Abstract
Mathematical models and recent data from ecological, observational, and experimental studies show that antiretroviral therapy (ART) is effective for both treatment and prevention of HIV, validating the treatment as prevention (TasP) approach. Data from a variety of settings, including resource-rich and -limited sites, show that patient attrition occurs at each stage of the human immunodeficiency virus (HIV) treatment cascade, starting with the percent unaware of their HIV infection in a population and linkage to care after diagnosis, assessment of ART readiness, receipt of ART, and finally long-term virologic suppression. Therefore, in order to implement TasP, we must first define practical and effective linkage to care, acceptability of treatment, and adherence and retention monitoring strategies, as well as the cost-effectiveness of such strategies. Ending this pandemic will require the combination of political will, resources, and novel effective interventions that are not only feasible and cost effective but also likely to be used in combination across successive steps on the HIV treatment cascade.
Keywords: HIV, ART, treatment as prevention, test-and-treat, treatment cascade
The debate about the prioritization of treatment vs prevention for human immunodeficiency virus (HIV) infection has ended. Beyond observational evidence, the landmark HIV Prevention Trial Network (HPTN) 052 study indicates that earlier initiation of antiretroviral therapy (ART) can decrease plasma HIV viral load and dramatically reduce the likelihood of sexual transmission [1]. HIV testing and immediate ART initiation for infected individuals is known as the treatment as prevention (TasP) strategy. Mathematical models [2] and ecological studies from North America [3, 4], the United Kingdom [5], and Africa [6] postulate effective control of the spread of the disease and ultimately the end of the HIV pandemic if a large number of infected persons can be effectively engaged on ART and kept virologically suppressed. However, data from developed and developing countries demonstrate that a substantial reduction in patient retention occurs between each stage of the HIV treatment continuum—from diagnosis and assessment of ART readiness to acceptability, receipt of initial ART, adherence, long-term retention in care, and treatment success as reflected by virologic suppression (Figure 1). It is essential that the treatment cascade challenges of TasP be addressed so as to achieve the goal of an AIDS-free generation. In this paper we provide an overview of selected challenges and discuss possible approaches to overcome them.
Figure 1.
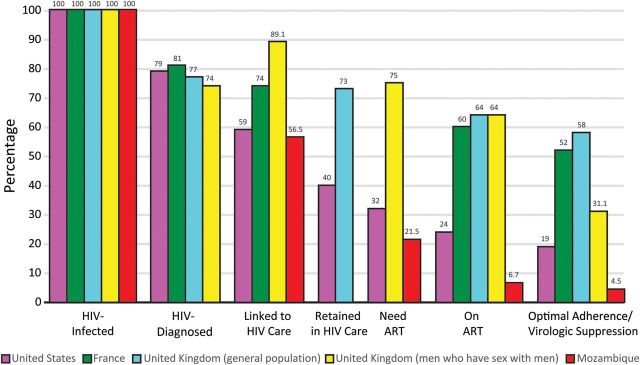
The spectrum of engagement in HIV care in United States, United Kingdom, France and Mozambique traversing from HIV acquisition to achievement of complete viral suppression [5, 7–10].
EARLY HIV TESTING AND LINKAGE TO CARE
For maximum clinical and public health benefits, ART should be initiated at an early stage of infection. Although it is challenging to identify patients during acute and early stages of HIV infection, novel testing strategies such as fourth generation HIV testing, which incorporates antigen to antibody testing, will increase the number of patients who are diagnosed early in HIV infection [11]. Late diagnosis and late ART initiation are major challenges as the majority of patients start ART late when the immune system is already compromised. In 2010, the median CD4+ T-cell count (cells/microliter) at ART initiation was <200 cells/μL in resource-limited settings and only around 200 cells/μL in resource-rich settings [12], and in contrast with the latest recommendations by the World Health Organization and European AIDS Clinical Society (CD4+ T-cell count >500 cells/µL), the International AIDS Society-USA, the US Department of Health and Human Services, and the French HIV treatment guidelines (irrespective of CD4+ T-cell count) [13–17]. Novel and emerging strategies to engage infected individuals early include point-of-care testing, self-testing, and home-based testing visits. A study in Kenya found that patients who accepted home testing had CD4+ T-cell counts that were 100 cells/µL or higher, on average, than those who visited voluntary testing centers [18]. As with all healthcare programs, widespread testing requires resources and trained individuals, and it is essential that testing programs consider and plan linkage to care, which is the next step in the treatment cascade.
