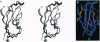Abstract
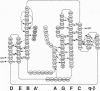
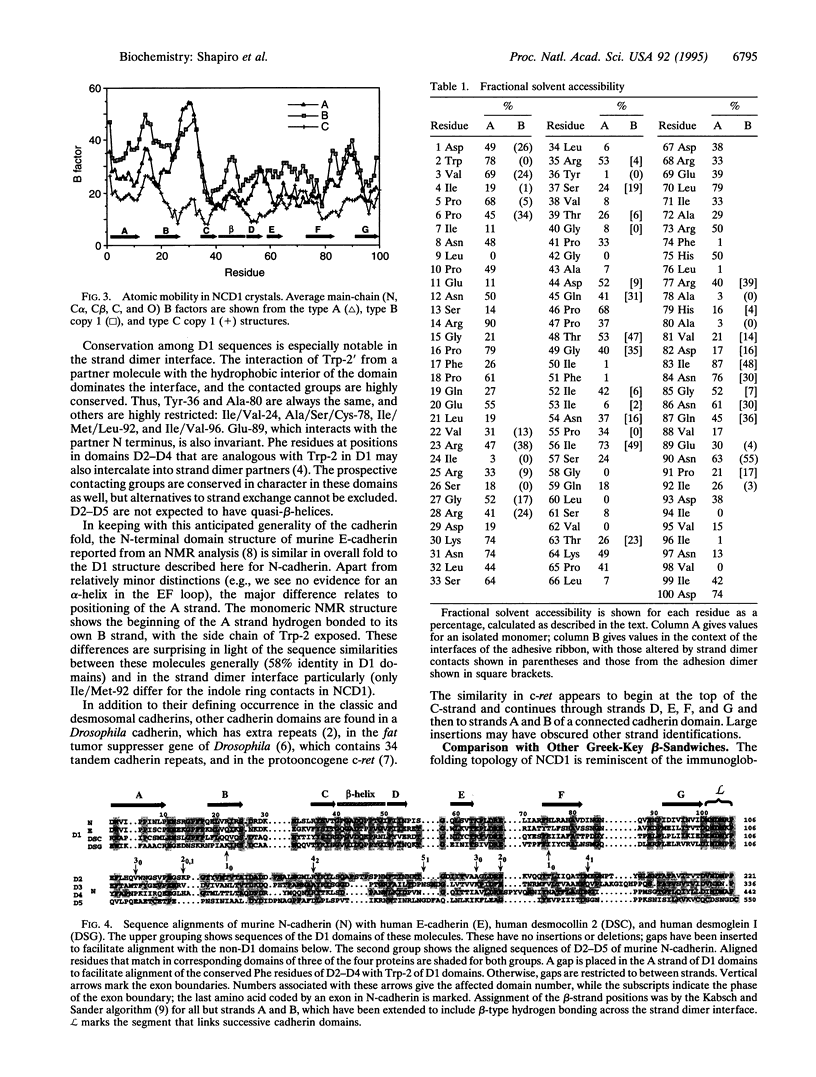
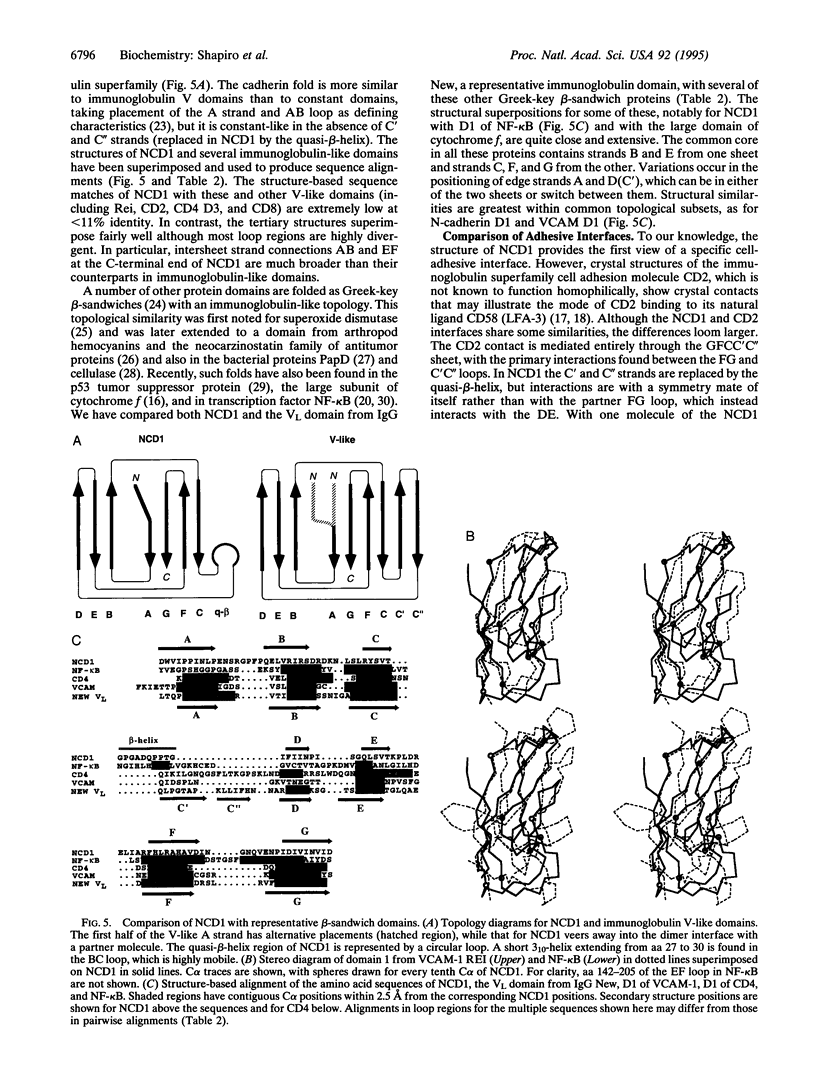
Cell-cell adhesion in zonula adherens and desmosomal junctions is mediated by cadherins, and recent crystal structures of the first domain from murine N-cadherin provide a plausible molecular basis for this adhesive action. A structure-based sequence analysis of this adhesive domain indicates that its fold is common to all extracellular cadherin domains. The cadherin folding topology is also shown to be similar to immunoglobulin-like domains and to other Greek-key beta-sandwich structures, as diverse as domains from plant cytochromes, bacterial cellulases, and eukaryotic transcription factors. Sequence similarities between cadherins and these other molecules are very low, however, and intron patterns are also different. On balance, independent origins for a favorable folding topology seem more likely than evolutionary divergence from an ancestor common to cadherins and immunoglobulins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson H. E., Royer W. E., Jr, Hendrickson W. A. Quantification of tertiary structural conservation despite primary sequence drift in the globin fold. Protein Sci. 1994 Oct;3(10):1706–1711. doi: 10.1002/pro.5560031009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodian D. L., Jones E. Y., Harlos K., Stuart D. I., Davis S. J. Crystal structure of the extracellular region of the human cell adhesion molecule CD2 at 2.5 A resolution. Structure. 1994 Aug 15;2(8):755–766. doi: 10.1016/s0969-2126(94)00076-x. [DOI] [PubMed] [Google Scholar]
- Cho Y., Gorina S., Jeffrey P. D., Pavletich N. P. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994 Jul 15;265(5170):346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- Ghosh G., van Duyne G., Ghosh S., Sigler P. B. Structure of NF-kappa B p50 homodimer bound to a kappa B site. Nature. 1995 Jan 26;373(6512):303–310. doi: 10.1038/373303a0. [DOI] [PubMed] [Google Scholar]
- Harpaz Y., Chothia C. Many of the immunoglobulin superfamily domains in cell adhesion molecules and surface receptors belong to a new structural set which is close to that containing variable domains. J Mol Biol. 1994 May 13;238(4):528–539. doi: 10.1006/jmbi.1994.1312. [DOI] [PubMed] [Google Scholar]
