularly MSM in the developed world, although knowledge about PrEP remains limited [23].
How to Improve PrEP?
There are multiple studies now under way to optimize the provision of PrEP, including addressing issues such as how infrequently can chemoprophylaxis be given so that individuals are less likely to have side effects; this may lead to cost savings and may be associated with improved adherence. The ADAPT study (HPTN 067) is looking at at-risk MSM in the United States and Thailand, and at-risk women in South Africa, and attempting to assess if adherence is better with pericoital dosing or whether fixed-event dosing (eg, on Friday and Monday for individuals who engage in high-risk behavior over the weekend) is preferable [24]. The iPergay study being conducted in France, Germany, and Quebec is enrolling high-risk MSM as a placebo-controlled study of peri-event dosing of chemoprophylaxis [25]. Other approaches for chemoprophylaxis are also being studied, such as the use of rectal microbicides (MTN017) and intravaginal rings (the RING and the ASPIRE studies; MTN020) [26]. Other approaches to provision of chemoprophylaxis include the use of injections with antiretrovirals. Rilpivirine (a nonnucleoside reverse transcriptase agent), and a new integrase inhibitor, GSK744, are each being studied for use as injectable PrEP, which might be administered as infrequently as quarterly [27].
Other approaches to optimize PrEP are focusing on adherence with studies that are trying the use of text messaging and other electronic interfaces through social media to improve medication adherence. The impetus for the use of electronic contact with participants to optimize adherence is based on work in East Africa with HIV-infected patients [28]. Work is also under way to determine the optimal ways to enhance PrEP acceptability and adherence, by attending to the social and cultural context of specific populations who might benefit from PrEP. For example, HPTN 073 is a demonstration project in 3 US centers focusing on African-American men who have sex with men and trying to develop a culturally appropriate intervention involving engagement of peers to help motivate engagement in care, addressing unmet common social needs [29, 30]. Studies focusing on adolescents are also under way: ATN 110 and 113 are studies in the Adolescent Trials Network that are offering open-label chemoprophylaxis to high-risk youth aged 15–22 years, and are evaluating the added benefit of either an evidence-based individualized behavioral intervention or a group intervention, based on the recognition that individualized attention or peer support might be particularly helpful to enhance medication adherence in high-risk youth [31].
Assessment of PrEP Effectiveness
Although the Food and Drug Administration approved the use of tenofovir-emtricitabine for chemoprophylaxis 2 years ago, uptake has been limited [32]. This may reflect the diffusion of innovations theory, which posits that with any new technological intervention, there may be a very small group of innovators and early adopters who begin the process of uptake before a new intervention becomes normative; time may be necessary for full uptake [33]. For example, zidovudine was demonstrated to be beneficial to prevent mother-to-child transmission of HIV by 1994; however, full uptake of prenatal HIV testing and antiretroviral chemoprophylaxis took at least 5 more years [34]. Although surveys of at-risk populations suggest that knowledge of PrEP remains quite low and that utilization is comparably low, many individuals indicate that they might be interested in PrEP if they were provided the sufficient information [23]. Another challenge to wider uptake of PrEP is the hesitancy of many medical providers to prescribe chemoprophylaxis. For example, a survey of Massachusetts physicians after the release of the CAPRISA-004 data indicated that the majority had heard of the study, and some knew that PrEP studies were under way, but the majority had concerns about risk compensation, transmission of drug resistance, and cost [35]. The initial reluctance of many providers to prescribe PrEP may be overcome by the development of medical education materials that assist providers in identifying those patients who are most likely to benefit from PrEP and through conversations with peers who have had experience in prescribing PrEP [36]. A recent study of PrEP use in the United States [36] involved a pharmacy record review of 55% of US pharmacies and found that between January 2011 and March 2013, <1800 patients were prescribed PrEP, but that utilization was increasing, particularly women. Prescriptions had been written in 49 US states and 700 US cities, with the largest number of prescriptions being written for individuals in the South. Only a little more than a third of the providers who prescribed PrEP also prescribed highly active antiretroviral therapy, presumably reflecting that the majority of PrEP prescribers in this series were not providing primary care for people living with HIV. These data did not evaluate individuals in clinical trials and demonstration projects, where drug would be provided outside of a pharmacy, but were informative nonetheless. Other recent studies also indicate that limited uptake of PrEP among MSM reflects limited awareness in use but high levels of potential interest [37].