HEALTH SYSTEMS AND HUMAN RESOURCES STRENGTHENING
Many countries that are greatly affected by HIV also have healthcare systems with limited financing and severe human resource constraints. Basic AIDS services include counseling and testing, HIV/tuberculosis treatment, prevention of mother-to-child transmission (PMTCT), and pediatric services. The efforts required to ensure ART education and adherence, safety, and retention in care further strain human resource capacity. Maintaining the drug and diagnostic supply chain is inherently challenging, particularly in low-income countries, and the increased demand resulting from TasP may exacerbate this [19]. With declining financial support from development partners, lower-income countries will have to determine if TasP merits more resource allocation than other health priorities. Simplification of the services and decentralization of care represents important steps toward reducing human resource limitations. Even in more affluent countries, it is unclear whether the healthcare systems have the human resources needed to meet contemporary HIV treatment and prevention service needs. It remains to be seen whether required resources can be allocated globally to achieve the promise of TasP.
Task-shifting initiatives that include community health workers or paraprofessionals can help when dealing with challenges related to healthcare shortages and in turn optimize ART adherence, retention in care, psychological concerns, and even income generation. Indeed, 3 randomized controlled trials (RCTs) reported noninferiority of ART care compared with physician in South Africa and Uganda [20–22]. Also, community-based workers who provide treatment and adherence support for people living with HIV/AIDS and tuberculosis [23] and who improve maternal and child health [24] have been documented. Finally, patient-nominated treatment supporters or HIV support groups can help others by serving as peer mentors, relating personal experiences and strategies for coping with individual, structural, and social barriers related to HIV/AIDS and its treatment [25–27]. Harnessing the HIV-infected community will be necessary to keep human resource costs low and to maximize the ongoing involvement of patients in care [19].
TREATMENT ACCEPTANCE, ADHERENCE, AND RETENTION IN HIV CARE
ART adherence is a strong predictor of virological, immunological, and clinical outcomes. In the HPTN 052 trial, virological suppression was near universal in the intervention group, reflecting intensive strategies to optimize adherence [1], which may not be the case outside the clinical trial setting [28]. Of 1.1 million people infected with HIV in the United States, only 328 000 (28%) have their viral loads sufficiently suppressed, largely due to inconsistent access to medical care and to ART and poor ART adherence and retention in care [7, 29]. Similar suboptimal HIV treatment cascades have been reported in France, United Kingdom, and Mozambique [5, 8–10] (Figure 1). These studies underscore the critical need to optimize linkage and retention in care and to maximize patient ART adherence as the critical determinants for the success of the test-and-treat strategy.
Active and continuing patient education as well as proactive monitoring of adherence will be critical, especially in the context of TasP where patients are in relatively good health and may question the need to take medicines, making them at high risk of treatment fatigue. Indeed, about 1 in 5 treatment-eligible HIV-infected individuals refused to initiate ART at a voluntary counseling and testing center in Soweto, South Africa. Interestingly, “feeling healthy” was given as the most common reason for refusing ART initiation [30]. Of more concern, recent observational data from Uganda [31] showed that individuals who start ART with a CD4+ T-cell count ≥250 cells/μL were more likely to experience treatment interruptions in the first 3 months of therapy and were more likely to have persistent viremia at 3 months compared with those who start with a CD4 cell count <250 cells/μL. Such treatment interruptions predispose patients to develop resistance to nonnucleoside reverse transcriptase inhibitors [32], which continue to be first-line therapy in middle- and low-income countries. While there are no published interventions aimed at improving ART adherence and retention in the explicit context of TasP, selected evidence-based interventions published in International Association of Physicians in AIDS Care (IAPAC) guidelines [26] have been proven effective and should be evaluated under TasP conditions, specifically for relatively healthy individuals who are being started on ART at higher CD4 T-cell count thresholds. Two RCTs in Kenya [33, 34] demonstrated that weekly text messages were efficacious in improving ART adherence. Simplification of ART regimens by reducing pill burden and frequency (eg, once-daily single-tablet regimen) should also be considered [35] as well as targeted ART adherence counseling [36, 37].