- Hatta K., Nose A., Nagafuchi A., Takeichi M. Cloning and expression of cDNA encoding a neural calcium-dependent cell adhesion molecule: its identity in the cadherin gene family. J Cell Biol. 1988 Mar;106(3):873–881. doi: 10.1083/jcb.106.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazes B., Hol W. G. Comparison of the hemocyanin beta-barrel with other Greek key beta-barrels: possible importance of the "beta-zipper" in protein structure and folding. Proteins. 1992 Mar;12(3):278–298. doi: 10.1002/prot.340120306. [DOI] [PubMed] [Google Scholar]
- Holden H. M., Ito M., Hartshorne D. J., Rayment I. X-ray structure determination of telokin, the C-terminal domain of myosin light chain kinase, at 2.8 A resolution. J Mol Biol. 1992 Oct 5;227(3):840–851. doi: 10.1016/0022-2836(92)90226-a. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Bränden C. I. Crystal structure of chaperone protein PapD reveals an immunoglobulin fold. Nature. 1989 Nov 16;342(6247):248–251. doi: 10.1038/342248a0. [DOI] [PubMed] [Google Scholar]
- Jones E. Y., Davis S. J., Williams A. F., Harlos K., Stuart D. I. Crystal structure at 2.8 A resolution of a soluble form of the cell adhesion molecule CD2. Nature. 1992 Nov 19;360(6401):232–239. doi: 10.1038/360232a0. [DOI] [PubMed] [Google Scholar]
- Jones E. Y., Harlos K., Bottomley M. J., Robinson R. C., Driscoll P. C., Edwards R. M., Clements J. M., Dudgeon T. J., Stuart D. I. Crystal structure of an integrin-binding fragment of vascular cell adhesion molecule-1 at 1.8 A resolution. Nature. 1995 Feb 9;373(6514):539–544. doi: 10.1038/373539a0. [DOI] [PubMed] [Google Scholar]
- Leahy D. J., Hendrickson W. A., Aukhil I., Erickson H. P. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992 Nov 6;258(5084):987–991. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]
- Mahoney P. A., Weber U., Onofrechuk P., Biessmann H., Bryant P. J., Goodman C. S. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell. 1991 Nov 29;67(5):853–868. doi: 10.1016/0092-8674(91)90359-7. [DOI] [PubMed] [Google Scholar]
- Martinez S. E., Huang D., Szczepaniak A., Cramer W. A., Smith J. L. Crystal structure of chloroplast cytochrome f reveals a novel cytochrome fold and unexpected heme ligation. Structure. 1994 Feb 15;2(2):95–105. doi: 10.1016/s0969-2126(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Müller C. W., Rey F. A., Sodeoka M., Verdine G. L., Harrison S. C. Structure of the NF-kappa B p50 homodimer bound to DNA. Nature. 1995 Jan 26;373(6512):311–317. doi: 10.1038/373311a0. [DOI] [PubMed] [Google Scholar]