Figure 1.
PrEP works, but adherence is critical. Abbreviations: FTC, emtricitabine; N/A, not applicable; PrEP, preexposure prophylaxis; TDF, tenofovir.
CONCLUSIONS
As of the end of 2013, the proof of concept that PrEP can decrease HIV incidence in diverse populations has been fully established. However, many scientific and implementation questions remain. Over the next few years, it will be critical to develop and test interventions to optimize PrEP delivery by prioritizing the ways in which there will be population impact, with increased appropriate uptake and the development of tools to support PrEP users to enhance their adherence and decrease risk reduction. It is also important that programs be developed to support potential PrEP providers to be able to more readily identify individuals who will most benefit from PrEP, to provide skills to providers that enable them to enhance patient adherence and to decrease risk behavior, and to assist them and PrEP users to make appropriate decisions regarding when to start and stop PrEP. These further developments will entail optimal use of new technology to sustain scalability and sustainability. At the same time, new clinical studies may suggest more parsimonious ways to use tenofovir-emtricitabine (eg, intermittent or episodic), or may support the use of other antiretroviral agents for PrEP such as maraviroc, nevirapine, rilpivirine, or newer integrase inhibitors, and may also support the use of different delivery systems including topical gels, vaginal rings, and injectable agents.
Some writers have voiced concerns that the cost of PrEP is sufficiently high, >$10 000/year for medication per person alone in the developed world and >$100/person in the developing world, that this is not a sustainable way to halt the spread of HIV. However, by identifying the individuals who might be most likely to become HIV infected, and by determining how to use PrEP for discrete periods of time during periods of greatest risk, the resources used for PrEP in the short term may prevent new cases of HIV in the longer term. The judicious use of PrEP, and further developments in the science, coupled with wider expansion of HIV testing, engagement in care, early initiation of treatment, and support for medication adherence for those living with HIV, may have a substantial impact in decreasing the >2 million new HIV infections per year that are anticipated in the near future.
Notes
Acknowledgments. The author acknowledges the assistance of Andrea Karis for technical assistance in the preparation of this manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases/Harvard Center for AIDS Research (grant number P30AI06354) and by the International Association of Providers of AIDS Care.
Supplement sponsorship. This article is published as part of a supplement entitled “Controlling the HIV Epidemic With Antiretrovirals,” sponsored by the International Association of Providers of AIDS Care.
Potential conflicts of interest. Author certifies no potential conflicts of interest.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips A, Baker J, Lundgren J. Are antiretrovirals enough for people living with HIV? Lancet. 2013;382:1466–7. doi: 10.1016/S0140-6736(13)62072-3. [DOI] [PubMed] [Google Scholar]
- 3.Audelin AM, Cowan SA, Obel N, Nielsen C, Jorgensen LB, Gerstoft J. Phylogenetics of the Danish HIV epidemic: the role of very late presenters in sustaining the epidemic. J Acquir Immune Defic Syndr. 2013;62:102–8. doi: 10.1097/QAI.0b013e318276becc. [DOI] [PubMed] [Google Scholar]
- 4.Birrell PJ, Gill ON, Delpech VC, et al. HIV incidence in men who have sex with men in England and Wales 2001–10: a nationwide population study. Lancet Infect Dis. 2013;13:313–8. doi: 10.1016/S1473-3099(12)70341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United Nations Joint Programme on HIV/AIDS. Geneva, Switzerland: UNAIDS; 2014. Global AIDS response progress reporting 2014: construction of core indicators for monitoring the 2011 UN political declaration on HIV/AIDS. [Google Scholar]