Of note, there are no gold standard tools for monitoring ART adherence as each method has its own advantages and limitations. Patient self-reporting tends to overestimate actual adherence compared with objective measures such as pill count, pharmacy refills, and electronic devices (eg, MEMScaps, Medi-Monitors). The assessment of therapeutic drug levels is not realistic in routine clinical practice and is costly. Despite these caveats, the IAPAC guidelines recommend the routine use of self-report or pharmacy refill records and maintains that other emerging techniques, such as real-time electronic monitoring, need further validation, refinement, or replacement in order to make them affordable for use in routine care [23]. There is also a need for monitoring entry into care. This is done by collating the data sources, establishing responsibilities for linkage, and monitoring retention in care using clinic-based administrative records and community outreach where feasible [23]. Similar to ART adherence, there is no gold standard for measuring retention in care. Commonly used measures include tracking missed visits or appointments, visit constancy, and gaps in care. The Human Resources and Services Administration HIV/AIDS Bureau performance measure captures whether a patient had 2 or more completed clinic visits separated by 3 or more months during a 12-month observation period [38]. Also, there are the Institute of Medicine recommendations for standardized cascade-related measures. These include the following: linkage—the proportion of people newly diagnosed with HIV who are linked to care within 3 months of diagnosis; retention in care—the proportion of people with diagnosed HIV infection who are in continuous care (2 or more visits for routine HIV care in the preceding 12 months at least 3 months apart); and viral suppression—the proportion of people with diagnosed HIV infection who have been on ART for 12 or more months and have a viral load below the level of detection [39]. Rosen and colleagues defined retention in resource-limited settings as the proportion of participants retained in care at 6, 12, and 24 months from baseline, measured by whether or not the participant attended a follow-up appointment within 1 to 3 months of the scheduled date [28].
Because there is limited published evidence on interventions to improve linkage and retention in care, in general, and even in the context of TasP, further research is critically needed in this area. The Antiretroviral Treatment and Access Study evaluated entry into and retention in care as part of a multisite RCT at several US care sites. Strengths-based case management sessions (up to 5 in a 90-day period) were compared with passive referrals for local care among patients with recently diagnosed HIV infection [40]. This resource-intense intervention showed that a significantly larger proportion of the case-managed participants visited an HIV clinician at least once during a 6-month period (78% vs 60%) and at least twice during a 12-month period (64% vs 49%). Another intervention evaluated patient navigators who were trained to help HIV-infected patients facilitate interactions with the healthcare system in the United States. An analysis of 4 patient navigation interventions showed that the proportion who had at least 2 visits in the previous 6 months increased from 64% at baseline to 87% at 6 months and to 79% at 12 months in the intervention group. In addition, the proportion of patients with undetectable HIV-1 RNA was 50% larger at 12 months than at baseline [41]. Of note, the Test and Link to Care-Plus (HPTN 065) study (Washington, DC, and New York City, NY [intervention communities] vs Chicago, IL; Miami, FL; Philadelphia, PA; and Houston, TX) is evaluating the feasibility of an enhanced community-based HIV testing, link-to-care and treat strategy and whether patient incentives are effective for linkage to care. Specifically, this study is investigating the effectiveness of financial incentives to improve the HIV care continuum at all steps: HIV testing ($25); linked to care ($125); completed initial visit and laboratory tests ($100); and plasma HIV-1 RNA level <400 copies/mL ($70) at 3-monthly visit [42]. This Pay-for-Performance-for-Patient (P4P4P) intervention using cash or cashlike incentives has been shown to be effective for smoking cessation [43] and weight loss [44], improving adherence to chronic medication in substance using patients [45], hepatitis [46], latent tuberculosis infection [47], and HIV infection [48, 49]. But, most of these studies were small and raised more questions about generalizability, feasibility, sustainability in real-life setting, ethical concerns, and cost-effectiveness, therefore calling for further research in HPTN 065.
