Abstract
Although epithelial morphogenesis is tightly controlled by intrinsic genetic programs, the microenvironment in which epithelial cells proliferate and differentiate also contributes to the morphogenetic process. The roles of the physical microenvironment in epithelial morphogenesis, however, have not been well dissected. In this study, we assessed the impact of the microenvironment on epithelial cyst formation, which often marks the beginning or end step of morphogenesis of epithelial tissues and the pathological characteristic of some diseases. Previous studies have demonstrated that Madin-Darby canine kidney (MDCK) epithelial cells form cysts when grown in a three-dimensional (3D) extracellullar matrix (ECM) environment. We have now further demonstrated that the presence of ECM in the 3D scaffold is required for the formation of properly polarized cysts. Also, we have found that the full interface of epithelial cells with the ECM environment (in-3D) is not essential for cyst formation, since partial contact (on-3D) is sufficient to induce cystogenesis. In addition, we have defined the minimal ECM environment or the physical threshold for cystogenesis under the on-3D condition. Only above the threshold can the morphological cues from the ECM environment induce cyst formation. Moreover, cyst formation under the on-3D condition described in this study actually defines a novel and more feasible model to analyze in vitro morphogenesis. Finally, we have found that, during cystogenesis, MDCK cells generate basal microprotrusions and produce vesicle-like structures to the basal extracellular space, which are specific to and correlated with cyst formation. For the first time, we have systematically elucidated the microenvironmental determinants for epithelial cystogenesis.
Keywords: Cystogenesis, Epithelial morphogenesis, Polarity, Three-dimensional culture
Introduction
An epithelial cyst is a spherical and polarized epithelial cell layer that encloses a lumen (Kroschewski, 2004). Organs such as kidney, lung, and mammary gland consist largely of epithelial tissues characterized by tubular and/or cystic epithelia. Hence, epithelial cysts and tubules are the two basic structural units of these organs (O'Brien et al., 2002; Kroschewski, 2004). The morphogenesis of epithelial tissues includes a series of morphogenetic events such as cystogenesis, tubulogenesis, and branching morphogenesis. In these events, cystogenesis, the morphogenic process of cysts, is usually associated with tubulogenesis and branching morphogenesis. For example, in development, the terminal differentiation of branched tubules in organs such as lung, mammary gland, and salivary gland forms alveoli or acini, which represent different forms of epithelial cystogenesis. In these cases, cystogenesis marks the completion of branching and tubular morphogenesis. Cystogenesis can also be independent of other morphogenetic processes. For example, thyroid consists mainly of epithelial follicles, which are the functional units of this organ and define a special form of cyst. During the pathogenesis of polycystic kidney disease, kidney epithelial cells form cyst-like structures, which are the pathological hallmark of the disease.
Morphologically, an epithelial cyst is a spherical cell aggregate consisting of a single layer of polarized epithelial cells and an enclosed, liquid-filled lumen. Formation of this well-polarized, multicellular, three-dimensional structure requires the establishment of three different plasma membrane surfaces –apical, lateral, and basal (Kroschewski, 2004). The apical surface faces the lumen, the basal surface faces the ECM, and the lateral surface faces adjacent cells (Drubin and Nelson, 1996; Mostov et al., 2000). In vitro, the epithelial cysts can further develop into tubules. Exposure of epithelial cysts formed by MDCK cells to hepatocyte growth factor (HGF) results in the transition of cysts into branching tubules, which resemble the in vivo renal tubulogenesis (Montesano et al., 1991a, b).
Although epithelial cystogenesis contributes to various physiological and pathological processes, the mechanism of epithelial cyst formation is poorly understood. Presumably, cystogenesis is determined intrinsically by specific genetic programs and signaling pathways. Cell-ECM interaction, cell-cell interaction, and soluble factor stimulation are considered to be three major extrinsic factors essential for cystogenesis (Grobstein, 1956; Saxen and Sariola, 1987; Laurie et al., 1989; Wang et al., 1990a, b). Among these extrinsic factors, the ECM plays a key role in the differentiation and morphogenesis of epithelia (Emerman and Pitelka, 1977; Emerman et al., 1979; Parry et al., 1987; Lin and Bissell, 1993). For example, attachment of epithelial cells to the ECM is required for the establishment of cellular polarity and subsequent morphogenesis (Nelson, 1992; Rodriguez-Boulan and Powell, 1992). After being exposed to the ECM 3D environment, MDCK epithelial cells typically undergo cystogenesis (Hall et al., 1982). Among various ECM proteins, collagen and laminin are expressed in the early developmental stage of organs such as kidney (Ekblom, 1989). For example, the polarized deposition of laminin is needed for the transition of mesenchyme to polarized epithelial cells during mouse kidney development (Sorokin et al., 1990). Also, laminin contributes to the cystotubulogenesis of kidney epithelium (Klein et al., 1988) and regulates the cystogenesis of polycystic kidney cells (Joly et al., 2006).
MDCK epithelial cells are derived from canine renal tubular epithelium (Simons and Fuller, 1985) and have been commonly used for in vitro studies of epithelial structure and function. Growing MDCK cells in a collagen type I 3D environment to form well polarized cysts has been a widely used model to analyze epithelial polarization (Wang et al., 1990a; Rahikkala et al., 2001). To determine the essential elements in epithelial cyst morphogenesis, we assessed the microenvironmental requirements for the cystogenesis of MDCK epithelial cells. By employing two-dimensional (2D) and 3D culture systems, we analyzed the physical requirements for in vitro epithelial cyst morphogenesis, compared MDCK cysts formed under different culture environments, and also determined whether or not the formation of cysts depends on specific cellular structures. Since the mechanism of epithelial cyst formation remains poorly understood, identifying and analyzing the microenvironmental elements that determine epithelial cyst morphogenesis will have significant impact on the understanding of the aforementioned physiological and pathological events.
Materials and methods
Cell lines, antibodies and reagents
MDCK cells were obtained from ATCC (Manassas, VA) and cultured in 5% CO2 at 37 ºC in Dulbecco’s minimal essential medium (DMEM) (Invitrogen, San Diego, CA) containing 10% fetal bovine serum, 100 unit/ml penicillin and 100 μg/ml streptomycin.
The antibodies used in this study include E-cadherin mAb (BD, San Diego, CA), β-catenin pAb (Santa Cruz Biotechnology, Santa Cruz, CA), human integrin α6 mAb GoH3 (BD), and human integrin β 1 mAb A1A5 (Hemler et al., 1984), human type IV collagen 7S domain mAb (Chemicon International, Temecula, CA), mouse laminin A chain mAb (Chemicon), mouse nidogen G1 domain mAb (Chemicon), and human laminin 5 or laminin β 3 subunit mAb 6F12 (Marinkovich et al., 1992). The fluorochrome or fluorochrome-conjugated reagents were Texas Red-X phalloidin (Molecular Probes, Eugene, OR), Hoechst 33258 (Sigma-Aldrich, St. Louis, MO), FITC-conjugated goat anti-rat IgG (Sigma-Aldrich), and FITC-conjugated goat anti-mouse IgG (Biosource International, Camarillo, CA). ECM proteins used in this study include recombinant basement membrane Matrigel, which is extracted from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma (BD); collagen gel (3D Collagen Culture Kit, Chemicon), which contains 85% type I collagen and 15% type III collagen proteins of chicken; mouse collagen type IV (Invitrogen, Carlsbad, CA); and mouse laminin 1 (Invitrogen).
In vitro cyst morphogenesis assay
For the morphogenesis experiments on the top of Matrigel or collagen gel, MDCK cells suspended in complete DMEM were added onto the surfaces of gels in a 48-well plate (104 cells/well) after gels were formed. Neither Matrigel nor collagen gel was added into the medium. For the morphogenesis experiments within Matrigel or collagen gel, the detached MDCK cells (104 cells/well) were resuspended with Matrigel or collagen gel (250 μl/well) on ice. The cells were dispersed into the gel solution by repetitive pipetting using precooled pipets, and the gel solutions containing dispersed cells were transferred to a 48-well plate and placed at 37 ºC in a cell culture incubator for 1 h for gelation.