- 6.Curran K, Ngure K, Shell-Duncan B, et al. ‘If I am given antiretrovirals I will think I am nearing the grave’: Kenyan HIV serodiscordant couples' attitudes regarding early initiation of antiretroviral therapy. AIDS. 2014;28:227–33. doi: 10.1097/QAD.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai AC, Bangsberg DR, Weiser SD. Harnessing poverty alleviation to reduce the stigma of HIV in sub-Saharan Africa. PLoS Med. 2013;10:e1001557. doi: 10.1371/journal.pmed.1001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paltiel AD, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48:806–15. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juusola JL, Brandeau ML, Owens DK, Bendavid E. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med. 2012;156:541–50. doi: 10.1059/0003-4819-156-8-201204170-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 13.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 14.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrazzo J, Ramjee G, Nair G. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine or vaginal tenofovir gel in the VOICE study (MTN 003). 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 16.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. CAPRISA 004 Trial Group. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson PL, Glidden DV, Liu A, et al. iPrEx Study Team. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosek SG, Siberry G, Bell M, et al. The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men. J Acquir Immune Defic Syndr. 2013;62:447–56. doi: 10.1097/QAI.0b013e3182801081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Underhill K, Mayer KH. Sexual behaviour among users of antiretroviral pre-exposure prophylaxis. Lancet Infect Dis. 2013;13:996–7. doi: 10.1016/S1473-3099(13)70251-2. [DOI] [PubMed] [Google Scholar]
- 21.Smith DK, Pals SL, Herbst JH, Shinde S, Carey JW. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421–7. doi: 10.1097/QAI.0b013e318256b2f6. [DOI] [PubMed] [Google Scholar]
- 22.Baeten JM, Haberer JE, Liu AY, Sista N. Preexposure prophylaxis for HIV prevention: where have we been and where are we going? J Acquir Immune Defic Syndr. 2013;63(suppl 2)):S122–129. doi: 10.1097/QAI.0b013e3182986f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krakower DS, Mimiaga MJ, Rosenberger JG, et al. Limited awareness and low immediate uptake of pre-exposure prophylaxis among men who have sex with men using an internet social networking site. PLoS One. 2012;7:e33119. doi: 10.1371/journal.pone.0033119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. HIV Prevention Trials Network. Available at: http://www.hptn.org/ Accessed 2 February 2014.
- 25. L'ANRS (Agence nationale de recherches sur le sida et les hépatites virales). Available at http://www.ipergay.fr/ Accessed 3 February 2014.
- 26. Microbicide Trials Network. Available at: http://www.mtnstopshiv.org/ . Accessed 3 February 2014.
- 27.Spreen W, Williams P, Margolis D, et al. First study of repeat dose co-administration of GSK1265744 and TMC278 long-acting parenteral nanosuspensions: pharmacokinetics, safety and tolerability in healthy adults. 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention,; Kuala Lumpur, Malaysia. 2013. [Google Scholar]
- 28.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–45. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 29.Koblin B, Mayer K, Eshelman S, et al. Correlates of HIV acquisition in a cohort of black men who have sex with men in the United States: HIV Prevention Trials Network (HPTN) 061. PLoS One. 2013;8:e70413. doi: 10.1371/journal.pone.0070413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer K, Wang L, Koblin B, et al. Concomitant socioeconomic, behavioral, and biological factors associated with the disproportionate HIV infection burden among black men who have sex with men in 6 U.S. cities. PLoS One. 2014 doi: 10.1371/journal.pone.0087298. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. AIDS Vaccine Advocacy Coalition. Available at: http://www.avac.org/ . Accessed 3 February 2014.
- 32.Rogers E. Diffusion of innovations. 5th ed. New York: Free Press,; 2003. [Google Scholar]
- 33.Lindegren ML, Byers RH, Jr, Thomas P, et al. Trends in perinatal transmission of HIV/AIDS in the United States. JAMA. 1999;282:531–8. doi: 10.1001/jama.282.6.531. [DOI] [PubMed] [Google Scholar]
- 34.White JM, Mimiaga MJ, Krakower DS, Mayer KH. Evolution of Massachusetts physician attitudes, knowledge, and experience regarding the use of antiretrovirals for HIV prevention. AIDS Patient Care STDS. 2012;26:395–405. doi: 10.1089/apc.2012.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krakower D, Mayer KH. What primary care providers need to know about preexposure prophylaxis for HIV prevention: a narrative review. Ann Intern Med. 2012;157:490–7. doi: 10.7326/0003-4819-157-7-201210020-00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawlings K, Mera R, Pechonkina A, et al. Gilead Sci FCC. Antiretroviral therapy: initiation, switch, novel combinations and outcomes. 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Denver, CO. 2013. [Google Scholar]
- 37.Holt M, Murphy D, Callander D, et al. HIV-negative and HIV-positive gay men's attitudes to medicines, HIV treatments and antiretroviral-based prevention. AIDS Behav. 2013;17:2156–61. doi: 10.1007/s10461-012-0313-z. [DOI] [PubMed] [Google Scholar]