ADDRESSING VULNERABLE POPULATIONS
The maximum benefits of a test-and-treat strategy are likely to be achieved by targeting those most at risk of transmitting the virus (Table 1). The WHO is now recommending that couples receive HIV testing and counseling, with support for disclosure, and that infected partners in serodiscordant couples be offered immediate ART, regardless of CD4+ T-cell count [16]. This guidance assumes that identification and retention in care of discordant couples are feasible, yet in many settings this strategy is challenging. In antenatal clinics, rates of serodiscordance range from 10% to 50%, but it is often difficult to identify and retain discordant couples in care.
Table 1.
Test-and-Treat: Target Population, Challenges, and Possible Solutions
| Population | Targeted Health Service | Challenge | Possible Intervention/Solution |
|---|---|---|---|
| General | Scale-up testing opportunities | Increasing early-stage testing and diagnosis; suboptimal linkage, adherence, and retention in care in real life; constraints on health systems and human resources | House-to-house testing in high-prevalence settings and opportunistic testing elsewhere; mobile phones weekly 2-way text messages reminders, targeted counseling, single-tablet-regimen to improve adherence; task shifting and possibly expansion of health staff to allow provision of ART in primary healthcare facilities, which house the majority of patients in need; maintaining the drug supply chain and uninterrupted provision of ART |
| Pregnant women | Antenatal care services | Poor adherence, especially postpartum, and poor retention in care; safety of efavirenz and tenofovir-based ART during pregnancy and while breastfeeding | Counseling; support for adherence as for general population, screening for mental health (e.g. post-partum depression), substance abuse, social support, and ART regimen simplification; case management. ART pharmacovigilance registries |
| Young women | Family planning services | One-third of pregnancies are among teenagers; many women with first pregnancy have not used contraception previously | Increased access to family planning services for young women; integration of HCT into family planning services |
| Men | Work places | Poor linkage, adherence, and retention in care | ART adherence support as per general population; male role models |
| Discordant couples | HCT | Family planning and early initiation of ART | Increased access to family planning services; early ART |
| Men who have sex with men | Special services | Stigma; criminalization in selected countries | Advocacy for human rights; specialized clinics for testing and treatment, ART adherence support as per general population |
| Female sex workers | Special services | Stigma; gender violence; illegal in selected countries | Advocacy for human rights; specialized clinics that include outreach services for testing and treatment; peer educators and expert patients |
| Intravenous drug users | Not prioritized in many countries with high HIV endemicity | Stigma; illegal in selected countries | Advocacy for human rights; specialized clinics that include outreach services for testing and treatment; directly administered antiretroviral therapy and similar services |
| Refugees | Not prioritized in many countries with high HIV endemicity | Not commonly supported through national healthcare systems | Advocacy for human rights; specialized clinics that include outreach services for testing and treatment |
| Prisoners | Not prioritized in many countries with high HIV endemicity | Stigma; poorly resourced health services | HCT services in prisons with linkage to care |
Abbreviations: ART, antiretroviral therapy; HCT, HIV counseling and testing.