Typically, the Matrigel and collagen gels were formed within 1 h. Once the gel formed, it was overlaid with complete DMEM. The media were changed every 2 days. On average, cysts were well developed after 6 days with Matrigel and 10 days with collagen gel. The morphology of MDCK cells and cysts was observed with an Olympus CK2 microscope equipped with the Olympus Phase Condenser ULWCD 0.30 contrast and photographed with an Olympus digital camera at a magnification of 10×.
For the morphogenesis experiments “on-2D” (or on the thin layer coating of Matrigel), MDCK cells were cultured according to the manufacturer’s protocol. Briefly, 50 μl and 150 μl per square centimeter of growth surface were used for thin gel and thick gel coating methods, respectively. For the thin coating, Matrigel was diluted to the desired concentrations using serum-free medium, from 2 mg/ml to 0.25 mg/ml, and added into the wells. The formation of MDCK cysts on the different coating volumes of Matrigel was observed as noted above.
For the morphogenesis experiments in methylcellulose gel, 105 MDCK cells were suspended in 5 ml methylcellulose gel (Sigma) or 5 ml methylcellulose gel containing 10 μg/ml type IV collagen and/or 10 μg/ml laminin 1, then overlaid with complete DMEM and cultured at 37 ºC. Cells were harvested at day 21, stained with β-catenin pAb and Texas Red-X phalloidin, and analyzed with confocal microscopy as described below.
Confocal microscopy
MDCK monolayers and cysts were fixed with 3% paraformaldehyde for 15 min, washed three times with phosphate-buffered saline (PBS), permeabilized with 0.1% Brij 98 for 2 min, and blocked with 20% goat serum in PBS for 1 h. The fixed and permeabilized cells and cysts were incubated with the staining cocktail, which contained E-cadherin mAb or β-catenin pAb and Texas Red-X phalloidin, for 1 h, followed by staining with FITC-conjugated goat anti-mouse IgG secondary antibody for 1 h. After each incubation, cells and cysts were washed with PBS three times, and each wash lasted 10 min. The whole procedure was performed at room temperature. After staining, coverslips were mounted on slides in FluroSave reagent (Calbiochem, Carlsbad, CA). The cells and cysts were examined under a Bio-Rad MRC-1024UV Confocal System (Bio-Rad) equipped with 63×/1.4 Plan-APOCHROMAT oil immersion objectives for x-y series and x-z series views. The horizontal serial optical sectioning or x-y plain sectioning was scanned at 2-μm intervals through the cysts (~30 sections/cyst) and executed on each sample. The samples were excited by the 488- and 543-nm lines of Kr-Ar lasers to examine FITC and Texas Red, respectively. Individual channels were scanned in a series to prevent cross-channel bleed-through. Images were captured using a digital camera and processed with Photoshop (Adobe Co., Mountain View, CA).
Electron microscopy
MDCK monolayers were cultured on 8.0-μm polycarbonate membranes (Nunc, Roskilde, DK) that were coated with 1:30 diluted Matrigel for 5 days, and MDCK cysts were cultured on membranes that were coated with 1:10 diluted Matrigel for 5 days. The cells were fixed with 2.5% glutaraldehyde in PBS for 1 h at room temperature. Then the monolayers and cysts were postfixed in 1% osmium tetraoxide/0.1M cacodylate buffer for 1 h at room temperature, rinsed briefly in deionized water, and dehydrated in graded solutions of ethanol (from 30% through 100% for 1 h each). The specimens were further infiltrated first with 50% Spurr in 100% ethanol overnight at room temperature and then 100% Spurr for 8 h during which Spurr was changed at least three times. The specimens were cured at 60 °C for 2 days. One-micron sections were cut using a Reichert Ultracut E microtome, followed by toluidine blue staining. The areas of interest were selected, sectioned at approximately 75 nm thickness, and poststained with 1% uranyl acetate and Ultrostain II with an LKB Ultrostainer. The specimens were observed and photographed on a JEOL 2000EX TEM at 60 kV.
Time-lapse video microscopy
MDCK cells were cultured on top of Matrigel-coated donut dishes (Nunc) for 24 h and then incubated in a microscopic stage environmental chamber (InVivo Scientific, St. Louis, MO) at 37 ºC under 5% CO2. Cells in a randomly selected field were continuously photographed for 60~72 h. The DIC digital images were captured every 30 min by using a C1Si confocal system on a TE2000-E inverted microscope (Nikon, New York, NY) with a 20× Planfluor objective (NA 0.5) (Nikon). For each time point, we collected a stack of optical sections spaced 3.4 μm apart. Typically,23 optical sections were sufficient to span an entire cyst. Laser illumination was minimized, and confocal scans were carried out in minimal timeframe. The digital images were viewed and analyzed using EZ-C1 3.00 FreeViewer program (Nikon).
Histochemical analysis
MDCK cysts were fixed directly with 1% paraformaldehyde in PBS (pH 7.4) for 1 h at room temperature, dehydrated in a graded ethanol series, and embedded in paraffin manually. Then 5 μm thick sections were cut on a Leica 2030 microtome. Slides were deparaffinized in xylene, rehydrated in graded alcohol, and stained with hematoxylin/eosin.
Results
MDCK cells undergo cyst morphogenesis both “on” and “in” an ECM 3D microenvironment
Three-dimensional environment is critical for the morphogenesis of MDCK cysts. Many studies have shown that MDCK cells can form polarized cysts when the cells are cultured in collagen gel, a 3D ECM environment (McAteer et al., 1987; Wang et al., 1994). The difference between the 3D culture and the classical culture (referred to as 2D culture herein) for adherent cells is that the adherent cells grow inside an ECM scaffold in 3D culture rather than on an ECM substratum in 2D culture. As epithelial cells grown on an ECM substratum in 2D culture typically do not undergo morphogenesis, the question arises whether MDCK cells need to be surrounded by ECM to form cysts. In other words, will they form cysts when cultured on 3D ECM environments such as collagen gel? We cultured MDCK cells both inside and on top of collagen gel to determine if they undergo cyst morphogenesis or cystogenesis. As shown in the upper half of Figure 1A, MDCK cells formed polarized cysts under both on-3D and in-3D experimental conditions, evidenced by the apical marker cortical F-actin, the basolateral marker E-cadherin, and the enclosed cavity or lumen. Confocal microscopy and histochemical analysis confirmed that the “cysts” observed under phase-contrast microscopy on-3D indeed contained lumen, became polarized, and were morphologically indistinguishable from the cysts formed in-3D. This result indicates that the in-3D environment is not necessary for cyst formation and that cysts can form under the on-3D condition. Although MDCK cells form cysts when cultured both on and in a 3D environment, the sizes of cysts vary under different conditions (Fig. 1B). The sizes of MDCK cysts formed under the “on” condition are in general larger than the ones under the “in” condition within a given culture period (Fig. 1B). In other words, when compared to the cysts formed on-3D, the ones formed in-3D take longer time to reach the same size. The cysts formed on Matrigel are typically larger than the one on collagen I gel (Fig. 1B). Of note, the cysts formed on-3D did not result from the “sinking” of MDCK cells into the 3D environment at the initial stage of cyst formation, based on the dynamic observation of the whole process of cyst formation using time-lapse video microscopy (data not shown). The ultrastructural analysis also demonstrated that cysts formed on-3D indeed largely remained on the surface of and were not encircled by the 3D ECM (Fig. 1C).
Fig. 1.
