Abstract
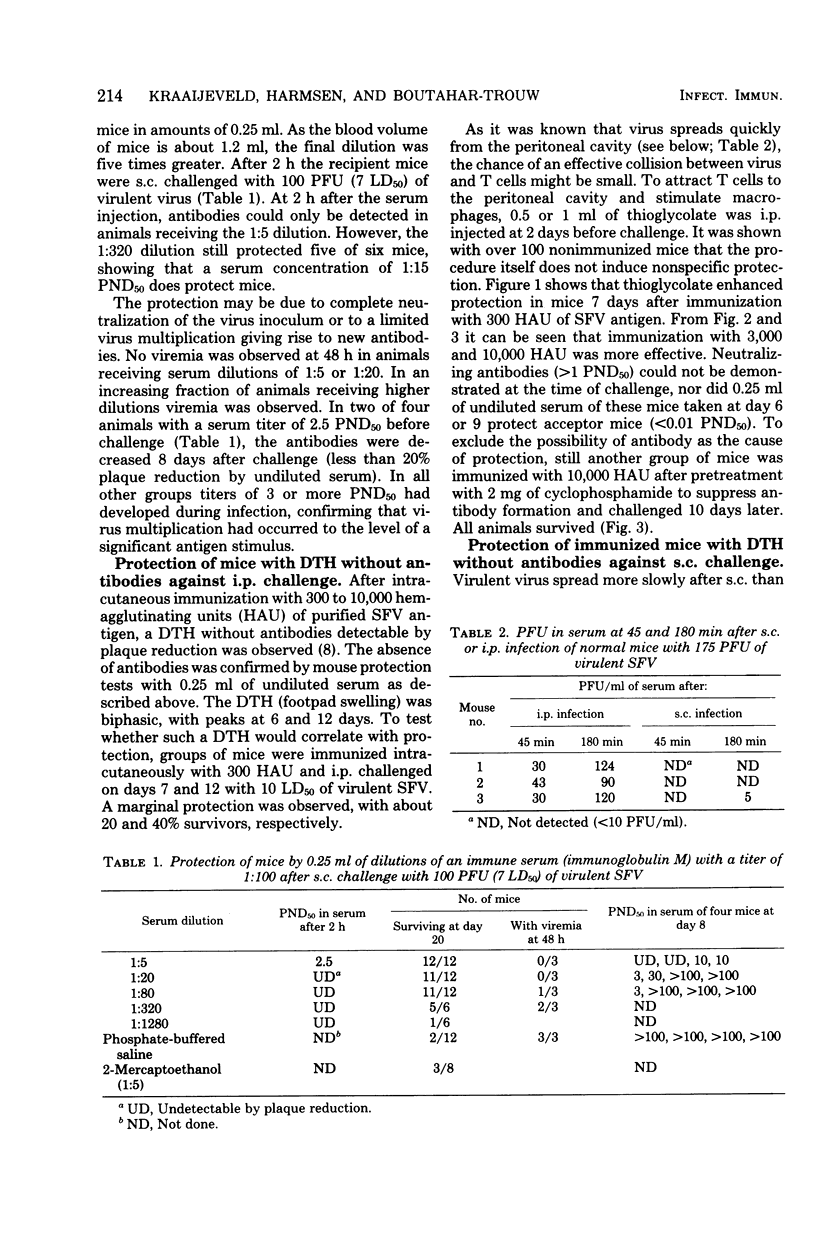
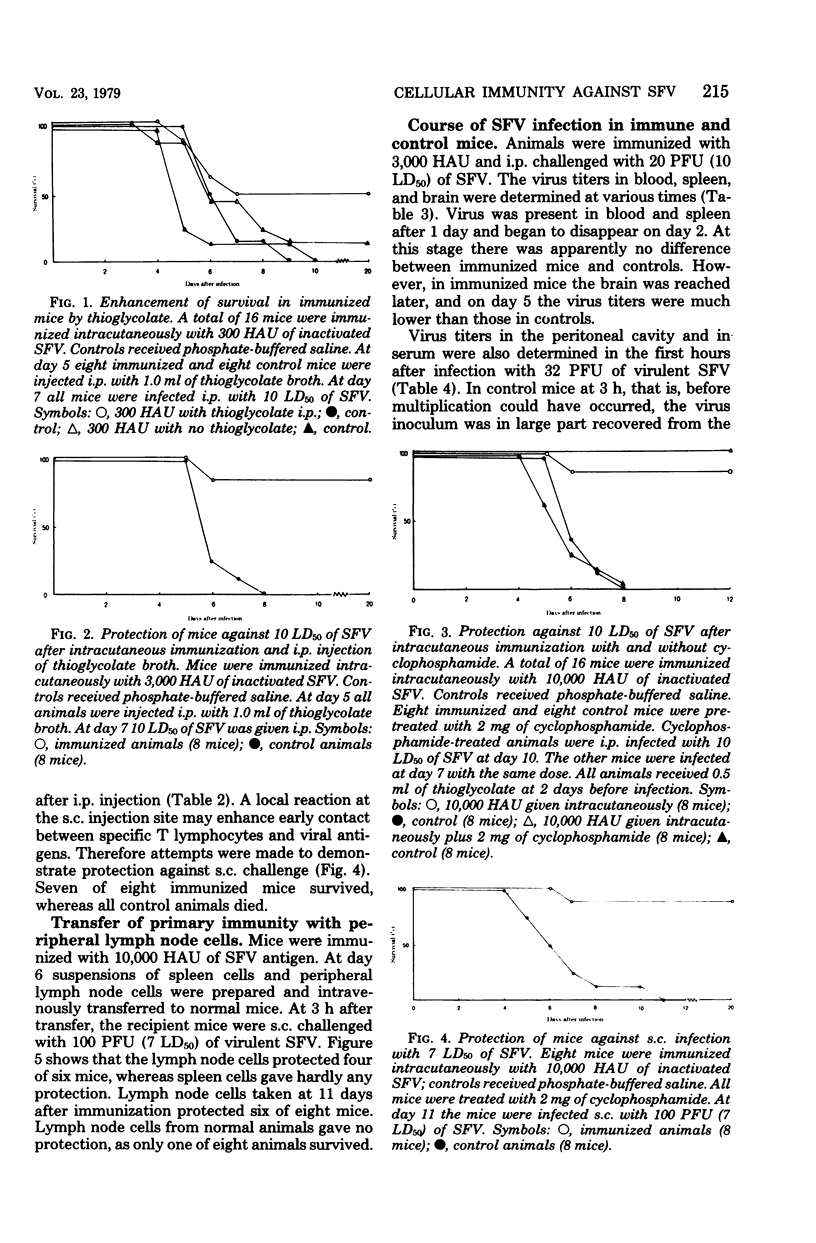
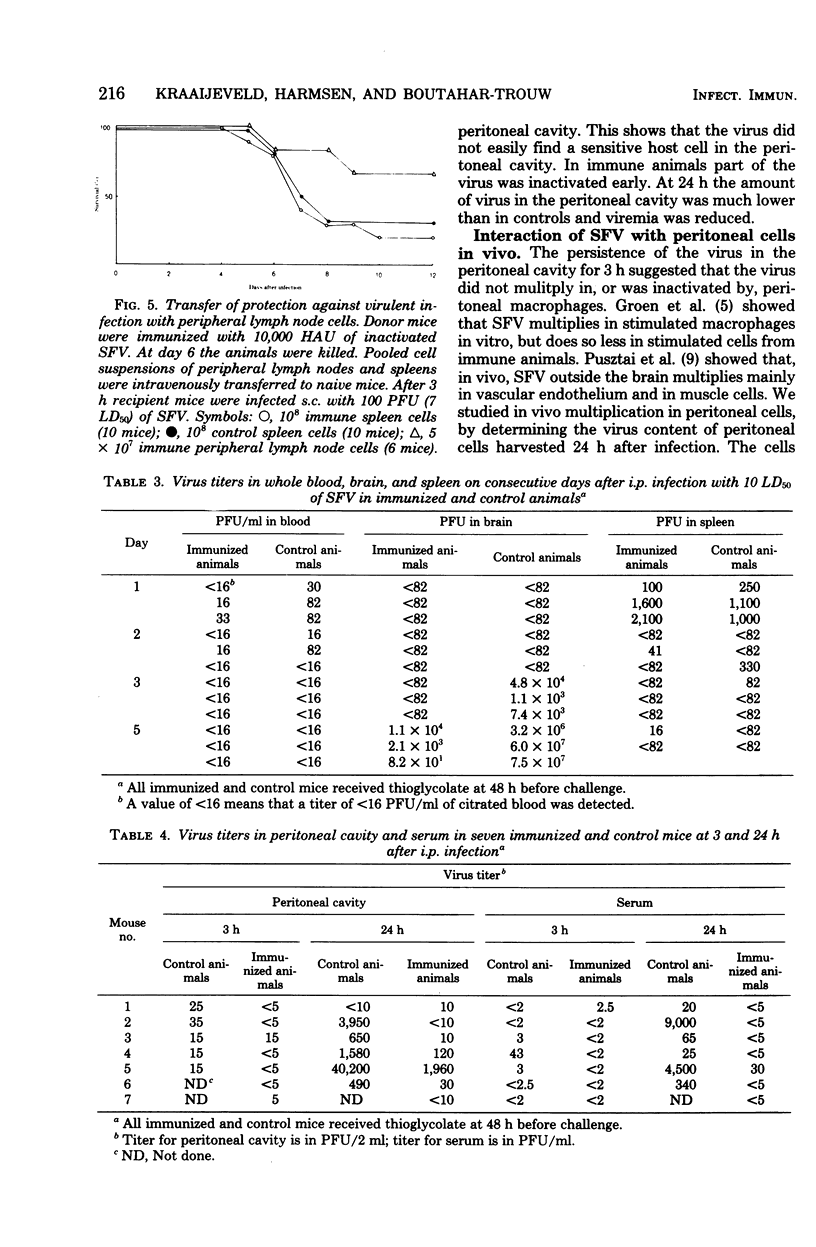
Intracutaneous immunization of BALB/c mice with purified inactivated Semliki Forest virus resulted in cellular immunity without detectable antibodies. The animals were protected against subcutaneous challenge, from which the challenge virus spreads slowly. After intraperitoneal challenge, which permits a rapid virus spread, the protection was marginal. Stimulation of the intraperitoneal cell population with thioglycolate before challenge resulted in complete protection. The protection could be transferred to normal mice with peripheral lymph node cells, but not with spleen cells. The course of the infection in immunized and normal mice was also studied. Semliki Forest virus does not multiply in peritoneal cells in vivo. In immunized mice part of the challenge virus in the peritoneal cavity was rapidly eliminated and viremia was reduced. After challenge, immunized mice produced less antibody than normal mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradish C. J., Allner K., Maber H. B. The virulence of original and derived strains of Semliki forest virus for mice, guinea-pigs and rabbits. J Gen Virol. 1971 Aug;12(2):141–160. doi: 10.1099/0022-1317-12-2-141. [DOI] [PubMed] [Google Scholar]