Also, as PMTCT programs move toward universal lifetime use of triple ART for all pregnant women regardless of CD4+ T-cell count (option B+), it will be crucial that women receive special assistance with adherence, retention in care, and maintenance of the uninterrupted provision of ART during pregnancy and postpartum while breastfeeding [7–9]. In a metaanalysis that involved 20 153 HIV-infected pregnant women, ART adherence during pregnancy and postpartum was significantly below levels recommended for adequate virological suppression. This pooled analysis of 52 studies estimated that only 73.5% of pregnant women had adequate ART adherence (>80%) and that the proportion of women with adequate adherence levels was larger during antepartum than postpartum [50–52]. A recent study from Malawi by Tenthani and colleagues, showed that of all women starting ART under Option B+ (N = 21 939) in Malawi, 17% were lost to follow-up 6 months after start ART. Most loss occurred in the first 3 months of therapy. Option B+ women starting ART during pregnancy were 5 times more likely (OR 5.0; 95% CI 4.2–6.1) than those meeting treatment criteria to not return after 1 clinic visit. Option B+ women starting ART during breast-feeding were 2 times more likely (OR 2.2; 95% CI 1.8–2.8) to miss first follow-up visit [53]. Innovative interventions on how to retain in care and improve ART adherence in HIV-infected pregnant women are sorely needed [9].
Other priority populations, including sex workers, injecting drug users, and men who have sex with men (MSM), will be more challenging to engage in settings where they experience stigma or legal problems (Table 1). Recent data from the United Kingdom shows that between 2006 and 2010, high and increasing ART coverage reduced the proportion of all diagnosed or undiagnosed infectious men from 47% to 35%. However, over the same period, the number of MSM living with HIV rose from 30 000 to 40 100 and the absolute number of infected MSM was unchanged. In 2010, an estimated two-thirds of the infected population was undiagnosed, suggesting that HIV transmission is largely driven by this population [5]. In the clinical trial setting, test-and-treat had the largest preventive effect of any intervention from the outset of the HIV pandemic; however, the real effectiveness of test-and-treat in communities is unknown. It is anticipated that test-and-treat in combination with male circumcision, condom promotion, and PMTCT has the potential to be a powerful prevention tool and is currently being evaluated through cluster community–randomized trials in Africa, notably the PopART Study in Zambia and South Africa [54], the TasP trial in Kwa-Zulu Natal, South Africa [55], and an HIV prevention program for Mochudi, Botswana [56].
COST AND COST EFFECTIVENESS
Resources for treatment of HIV-infected people remain limited, and 10 million individuals worldwide are eligible but cannot yet access ART. Therefore, the highest priority must be to ensure that eligible patients receive ART, while at the same time guaranteeing sustainable access for those initiated early. Cost-effectiveness evaluations of the global TasP strategy as a single intervention have been controversial, but a combination of multiple component interventions may prove more cost effective and is being evaluated in both developed and developing countries [57, 58]. Walensky and colleagues recently reported that in South Africa, early ART provided cost savings over a 5-year period, while in both South Africa and India, early ART was projected to be very cost effective over a lifetime in serodiscordant couples [59]. Therefore, with individual, public health, and economic benefits, there is a compelling case for early ART in high-risk HIV transmission groups as well as other vulnerable groups (Table 1).
CONCLUSIONS
While there is much advocacy for an AIDS-free generation, many important challenges lie ahead. With shifting international priorities, a TasP strategy may be feasible in settings with enthusiastic and sustained local political support but less realistic in other settings. In a well-resourced setting with matching political support, vibrant social involvement, and substantial health infrastructure, an AIDS-free generation may become a reality. The history of HIV has already taught us that with innovative research, dedicated resources, and focused ambition, challenges can be overcome. Ending this pandemic will take extreme dedication and the combination of of political will, resources, and novel effective interventions. These interventions must be cost effective, amenable to widespread dissemination and implementation, and tailored to a wide range of global communities.
Notes
Acknowledgments. The authors wish to thank Professor Christine Katlama, MD, PhD, Maladies Infectieuses et Tropicales, Unité VIH et Immunodépression, Hôpital Pitié Salpêtrière and Unité INSERM, Université Pierre et marie Curie, Paris, France, for helping with documentation of HIV cascade data from France, and Caroline Connor, MSc, for administrative and logistical support.