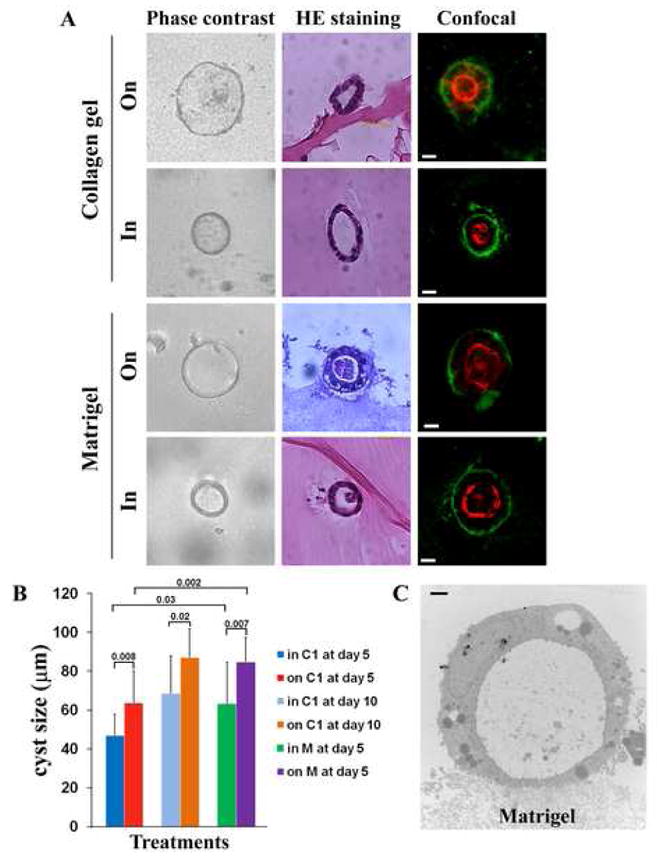
Epithelial cyst formation in and on 3D ECM environments. (A) MDCK cells were cultured within and on top of matrix (5 days for Matrigel and 14 days for collagen gel). The cells were plated in 24-well plates (104 cells/well). MDCK cells formed cyst-like cell clumps at 5 days after culturing with 3D matrix environments. The cyst-like cell clumps were directly photographed under a phase-contrast microscope (10×, left panel); paraffin-embedded, sectioned (5 μm), HE stained, and photographed (10×, middle panel); and stained with anti-integrin β 1 mAb and Texas Red-X phalloidin and photographed with confocal microscopy to confirm cyst formation (Bars: 20 μm, right panel). MDCK cysts were formed either on the top of or within Matrigel or collagen gels. Note that no monolayers were formed on 3D substrates and the rough texture on the surface of 3D matrices probably results from the retraction of collagen gel or Matrigel during cyst formation. (B) The diameters of MDCK cysts formed either on or in collagen I gel or Matrigel were measured using scale bars in Photoshop and compared statistically. At least 10 cysts were measured in each group and the quantifications were based on the results of 3 independent experiments. The data are expressed as the mean + SD, and the p values (two-tailed Student T test) are indicated in the bar graph. Since cysts are typically well developed either on or in Matrigel at day 5, we did not analyze them on or in Matrigel at day 10. (C) MDCK cells were cultured on Matrigel for 5 days to form cysts. The cells were then fixed, thin-sectioned, and analyzed under a transmission electron microscope, as described in Materials and methods. Bar: 2 μm.
Cystogenesis can be induced by different ECM microenvironments
Although collagen gel has been commonly used as 3D matrix to induce MDCK cyst formation, we questioned the necessity of type I collagen for MDCK cyst formation. We tested the ability of Matrigel, a recombinant basement membrane secreted by Engelbreth-Holm-Swarm (EHS) tumor cells, to induce cystogenesis. Matrigel contains laminin 1, collagen IV, entactin/nidogen, and heparin sulfate (Kleinman and Martin, 2005), a set of matrices completely different from the collagen gel. As shown in the lower half of Figure 1, when MDCK cells were cultured in Matrigel, they formed polarized cysts, evidenced by the apical distribution of F-actin, basal distribution of integrin β 1 subunit, and the existence of lumen. Other basolateral markers such as β-catenin and E-cadherin were also found in the basolateral membrane (data not shown), further confirming the polarization of the cysts. This result is consistent with earlier observations (Zinkl, 1996; Rahikkala et al., 2001) and indicates that epithelial cystogenesis can occur in different 3D matrix environments. In addition, MDCK cells also underwent cystogenesis on Matrigel (Fig. 1), supporting our above conclusion. Notably, the timeframes for MDCK cells to form cysts in or on Matrigel are different from those in or on collagen gel. When cultured with Matrigel, cyst formation takes approximately 6 days on the average; but with collagen, the average time is approximately 10 days, suggesting the mechanistic difference of the two cyst-forming processes or the quantitative difference of the efficient ligands present in these two different 3D matrix environments.
An ECM-free 3D scaffold is not sufficient to induce cystogenesis
MDCK cells form cysts only under (either in or on) 3D culture conditions. It remains unknown whether only the 3D environment (or 3D scaffold) is sufficient for cystogenesis or whether the ECM together with the 3D environment is required for cystogenesis. To address this issue, we cultured MDCK cells in 2.8% DMEM-saturated methylcellulose, a 3D scaffold without any ECM components. We found that most MDCK cells died after being cultured in methylcellulose gel. Only a few cells, which constituted less than 0.1% of the entire cell population, remained alive, kept proliferating, and formed cell aggregations upon prolonged culture (14–21 days). Confocal and immunofluorescence analyses of these cell aggregates confirmed that they do not contain lumen (Fig. 2A) and therefore do not qualify as cysts. These aggregates did not transform into cysts in the continued culture for another one or two weeks (data not shown). Thus, MDCK cells form cysts only in a 3D ECM environment not in a 3D scaffold, indicating that the mere 3D scaffold is not sufficient for the induction of MDCK cyst formation and the presence of ECM components in the 3D scaffold is necessary for cystogenesis.
Fig. 2.
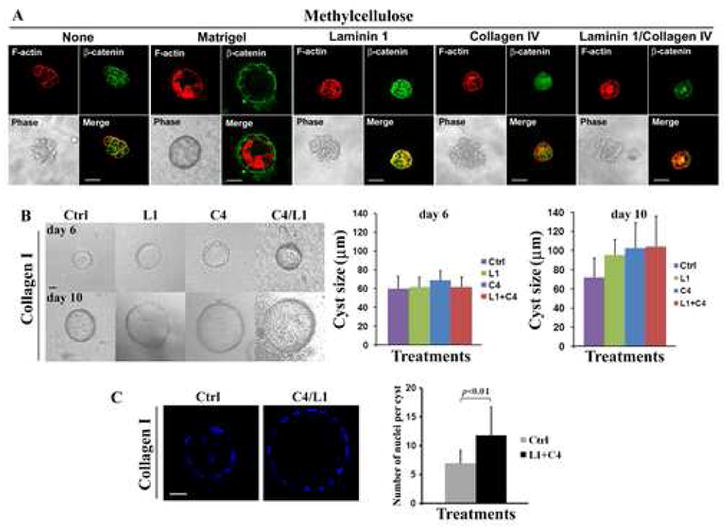
An ECM-free 3D scaffold cannot induce MDCK cells to form polarized cysts. (A) MDCK cells were cultured for 21 days in 2.8% methylcellulose gel or 2.8% methylcellulose gel containing either 1:10 diluted Matrigel, 10 μg/ml laminin 1, 10 μg/ml collagen IV, or 10 μg/ml collagen IV and 10 μg/ml laminin 1. The cell clumps or cysts formed in 3D culture were observed with phase-contrast (10×) and confocal immunofluorescence microscopy. Cells were stained with anti-β-catenin pAb (green) and Texas Red-conjugated phalloidin (red). The confocal images in each group show the same cyst or aggregate while the phase-contrast images display different ones. (B) MDCK cells were cultured in either collagen gel alone (control or Ctrl) or collagen gel containing either 10 μg/ml laminin 1 (L1), 10μg/ml collagen IV (C4), or 10 μg/ml collagen IV and 10 μg/ml laminin 1 (L1+C4) for 6 or 10 days. Images were captured using phase-contrast microscopy. MDCK cyst sizes were assessed in diameter and compared statistically. At least 13 cysts were measured in each group and the quantifications were based on the results of 3 independent experiments. The data are expressed as the mean + SD. p < 0.01 for Ctrl versus L1, C4, or L1+C4 at day 10 (two-tailed Student T test). (C) MDCK cysts were formed in collagen I gel (Ctrl) and in collagen gel containing 10 μg/ml collagen IV and 10 μg/ml laminin 1 (L1+C4). After 10 days, the cysts were stained with Hoechst 33258 at 37°C for 1 h, fixed, and then analyzed under a confocal microscope. For each cyst, a stack of optical sections spaced 2 μm apart was collected. Cell numbers were quantitated by counting nuclei in a cyst at the focal plane with longest diameter. Data are expressed as means + SD. All micrograph size bars: 20 μm.