- Oda H., Uemura T., Harada Y., Iwai Y., Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol. 1994 Oct;165(2):716–726. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- Overduin M., Harvey T. S., Bagby S., Tong K. I., Yau P., Takeichi M., Ikura M. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science. 1995 Jan 20;267(5196):386–389. doi: 10.1126/science.7824937. [DOI] [PubMed] [Google Scholar]
- Puttagunta S., Mathur M., Cowin P. Structure of DSG1, the bovine desmosomal cadherin gene encoding the pemphigus foliaceus antigen. Evidence of polymorphism. J Biol Chem. 1994 Jan 21;269(3):1949–1955. [PubMed] [Google Scholar]
- Reid R. A., Hemperly J. J. Human N-cadherin: nucleotide and deduced amino acid sequence. Nucleic Acids Res. 1990 Oct 11;18(19):5896–5896. doi: 10.1093/nar/18.19.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C., Thomas K. A., Silverton E. W., Davies D. R. Similarity of three-dimensional structure between the immunoglobulin domain and the copper, zinc superoxide dismutase subunit. J Mol Biol. 1976 Apr 5;102(2):221–235. doi: 10.1016/s0022-2836(76)80050-2. [DOI] [PubMed] [Google Scholar]
- Ryu S. E., Truneh A., Sweet R. W., Hendrickson W. A. Structures of an HIV and MHC binding fragment from human CD4 as refined in two crystal lattices. Structure. 1994 Jan 15;2(1):59–74. doi: 10.1016/S0969-2126(00)00008-3. [DOI] [PubMed] [Google Scholar]
- Schneider R. The human protooncogene ret: a communicative cadherin? Trends Biochem Sci. 1992 Nov;17(11):468–469. doi: 10.1016/0968-0004(92)90490-z. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Fannon A. M., Kwong P. D., Thompson A., Lehmann M. S., Grübel G., Legrand J. F., Als-Nielsen J., Colman D. R., Hendrickson W. A. Structural basis of cell-cell adhesion by cadherins. Nature. 1995 Mar 23;374(6520):327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Shrake A., Rupley J. A. Environment and exposure to solvent of protein atoms. Lysozyme and insulin. J Mol Biol. 1973 Sep 15;79(2):351–371. doi: 10.1016/0022-2836(73)90011-9. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Teplyakov A., Obmolova G., Wilson K., Kuromizu K. Crystal structure of apo-neocarzinostatin at 0.15-nm resolution. Eur J Biochem. 1993 Apr 15;213(2):737–741. doi: 10.1111/j.1432-1033.1993.tb17814.x. [DOI] [PubMed] [Google Scholar]
- Wagner G. E-cadherin: a distant member of the immunoglobulin superfamily. Science. 1995 Jan 20;267(5196):342–342. doi: 10.1126/science.7824932. [DOI] [PubMed] [Google Scholar]