Financial support. J. B. N. receives research grant support from the National Institutes of Health/National Institutes for Allergy and Infectious Disease, the AIDC Clinical Trial Group (ACTG)/Stellenbosch University Clinical Trial Unit (2UM1AI069521-08); the US President Emergency Plan for AIDS Relief (PEPFAR) (T84HA21652-01-00) for Medical Education Partnership Initiative; the European Developing Countries Clinical Trial Partnership Senior Fellowship Award (TA-08-40200-021); and the Wellcome Trust Southern Africa Consortium for Research Excellence (WT087537MA). E. J. M. receives salary support from the Canadian Institutes of Health Research through a Canada Research Chair. O. A. U. receives grant support from Marie Curie International Postdoc Program, Swedish Research Council for Health, Working Life and Welfare (2012-0064).
Supplement sponsorship. This article is published as part of a supplement entitled “Controlling the HIV Epidemic With Antiretrovirals,” sponsored by the International Association of Providers of AIDS Care.
Role of the Sponsors. No funding agency had any role in the collection, management, analysis, or interpretation of any data or in the preparation, review, or approval of this manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 3.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PloS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown AE, Gill ON, Delpech VC. HIV treatment as prevention among men who have sex with men in the UK: is transmission controlled by universal access to HIV treatment and care? HIV Med. 2013;14:563–70. doi: 10.1111/hiv.12066. [DOI] [PubMed] [Google Scholar]
- 6.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–71. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delpech V, Brown A, Conti S, Polavarapu V, Yin Z. Reducing onward transmission: viral suppression among key population groups living with HIV in the United Kingdom [Abstract 018]. 19th Annual Conference of the British HIV Association; Manchester, UK: 2013. [Google Scholar]
- 9.Micek MA, Gimbel-Sherr K, Baptista AJ, et al. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. J Acquir Immune Defic Synd. 2009;52:397–405. doi: 10.1097/QAI.0b013e3181ab73e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Supervie V, Costagliola D. The spectrum of engagement in HIV care in France: strengths and gaps [Abstract #: 1030]. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA: 2013. [Google Scholar]
- 11.Wouters K, Fransen K, Beelaert G, et al. Use of rapid HIV test in low threshold center in Antwerp, Belgium, 2007–2012. Int J STD AIDS. 2014 doi: 10.1177/0956462414526705. [published onle ahead of print 3 April 2014]. [DOI] [PubMed] [Google Scholar]
- 12.Mugglin C, Althoff KN, Wools-Kaloustian K, et al. Immunodeficiency at the start of ART: global view. 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA: 2012. [Google Scholar]
- 13.European AIDS Clinical Society. European AIDS Clinical Society Guidelines version 7. 2013. Available at: http://www.eacsociety.org/Portals/0/Guidelines_Online_131014.pdf . Accessed 8 May 2014.
- 14.Panel on Antiretroviral Guidelines for Adults and Adolescents. Recommendation on Integrase Inhibitor Use in Antiretroviral Treatment-Naive HIV-Infected Individuals from the HHS Panel on Antiretroviral Guidelines for Adults and Adolescents Department of Health and Human Services. 2013. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf . Accessed 8 May 2014.
- 15.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Geneva, Switzerland: World Health Organization; 2013. Antiretroviral Treatment Guidelines in HIV-infected Adults and Adolescents. [Google Scholar]
- 17.Department of STD, AIDS, and Viral Hepatitis. Saude: Ministerio da Saude. Available at: http://www.aids.gov.br/en/noticia/2013/france-adopts-new-national-policy-test-and-treat-hiv . Accessed 8 May 2014.