Because MDCK cells survive and proliferate but do not form cysts in methylcellulose gel, this system is ideal for reconstituting cyst formation. To determine which ECM component(s) in the basement membrane initiates cystogenesis, we reconstituted methylcellulose gel with individual basement membrane ECM proteins. When Matrigel was mixed with the methylcellulose gel at the minimal concentration, MDCK cells formed cysts (Fig. 2A), indicating that methylcellulose gel does not contain cystogenesis-suppressive factor(s) and can induce cystogenesis only in the presence of ECM components. When we added laminin 1, collagen IV, or laminin 1 and collagen IV into methylcellulose, MDCK cells still formed cell aggregates but not cysts (Fig. 2A), suggesting that either laminin 1 or collagen IV alone or together is not responsible, at least not sufficient, for inducing MDCK cystogenesis under the given experimental conditions. To further determine the roles of laminin 1 and collagen IV in cyst development, we reconstituted the collagen I gel with laminin 1 and/or collagen IV at the concentration of 10 μg/ml. Interestingly, the cysts formed in collagen I gel containing laminin 1, collagen IV, or laminin 1 and collagen IV were significantly larger than the ones in collagen I gel alone at day 10, though they were similar in size at day 6 (Fig. 2B). This result suggests that laminin 1 and collagen IV are the ECM components in Matrigel responsible for the more rapid cystogenesis on or in Matrigel. This result also suggests that laminin 1 and collagen IV accelerate cyst formation and/or enlargement, probably through promoting MDCK cell proliferation. Indeed, by quantifying the cell numbers in cysts, we found that markedly more cells are present in the cysts formed in collagen I gel containing laminin 1 (data not shown), collagen IV (data not shown), or laminin 1 and collagen IV (Fig. 2C), indicating both ECM proteins facilitate cell proliferation during cystogenesis.
Minimal requirements of the 3D microenvironment for MDCK cyst formation
Since MDCK cells form cysts only on a 3D, but not a 2D, environment, we reasoned that the “on-3D” environment contains the minimal requirement(s) for cyst morphogenesis. To determine the factor(s) or parameters critical for morphogenesis in the “on-3D” environment, we first tested cyst formation on Matrigel with different thicknesses. The minimal volume of Matrigel that we could apply in the assay was 50 μl/cm2 surface area (data not shown), which gives rise to a 3D matrix with the theoretical height of 0.5 mm. The theoretical height was calculated using the formula V/π · r2, in which V is the volume of Matrigel and r is the radius of the well. On the 0.5-mm thick Matrigel, MDCK cells could form cysts, indicating that the minimal thickness of Matrigel for cystogenesis is less than or equal to 0.5 mm. Due to the viscosity of Matrigel, it is practically impossible to apply a volume less than 50 μl/cm2 surface area for the cystogenesis assay. Then we cultured MDCK cells with the Matrigel that was diluted with DMEM to determine the minimal concentration of Matrigel that can induce MDCK cells to form cysts. As shown in Figure 3A, the minimal concentration for cysts to form on Matrigel was 1 mg/ml or 1:10 dilution of Matrigel. At concentrations lower than 1 mg/ml, no cysts but only monolayers were formed (Fig. 3A). At a concentration of 1 mg/ml, we further measured the minimal thickness of the diluted Matrigel for cyst formation. By testing the Matrigel of 1 mg/ml at 50 μl, 30 μl, 25 μl, 15 μl, and 0μl/well (i.e., 156 μl/cm2, 94 μl/cm2, 78 μl/cm2, 47 μl/cm2, and 0 μl/cm2, respectively), we found that 25 μl/well of the 96-well plate or 78 μl/cm2 is the minimal volume of Matrigel for cyst formation (Fig. 3B). As the gel forms a meniscus in the well, there are two thicknesses or heights for a given volume: one thickness is the height on the edge of the meniscus (H1) and the other one is the height in the center of the meniscus (H2). We measured H1 and H2 of each volume of Matrigel using a mini-scale ruler. The minimal thickness for cystogenesis should range between heights H1 and H2. We predicted that the minimal thickness might be close to the theoretical height within the range of H1 and H2. After measuring heights H1 and H2 at different volumes (Fig. 3C, D), we obtained the minimal thickness range of Matrigel (1 mg/ml) for cyst formation in the wells of 96-well plates, i.e., a range from 0.17 mm (H2) to 2.31 mm (H1) with a mean thickness of 0.8 mm.
Fig. 3.
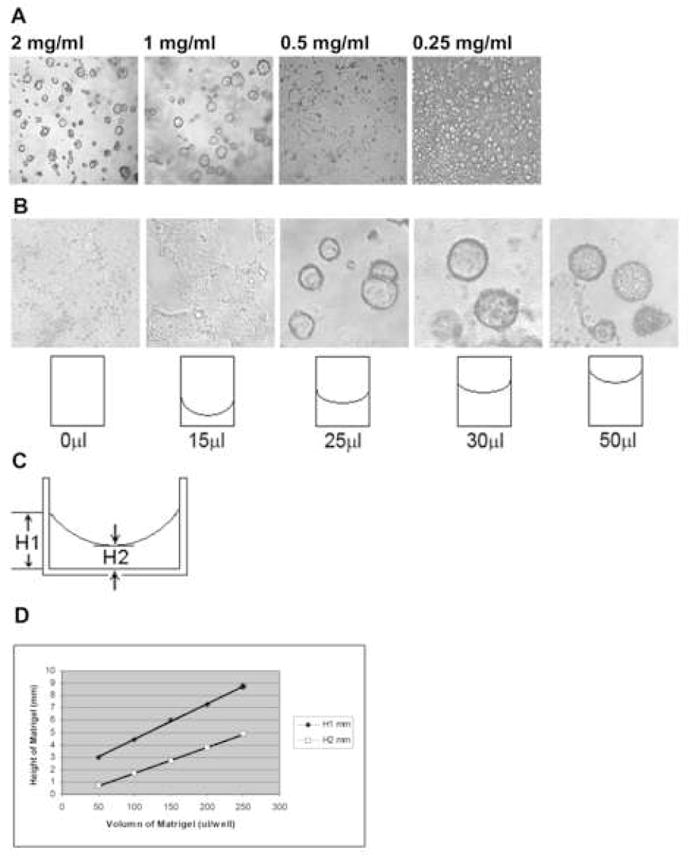
Definition of 2D and 3D matrix environments based on epithelial cyst formation. MDCK cells were cultured for 5 days in wells of a 96-well plate, which were coated with different concentrations (A) or volumes (B) of Matrigel. The images display representative results from 3 individual experiments. A minimal concentration of 1 mg/ml Matrigel is required for MDCK cells to form cysts (A). The minimal volume of Matrigel (1 mg/ml) for cyst formation is 25 μl (B). The monolayers visible in the images of “on-30 or -50 μl Matrigel” were formed by cells that had migrated to the bottom of the well and were located neither in nor on the Matrigel scaffold. (C) Sketch map of two thicknesses for a given volume of Matrigel that forms a meniscus in the well. (D) Relation between volume and height (H1 and H2) of Matrigel (1 mg/ml) in a well of a 96-well plate.
Polarization is neither sufficient nor specific for cystogenesis
Besides the presence of lumen, matured epithelial cysts are properly polarized. As shown in Figure 1, we demonstrated that MDCK cells form the polarized cysts at different ECM conditions either “on” or “in” a 3D ECM environment. For the MDCK cysts formed at the minimal ECM environment, it remains to be determined whether or not the cysts are properly polarized. Also, it is important to know if polarization is a key feature for cystogenesis. By using F-actin as an epithelial apical marker and E-cadherin as a basolateral marker, we found that the cysts formed on the minimal 3D Matrigel were polarized (Fig. 4A). Other basolateral markers such as β-catenin and integrin β 1 subunit were also found in the basolateral membrane (data not shown). For the monolayers formed on the 2D environment (1:30 dilution of Matrigel), MDCK cells were polarized as expected (Fig. 4A). These results demonstrated that polarization is likely needed but not sufficient for cystogenesis, because both monolayers and cysts of MDCK cells can be polarized. To distinguish from the classical 2D environments such as cell culture dishes, we designate the 3D ECM environment under the morphogenic threshold such as the 1:30-diluted Matrigel as the non-morphogenic 3D.
Fig. 4.
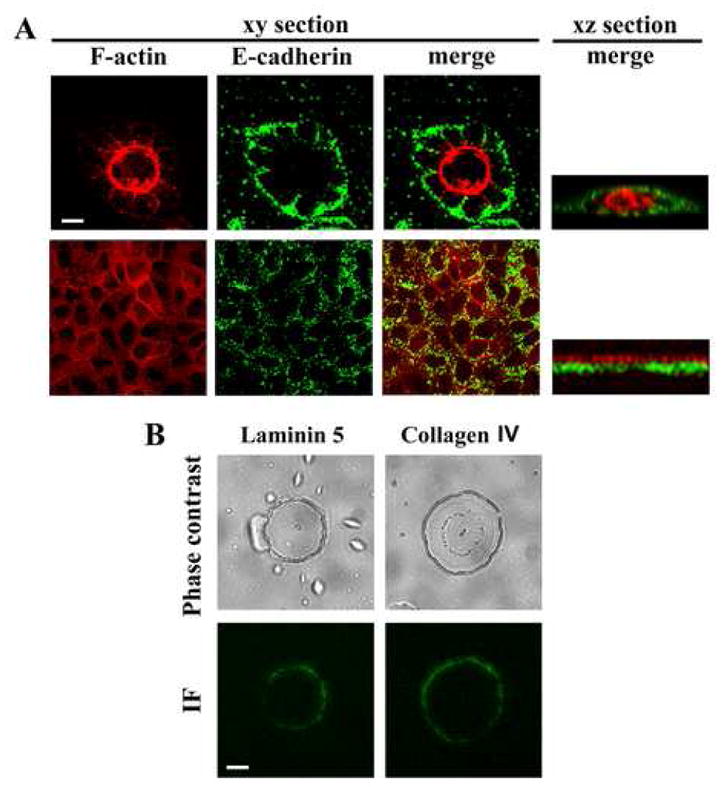
Polarization of MDCK cells on 2D and 3D ECM environments. (A) MDCK cells were cultured either on 3D (1:10 diluted Matrigel at the minimal volume for cystogenesis) or 2D (equivalent volume of 1:30 diluted Matrigel) ECM environments. MDCK cells formed cysts on 3D (top panel) and monolayers on 2D (bottom panel) ECM environments, respectively. The cysts and monolayers were stained for basolateral membranes with anti-E-cadherin mAb (green) and for apical membranes with Texas Red-conjugated phalloidin (red). Stacks of confocal images were collected as the x-y and x-z sections. (B) MDCK cysts formed on Matrigel were isolated at day 6, fixed and immunofluorescence-stained for collagen IV and laminin 5. Bars: 10 μm (A), 20 μm (B).
Although the cysts formed on 3D are polarized based on the distributions of the cellular markers for polarization as described above, it remains unknown whether the cysts deposit basement membrane components, the extracellular markers for polarization, around the entire cyst or only at the cyst-3D interface. By analyzing epithelial basement membrane components laminin 5 and collagen IV, we found these proteins were indeed present at the entire abluminal side of the cysts formed on Matrigel (Fig. 4B). Since laminin 5 does not exist in Matrigel, the presence of laminin 5 at the outer surface of cysts indicates that the local contact under the on-3D condition can induce the global deposition of basement membrane components. This result also further supports the conclusion of the complete polarization of the cyst formed on-3D.
Specific subcellular structures during MDCK cyst formation
Based on the differential abilities of MDCK cells to form epithelial cysts under 3D and 2D ECM environmental conditions, we hypothesized that the minimal 3D ECM environment may trigger MDCK cells to form some unidentified subcellular structures essential or important for cystogenesis. To determine whether these structures exist, we analyzed the interface of cyst and Matrigel in x-z section series of confocal micrographs and found no detectable subcellular structure at the interface (Fig. 4, x-z section panel). Moreover, no difference was found between the interface of cyst and Matrigel and the one of monolayer and Matrigel (Fig. 4, x-z section panel).
Since the analysis of subcellular structures using light microscopy is limited by its resolution, we further examined the ultrastructure of MDCK monolayers formed on the 2D environment versus MDCK cysts formed on the 3D environment by using electron microscopy (EM). Under the “on-2D” Matrigel condition, the MDCK monolayer developed microvilli at the apical surface and cell-cell contacts at lateral cell borders (Fig. 5A). No unique subcellular structures were found at the interface of the basal plasma membrane of the MDCK monolayer and Matrigel. In MDCK cysts formed on top of Matrigel, microvilli were lined up at the apical or luminal side of the cysts (Fig. 5B), similar to those seen in the monolayer. The presence of microvilli at the apical side of cysts is consistent with results previously reported (Schwimmer and Ojakian, 1995) and further confirms the polarization of cysts formed on minimal 3D Matrigel. Notably, some microprotrusion structures or foot processes were detected on the outer surface or abluminal side of MDCK cysts. These microprotrusion structures were located at the basal plasma membrane independently of a direct contact between plasma membrane and Matrigel (Fig. 5C, D). We also found some microvesicular structures around the cysts, which were likely secreted by MDCK cells during or after cystogenesis. These structures were specific to cysts and were not found in monolayers (Fig. 5A). Thus, MDCK cysts contain specific subcellular structures that may be involved in cystogenesis.
Fig. 5.
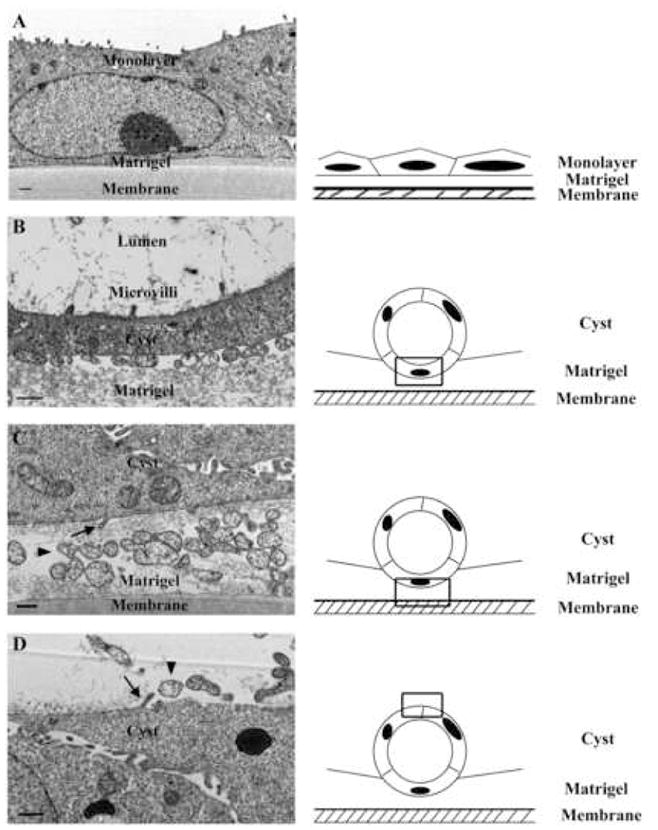
Ultrastructural analysis of MDCK cysts. MDCK cells were cultured on 8-μm polycarbonate membranes coated with DMEM-diluted Matrigel for 5 days to form monolayers or cysts. The cells were then fixed, thin-sectioned and analyzed under a transmission electron microscope. The images represent the results from one out of three individual experiments. (A) Monolayer on 1:30 diluted Matrigel. This concentration of Matrigel is lower than the minimal concentration for cyst formation. After 5-day culture, the underlying Matrigel probably had been largely absorbed by the MDCK cells. (B-D) Cysts formed on a 1:10 diluted Matrigel. Frames in the drawings on the right indicate the regions visible in the images on the left. Arrows indicate the microprotrusion structures located at the basal plasma membrane of cysts, and arrowheads point to the microvesicular structures around the cysts. Bars: 500 nm.