- 18.Wachira J, Kimaiyo S, Ndege S, Mamlin J, Braitstein P. What is the impact of home-based HIV counseling and testing on the clinical status of newly enrolled adults in a large HIV care program in Western Kenya? Clin Inf Dis. 2012;54:275–81. doi: 10.1093/cid/cir789. [DOI] [PubMed] [Google Scholar]
- 19.Lynch S, Ford N, van Cutsem G, et al. Public health. Getting HIV treatment to the most people. Science. 2012;337:298–300. doi: 10.1126/science.1225702. [DOI] [PubMed] [Google Scholar]
- 20.Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380:889–98. doi: 10.1016/S0140-6736(12)60730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matovu F, Wabwire D, Nakibuuka J, Mubiru M, Bagenda D, Musoke P. Efficacy of using peer counselors and nurses to support adherence to HAART among HIV-1-infected patients at the prevention of MTCT program, Mulago Hospital, Kampala, Uganda: a randomized non-inferiority interventional trial. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA: 2011. Paper 1016. [Google Scholar]
- 22.Sanne I, Orrell C, Fox MP, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010;376:33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson MA, Mugavero MJ, Amico KA, et al. Guidelines for improving entry into and retention in care for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156:817–33. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewin S, Munabi-Babigumira S, Glenton C, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst Rev. 2010;17:CD004015. doi: 10.1002/14651858.CD004015.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duwell MM, Knowlton AR, Nachega JB, et al. Patient-nominated, community-based HIV treatment supporters: patient perspectives, feasibility, challenges, and factors for success in HIV-infected South African adults. AIDS Patient Care STDs. 2013;27:96–102. doi: 10.1089/apc.2012.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nachega JB, Chaisson RE, Goliath R, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 2010;24:1273–80. doi: 10.1097/QAD.0b013e328339e20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachega JB, Knowlton AR, Deluca A, et al. Treatment supporter to improve adherence to antiretroviral therapy in HIV-infected South African adults. A qualitative study. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S127–33. doi: 10.1097/01.qai.0000248349.25630.3d. [DOI] [PubMed] [Google Scholar]
- 28.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296:679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 29.Vital signs: HIV prevention through care and treatment—United States. MMWR Morb Mortal Wkly Rep. 2011;60:1618–23. [PubMed] [Google Scholar]
- 30.Katz IT, Essien T, Marinda ET, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25:2177–81. doi: 10.1097/QAD.0b013e32834b6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adakun SA, Siedner MJ, Muzoora C, et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. J Acquir Immune Defic Syndr. 2013;62:317–21. doi: 10.1097/QAI.0b013e3182800daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–31. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 33.Lester RT, Mills EJ, Kariri A, et al. The HAART cell phone adherence trial (WelTel Kenya1): a randomized controlled trial protocol. Trials. 2009;10:87. doi: 10.1186/1745-6215-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pop-Eleches C, Thirumurthy H, Habyarimana JP, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25:825–34. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58:1297–307. doi: 10.1093/cid/ciu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung MH, Richardson BA, Tapia K, et al. A randomized controlled trial comparing the effects of counseling and alarm device on HAART adherence and virologic outcomes. PLoS Med. 2011;8:e1000422. doi: 10.1371/journal.pmed.1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradier C, Bentz L, Spire B, et al. Efficacy of an educational and counseling intervention on adherence to highly active antiretroviral therapy: French prospective controlled study. HIV Clin Trials. 2003;4:121–31. doi: 10.1310/brbv-3941-h1pp-ndry. [DOI] [PubMed] [Google Scholar]
- 38.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDs. 2010;24:607–13. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Institute of Medicine. Washington, DC: The National Academies Press; 2012. Indicators Related to Continuous HIV Care and Access to Supportive Services. Monitoring HIV Care in the United States: Indicators and Data Systems. [PubMed] [Google Scholar]
- 40.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–31. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 41.Bradford JB, Coleman S, Cunningham W. HIV System Navigation: an emerging model to improve HIV care access. AIDS Patient Care STDs. 2007;21(Suppl 1):S49–58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 42.HIV Prevention Trials Network (HPTN) HPTN 065 (TLC-Plus) Study Status. http://www.hptn.org/web%20documents/HPTN065/WES065OverallUpdate26Jun12.pdf. Accessed 29 January 2014.