To confirm that the subcellular structures surrounding MDCK cysts were secreted by MDCK cells during cyst formation, we characterized cystogenesis by using live imaging analysis. Under time-lapse video microscopy, we found that MDCK cells indeed project subcellular structures from the outer surface and eventually release them into surroundings, for example, during cyst formation on Matrigel (Fig. 6A, top panel and the movie at http://sam77783.googlepages.com/movie1.avi) and after the cyst was formed in Matrigel (Fig. 6A, bottom panel and the movie at http://sam77783.googlepages.com/movie2.avi). We also found that some MDCK cysts generate protrusive structures from the outer surface after the cysts were formed either on or in Matrigel (Fig. 6B and the movie at http://sam77783.googlepages.com/movie3.avi for on Matrigel and http://sam77783.googlepages.com/movie4.avi for in Matrigel). The filopodia-like protrusive structure, however, likely represents a different cellular process from the aforementioned, microvilli-like microprotrusion structures seen under EM (Fig. 5) because of the discrepancy in size. Nevertheless, both protrusive structures underscore the involvement of cellular protrusive activity during cyst formation and/or development. Both filopodia-like protrusive structure and cyst-released subcellular structure may be captured in the images in recent publications (Zeng et al., 2006; Martin-Belmonte et al., 2007; Tanimizu et al., 2007; Yu et al., 2007) though they did not catch the attentions in those studies. Interestingly, MDCK cysts formed on Matrigel do not stay static but actually rotate dynamically on Matrigel (our unpublished data) as earlier reported (Zeng et al., 2006), which may explain the existence of microprotrusion structures at the cyst outer surface which is not in direct contact with Matrigel (Fig. 5D). Under video microscopy, we found that the bleb-like structures described above did not result from a “torn off” process caused by the rotation. Also, this rotation process did not result in a significant repositioning of the cysts formed either on or in Matrigel. Thus, the bleb-like structures were not derived from the rolling of cysts on Matrigel either.
Fig. 6.
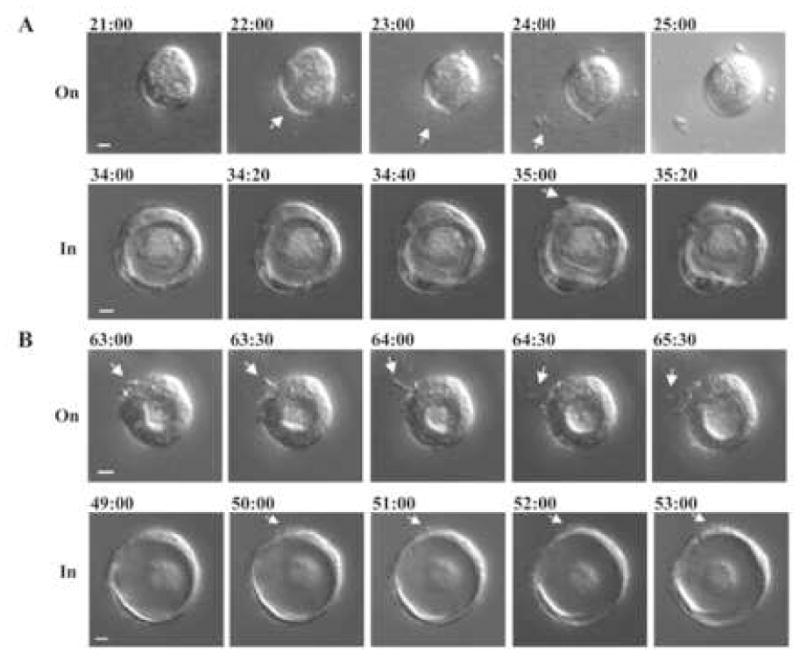
Live-cell imaging of MDCK cystogenesis. MDCK cells were cultured either on or in Matrigel. DIC images were captured using time-lapse video microscopy in constant time intervals as indicated. The pictures display an X-Y focal plane that traverses the cyst. The number on the top of each image denotes the time (h:min) from the onset of time-lapse video microscopy, i.e.,1 day after plating cells on or 2 days after plating in Matrigel. (A) Arrows indicate the cellular structures that were released before or after a cyst was formed on Matrigel. (B) Arrows indicate the cellular structures that protruded from the cyst after a cyst was formed. Bars: 10 μm.
Discussion
Multicellular, spherical, polairzed cyst formation of MDCK cells in collagen gel has been used to study epithelial polarization and morphogenesis (Hall et al., 1982; McAteer et al., 1987; Warren and Nelson, 1987; Mangoo-Karim et al., 1989; Wang et al., 1990a, b; Montesano et al., 1991a; Rahikkala et al, 2001; Yu et al., 2005). Because of the simplicity of its morphogenetic process, MDCK cyst formation is an ideal model for determining the cellular events critical for epithelial morphogenesis and the molecular mechanism(s) governing it. The signaling involved in MDCK cyst formation has emerged from various studies (Mangoo-Karim et al., 1989; Orellana and Marfella-Scivittaro, 2000; O'Brien et al., 2001; Schramek et al., 2003; Yu et al., 2005). The importance of the microenvironment in cystogenesis is well documented (Lin and Bissell, 1993; Kleinman et al., 2003). The environmental determinants for MDCK cystogenesis, however, have not been systematically assessed (Wang et al., 1990a, 1994). For example, is the cell embedment in a 3D physical environment necessary for cystogenesis? Is ECM material in the microenvironment required for cystogenesis? Is any specific cellular structure formed in response to the environmental morphogenic cues during cystogenesis?
By addressing these basic questions of morphogenesis, we have identified several microenvironmental determinants of MDCK cystogenesis. These observations provide unique insight into the general principle of epithelial morphogenesis. First, we showed that, under both on- and in-3D ECM environment conditions, MDCK cells form properly polarized cysts. Hence, the full cellular exposure to ECM is not essential for cystogenesis, at least in vitro. Second, we found that an ECM-free 3D scaffold failed to induce cystogenesis. Hence, an ECM environment is an essential extrinsic element during epithelial morphogenesis. Cystogenesis, however, can be induced at different ECM environments, though the morphogenetic processes exhibit different kinetics. Moreover, we have refined the definitions of 3D and 2D ECM environments based on MDCK cystogenesis. This parameter-based description of 3D and 2D redefines in vitro morphogenesis models. Finally, by applying this newly defined 3D and 2D model, we identified basal microprotrusions and secreted miniorganelles during cystogenesis, which are coordinated with, specific for, and potentially responsible for MDCK cyst formation.
The microenvironmental determinants of epithelial morphogenesis
The morphogenetic processes of MDCK cyst formation include the formation of an enclosed multicellular sphere, the generation of a lumen, and the polarization of the cyst shell. To facilitate the analysis of cystogenesis, we classify MDCK cysts into rudimentary, properly polarized, and aberrantly polarized cysts. The hypothetic cyst without polarization is referred to here as rudimentary cyst, the basic form of cyst. Since MDCK cells originated from kidney tubular epithelial cells, the cyst with the lumen-faced apical membrane is considered properly polarized, while the cyst with the lumen-faced basal membrane is aberrantly polarized. The cysts typically seen in either physiological or pathological conditions in vivo are the properly polarized cysts, such as thyroid follicles and renal cysts.
In 3D scaffolds without ECM, we found that MDCK cells form only cell aggregates, indicating that the 3D scaffold alone is not sufficient to induce MDCK cyst formation. Although earlier studies found that ECM is important for epithelial cyst formation, our study for the first time clearly concluded that an ECM 3D environment is essential for cystogenesis. MDCK cysts formed in a 3D ECM environment are properly polarized (Hall et al., 1982; O'Brien et al., 2002; Kroschewski, 2004; this study). However, MDCK cells form aberrantly polarized cysts when cultured in suspension (Wang et al., 1990a, b). In collagen gel, the aberrantly polarized cysts are reversed to properly polarized cysts through a β 1 integrin-dependent repolarization process (Wang et al., 1990a; Ojakian and Schwimmer, 1994). In this case, the ECM 3D environment appears to be inessential for the formation of an enclosed multicellular sphere and the generation of lumen but only critical for proper polarization. Notably, the lumen in properly polarized cysts is filled with fluid (McAteer et al., 1987; Mangoo-Karim et al., 1989), while the lumen in aberrantly polarized cysts contains ECM material (Wang et al., 1990a). Thus, the MDCK cysts formed in suspension may not actually contain the authentic lumen or represent typical cystogenesis. Even for the aberrantly polarized cysts, the ECM deposited into the “lumen” by the enclosed epithelial cells is still likely to be essential for the “lumen” formation or aberrant cystogenesis. Nevertheless, the ECM 3D environment determines, at least, the morphogenesis of properly polarized MDCK cysts by triggering lumen formation and polarization. Besides lumen formation and polarization, interestingly, our study also identified microprotrusions and released microvesicles at the abluminal side during cystogenesis, both of which may potentially play roles in cystogenesis.
MDCK cells form cysts under different ECM 3D environments. Most of earlier studies used collagen gel for cystogenesis, while a few studies found that MDCK cells could also form cysts in Matrigel (Bao and Hughes, 1995; Rahikkala et al., 2001). Like the cysts formed in collagen gel, the ones in Matrigel are properly polarized (Rahikkala et al., 2001). The results from this study fully agree with those earlier observations. Thus, the composition of ECM in 3D scaffolds is not critical for MDCK cystogenesis. The only difference in cyst formation between collagen I gel and Matrigel under the light microscope is the kinetics, i.e., cysts are formed and/or enlarged more rapidly with Matrigel than with collagen I gel. Likely, Matrigel readily contains the cystogenesis-essential ECM components such as laminin, which is needed to form properly polarized MDCK cysts (O’Brien, 2001). With collagen I gel, MDCK cells probably need to synthesize and deposit the ECM components crucial for cystogenesis, and therefore cysts are formed more slowly. Consistent with this interpretation, we found that the ECM from Matrigel promotes cyst formation and/or enlargement in collagen gel. This interpretation, however, may not explain why a methylcellulose 3D scaffold does not support cystogenesis, since one would also predict that MDCK cells could synthesize and deposit ECM in methylcellulose. Alternatively, if the morphogenic signals solicited by ECM vary in amplitude from one ECM 3D environment to the other, the rate of cystogenesis would also be different.
Quantification and redefining of ECM 3D microenvironment based on cystogenesis
Although MDCK cystogenesis in 3D collagen gels is well studied, the requirement of an in-3D physical environment for cystogenesis remained unknown. Under physiological conditions, morphogenesis can also occur on top of 3D ECM environments. For example, neurotube formation during embryonic development actually occurs on, not in, a 3D ECM environment. Surprisingly, MDCK cystogenesis on 3D has never been assessed. Our observation indicates that the full interface of MDCK cells with the 3D ECM microenvironment is not essential for cyst formation, and a partial interface is sufficient to induce lumen formation and proper polarization. In other words, local polarization of MDCK cells is sufficient to trigger global polarization of whole cysts. However, since MDCK cells seeded on 3D matrices keep rotating during cystogenesis, the rotating cysts may drag ECM proteins from the underlying 3D microenvironment to coat the cyst outer surface. Also possible, MDCK cells in a rudimentary cyst secrete ECM proteins to the outer surface. Thus, alternatively, the ECM proteins surrounding the outer surface of cysts trigger the global polarization of MDCK cells during cystogenesis on 3D matrices. Notably, the cysts formed on a 3D ECM environment are larger than the ones formed in such an environment. Probably the cells in-3D grow in a relatively hypoxic environment, which could decrease the proliferation rate.
The finding that an on-3D environment is sufficient for cyst formation and the fact that MDCK cells typically form monolayers on culture plates, i.e. a 2D substratum environment, led us to define the boundary between 2D and 3D. We have determined the minimal ECM 3D environment, i.e., the minimal concentration and thickness of ECM able to trigger cystogenesis. A remaining question is how the quantitative changes in ECM environment result in the qualitative difference in MDCK cell behavior: cyst versus monolayer. The mechanism behind these morphogenetic thresholds is apparently unknown but likely involves the elasticity and stiffness of the ECM, the quantity of ECM components, and a spatial element. For example, the minimal thickness of Matrigel may provide MDCK cells a spatial cue or sufficient room or the third dimension to develop the subcellular structures needed for cyst formation. The microvesicular structures around the cysts and the microprotrusion structures at cyst outer membranes may belong to this kind of structure. Experiments to address whether these structures determine cystogenesis are now in progress. We predict that microprotrusions share structural similarity with invadopodia, which are used by cancer cells to sense the microenvironmental milieu, and function as local centers to sense the spatial cue and transduce the ECM-solicited signaling, leading to the formation of polarized cysts. The microvesicular structures may be derived from the vesicular trafficking related to the establishment of polarization during cystogenesis.
Based on our study, the ECM 3D microenvironment is no longer an abstract term and can be defined by physical parameters. ECM 3D microenvironment is a relative term, which depends on ECM composition and concentration and possibly varies among cell types. In many studies, experiments are often called 3D analysis when Matrigel or collagen gel is used. We suggest that the ECM 3D microenvironment in vitro should specifically refer to the ECM culture condition in which cells undergo morphogenesis or form cellular structures typically not seen at 2D culture conditions. Otherwise, the culture condition should not be considered to represent 3D or “morphogenic 3D” even when Matrigel or collagen gel is used. To distinguish 2D, we call it “non-morphogenic 3D”. In other words, when used below morphogenetic thresholds, Matrigel or collagen gel cannot be considered 3D ECMs but fall into the “non-morphogenic 3D” category.
A novel in vitro model for epithelial morphogenesis
Departing from the cystogenesis on 3D, our study has established an in vitro model for the quantitative analysis of 2D and 3D environments for morphogenesis and, possibly, other cellular functions such as stem cell-niche interaction. From the comparative analyses of cell behaviors on cue-less and morphogenic 3D environments, this model could be used for more specific and quantitative assessment of the contributions of extrinsic factors such as growth factors, cell-ECM contact, and cell-cell adhesion to epithelial morphogenesis. This model could also be readily used in differential analyses of cue-less and morphogenic 3D environments to more feasibly identify and characterize intrinsic regulators for epithelial morphogenesis. More importantly, this model will allow us to more authentically dissect cellular events crucial for morphogenesis. At last, if the “on-3D” system indeed induces functional morphogenesis, it could be applied to tissue engineering and stem cell differentiation for higher efficiency and better feasibility.
Search for the critical cell-ECM adhesion event(s) in MDCK cystogenesis
The addition of laminin 1 and/or collagen IV at the concentration of 10 μg/ml into the 3D scaffold methylcellulose failed to reconstitute cyst formation. This result does not necessarily exclude the essentiality of these ECM proteins in cystogenesis. For example, the concentrations of laminin 1 and collagen IV added into methylcellulose were much less than in Matrigel, and they may not reach the quantity threshold to induce cystogenesis. However, the fact that the same amount of laminin had a significant effect on cyst formation in collagen gel argues against this possibility. On the other hand, if laminin 1 and/or collagen IV were indeed required for cyst formation, we would predict that MDCK cells should synthesize these ECM proteins when or before they undergo cystogenesis in or on collagen gel, which contains no laminin 1 or collagen IV. In addition, if the coating efficiency of ECM components on methylcellulose is low, the amount of laminin 1 and collagen IV that was added into methylcellulose could have been too low to induce cyst formation. A recent study demonstrated that the mechanical elasticity provided by ECM proteins within 3D scaffolds directs stem cell lineage specification (Engler et al., 2006). Even if laminin 1 or collagen IV were the ECM components critical for morphogenesis, the elasticity of laminin 1- and/or collagen IV-containing methylcellulose could not be suitable for cyst formation. Therefore, to efficiently couple ECM components to 3D scaffolds and identify a 3D scaffold with appropriate elasticity will be the goal of upcoming studies. Because of the unavailability of entactin/nidogen, we could not investigate its role in MDCK cystogenesis in methylcellulose gels. We predict that nidogen alone would not be sufficient for MDCK cystogenesis, because the ablation of the nidogen gene displays neurological abnormalities but does not result in aberrant epithelial morphogenesis (Dong et al., 2002).