- 43.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360:699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 44.Volpp KG, John LK, Troxel AB, et al. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300:2631–7. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stitzer ML, Polk T, Bowles S, Kosten T. Drug users' adherence to a 6-month vaccination protocol: effects of motivational incentives. Drug Alcohol Depend. 2010;107:76–9. doi: 10.1016/j.drugalcdep.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seal KH, Kral AH, Lorvick J, et al. A randomized controlled trial of monetary incentives vs outreach to enhance adherence to the hepatitis B vaccine series among injection drug users. Drug Alcohol Depend. 2003;71:127–31. doi: 10.1016/s0376-8716(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 47.Malotte CK, Hollingshead JR, Larro M. Incentives vs outreach workers for latent tuberculosis treatment in drug users. Am J Prev Med. 2001;20:103–7. doi: 10.1016/s0749-3797(00)00283-x. [DOI] [PubMed] [Google Scholar]
- 48.Haug NA, Sorensen JL. Contingency management interventions for HIV-related behaviors. Current HIV/AIDS Rep. 2006;3:154–9. doi: 10.1007/s11904-006-0010-5. [DOI] [PubMed] [Google Scholar]
- 49.Rosen MI, Dieckhaus K, McMahon TJ, et al. Improved adherence with contingency management. AIDS Patient Care and STDS. 2007;21:30–40. doi: 10.1089/apc.2006.0028. [DOI] [PubMed] [Google Scholar]
- 50.Kaplan R, Orrell C, Zwane E, Bekker LG, Wood R. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS. 2008;22:1679–81. doi: 10.1097/QAD.0b013e32830ebcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myer L, Zulliger R, Bekker LG, Abrams E. Systemic delays in the initiation of antiretroviral therapy during pregnancy do not improve outcomes of HIV-positive mothers: a cohort study. BMC Pregnancy Childbirth. 2012;12:94. doi: 10.1186/1471-2393-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26:2039–52. doi: 10.1097/QAD.0b013e328359590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tenthani L, Haas AD, Tweya H, et al. Ministry of Health in Malawi and IeDEA Southern Africa. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (“Option B+”) in Malawi. AIDS. 2014;28:589–98. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imperial College London. PopART—reducing HIV transmission at a population level. Available at: http://www1.imperial.ac.uk/departmentofmedicine/divisions/infectiousdiseases/infectious_diseases/hiv_trials/hiv_prevention_technologies/popart/ Accessed 29 January 2014.
- 55.KwaZulu-Natal- Africa Centre. Treatment As Prevention (TasP) trial. Available at: http://www.africacentre.ac.za/NewsArchives/2010Archives/FrenchAIDSResearchAgencyawardsAfricaCentre/tabid/439/Default.aspx. Accessed 29 January 2014.
- 56.Labome.Org. An HIV prevention program for Mochudi, Botswana. Available at: http://www.labome.org/grant/r01/ai/an/hiv/an-hiv-prevention-program-for-mochudi--botswana-7680502.html. Accessed 29 January 2014.
- 57.Barnighausen T, Bloom DE, Humair S. Economics of antiretroviral treatment vs. circumcision for HIV prevention. Proc Natl Acad Sci U S A. 2012;109:21271–6. doi: 10.1073/pnas.1209017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Granich R, Kahn JG, Bennett R, et al. Expanding ART for treatment and prevention of HIV in South Africa: estimated cost and cost-effectiveness 2011–2050. PloS One. 2012;7:e30216. doi: 10.1371/journal.pone.0030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walensky RP, Ross EL, Kumarasamy N, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013;369:1715–25. doi: 10.1056/NEJMsa1214720. [DOI] [PMC free article] [PubMed] [Google Scholar]