In conclusion, we have identified several microenvironmental requirements, made a few novel descriptive observations, and reached some mechanistic conclusions for epithelial cystogenesis in this study. For example, the local contact between MDCK cells and 3D ECM induces global polarization of the entire enclosed epithelial shell. In addition, it appears that a 3D microenvironment unable to solicit cellular signaling and/or provide amenable mechanical elasticity cannot trigger MDCK cystogenesis. Furthermore, the polarization of MDCK cells is uncoupled from the lumen and enclosed sphere formations under the non-morphogenic 3D ECM condition. Moreover, like polarization and lumen formation, secreting and protruding microstructures are the inherent processes of cystogenesis. Finally, laminin 1 and collagen IV in 3D ECM microenvironments promote cyst formation and/or enlargement. These findings will provide important insight into the mechanism of epithelial morphogenesis.
Acknowledgments
The authors are grateful to Drs. Seema Khurana, Valery Kukekov and David Armbruster for critical review of the manuscript. This work was supported by NIH Grant CA96991 (to X.A. Zhang). We also thank Drs. Valery Kukekov and Tanya Ignatova for providing methylcellulose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bao Q, Hughes RC. Galectin-3 expression and effects on cyst enlargement and tubulogenesis in kidney epithelial MDCK cells cultured in three-dimensional matrices in vitro. J Cell Sci. 1995;108:2791–2800. doi: 10.1242/jcs.108.8.2791. [DOI] [PubMed] [Google Scholar]
- Dong L, Chen Y, Lewis M, Hsieh JC, Reing J, Chaillet JR, Howell CY, Melhem M, Inoue S, Kuszak JR, DeGeest K, Chung AE. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab Invest. 2002;82:1617–1630. doi: 10.1097/01.lab.0000042240.52093.0f. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Ekblom P. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J. 1989;3:2141–2150. doi: 10.1096/fasebj.3.10.2666230. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Burwen SJ, Pitelka DR. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell. 1979;11:109–119. doi: 10.1016/0040-8166(79)90011-9. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Inductive tissue interaction in development. Adv Cancer Res. 1956;4:187–236. doi: 10.1016/s0065-230x(08)60725-3. [DOI] [PubMed] [Google Scholar]
- Hall HG, Farson DA, Bissell MJ. Lumen formation by epithelial cell lines in response to collagen overlay: a morphogenetic model in culture. Proc Natl Acad Sci USA. 1982;79:4672–4676. doi: 10.1073/pnas.79.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME, Sanchez-Madrid F, Flotte TJ, Krensky AM, Burakoff SJ, Bhan AK, Springer TA, Strominger JL. Glycoproteins of 210,000 and 130,000 m. w on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984;132:3011–3018. [PubMed] [Google Scholar]
- Joly D, Berissi S, Bertrand A, Strehl L, Patey N, Knebelmann B. Laminin 5 regulates polycystic kidney cell proliferation and cyst formation. J Biol Chem. 2006;281:29181–29189. doi: 10.1074/jbc.M606151200. [DOI] [PubMed] [Google Scholar]
- Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14:526–532. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Kroschewski R. Molecular mechanisms of epithelial polarity: about shapes, forces, and orientation problems. News Physiol Sci. 2004;19:61–66. doi: 10.1152/nips.01501.2003. [DOI] [PubMed] [Google Scholar]
- Laurie GW, Horikoshi S, Killen PD, Segui-Real B, Yamada Y. In situ hybridization reveals temporal and spatial changes in cellular expression of mRNA for a laminin receptor, laminin, and basement membrane (type IV) collagen in the developing kidney. J Cell Biol. 1989;109:1351–1362. doi: 10.1083/jcb.109.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- Mangoo-Karim R, Uchic M, Lechene C, Grantham JJ. Renal epithelial cyst formation and enlargement in vitro: dependence on cAMP. Proc Natl Acad Sci USA. 1989;86:6007–6011. doi: 10.1073/pnas.86.15.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem. 1992;267:17900–17906. [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAteer JA, Evan AP, Gardner KD. Morphogenetic clonal growth of kidney epithelial cell line MDCK. Anat Rec. 1987;217:229–239. doi: 10.1002/ar.1092170303. [DOI] [PubMed] [Google Scholar]
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991a;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Montesano R, Schaller G, Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell. 1991b;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Regulation of cell surface polarity from bacteria to mammals. Science. 1992;258:948– 955. doi: 10.1126/science.1439806. [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Ojakian GK, Schwimmer R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J Cell Sci. 1994;107:561–576. [PubMed] [Google Scholar]
- Orellana SA, Marfella-Scivittaro C. Distinctive cyclic AMP-dependent protein kinase subunit localization is associated with cyst formation and loss of tubulogenic capacity in Madin-Darby canine kidney cell clones. J Biol Chem. 2000;275:21233–21240. doi: 10.1074/jbc.M001964200. [DOI] [PubMed] [Google Scholar]
- Parry G, Cullen B, Kaetzel CS, Kramer R, Moss L. Regulation of differentiation and polarized secretion in mammary epithelial cells maintained in culture: extracellular matrix and membrane polarity influences. J Cell Biol. 1987;105:2043–2051. doi: 10.1083/jcb.105.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahikkala M, Sormunen R, Eskelinen S. Effects of src kinase and TGFbeta1 on the differentiation and morphogenesis of MDCK cells grown in three-dimensional collagen and Matrigel environments. J Pathol. 2001;195:391–400. doi: 10.1002/path.949. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- Schramek H, Feifel E, Marschitz I, Golochtchapova N, Gstraunthaler G, Montesano R. Loss of active MEK1-ERK1/2 restores epithelial phenotype and morphogenesis in transdifferentiated MDCK cells. Am J Physiol Cell Physiol. 2003;285:C652–661. doi: 10.1152/ajpcell.00463.2002. [DOI] [PubMed] [Google Scholar]
- Schwimmer R, Ojakian GK. The alpha 2 beta 1 integrin regulates collagen-mediated MDCK epithelial membrane remodeling and tubule formation. J Cell Sci. 1995;108:2487–2498. doi: 10.1242/jcs.108.6.2487. [DOI] [PubMed] [Google Scholar]
- Simons K, Fuller SD. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Sorokin L, Sonnenberg A, Aumailley M, Timpl R, Ekblom P. Recognition of the laminin E8 cell-binding site by an integrin possessing the alpha 6 subunit is essential for epithelial polarization in developing kidney tubules. J Cell Biol. 1990;111:1265–1273. doi: 10.1083/jcb.111.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimizu N, Miyajima A, Mostov KE. Liver progenitor cells develop cholangiocyte-type epithelial polarity in three-dimensional culture. Mol Biol Cell. 2007;18:1472–1479. doi: 10.1091/mbc.E06-09-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990a;95:137–151. doi: 10.1242/jcs.95.1.137. [DOI] [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. II Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990b;95:153–165. doi: 10.1242/jcs.95.1.153. [DOI] [PubMed] [Google Scholar]
- Wang AZ, Wang JC, Ojakian GK, Nelson WJ. Determinants of apical membrane formation and distribution in multicellular epithelial MDCK cysts. Am J Physiol. 1994;267:C473–481. doi: 10.1152/ajpcell.1994.267.2.C473. [DOI] [PubMed] [Google Scholar]
- Warren SL, Nelson WJ. Nonmitogenic morphoregulatory action of pp60v-src on multicellular epithelial structures. Mol Cell Biol. 1987;7:1326–1337. doi: 10.1128/mcb.7.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O'Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Fang X, Ewald A, Wong K, Hunt CA, Werb Z, Matthay MA, Mostov K. Formation of cysts by alveolar type II cells in three-dimensional culture reveals a novel mechanism for epithelial morphogenesis. Mol Biol Cell. 2007;18:1693–1700. doi: 10.1091/mbc.E06-11-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng D, Ferrari A, Ulmer J, Veligodskiy A, Fischer P, Spatz J, Ventikos Y, Poulikakos D, Kroschewski R. Three-dimensional modeling of mechanical forces in the extracellular matrix during epithelial lumen formation. Biophys J. 2006;90:4380–4391. doi: 10.1529/biophysj.105.073494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkl GM, Zuk A, van der Bijl P, van Meer G, Matlin KS. An antiglycolipid antibody inhibits Madin-Darby canine kidney cell adhesion to laminin and interferes with basolateral polarization and tight junction formation. J Cell Biol. 1996;133:695–708. doi: 10.1083/jcb.133.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]