- Doherty P. C. Quantitative studies of the inflammatory process in fatal viral meningoencephalitis. Am J Pathol. 1973 Dec;73(3):607–622. [PMC free article] [PubMed] [Google Scholar]
- Epstein L. B., Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. II. Identification of the cell type responsible for interferon production and cell proliferation in response to mitogens. Cell Immunol. 1974 Jun;12(3):407–421. doi: 10.1016/0008-8749(74)90097-5. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Metselaar D., Kirya G. B., Timms G. L. Investigations into yellow fever virus and other arboviruses in the northern regions of Kenya. Bull World Health Organ. 1970;42(5):787–795. [PMC free article] [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D., Mackaness G. B. The mediator of cellular immunity. II. Migration of immunologically committed lymphocytes into inflammatory exudates. J Exp Med. 1971 Feb 1;133(2):400–409. doi: 10.1084/jem.133.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai R., Gould E. A., Smith H. Infection patterns in mice of an avirulent and virulent strain of Semliki Forest virus. Br J Exp Pathol. 1971 Dec;52(6):669–677. [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., Manejias R. E., Russo M., Abbey E. E. Increased spreading of macrophages from mice treated with interferon inducers. Cell Immunol. 1977 Mar 1;29(1):86–95. doi: 10.1016/0008-8749(77)90277-5. [DOI] [PubMed] [Google Scholar]
- Rodda S. J., White D. O. Cytotoxic macrophages: a rapid nonspecific response to viral infection. J Immunol. 1976 Dec;117(6):2067–2072. [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. The immunosuppressive effect of type II mouse interferon preparations on antibody production. Cell Immunol. 1977 Dec;34(2):193–206. doi: 10.1016/0008-8749(77)90243-x. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Friedman H., Mills L., Zayon G. Suppression of murine virus leukaemogenesis by thioglycollate, a bacteriological culture medium that affects macrophage peroxidase. Nature. 1975 May 22;255(5506):343–344. doi: 10.1038/255343a0. [DOI] [PubMed] [Google Scholar]
- van der Groen G., Vanden Berghe D. A., Pattyn S. R. Interaction of mouse peritoneal macrophages with different arboviruses in vitro. J Gen Virol. 1977 Feb;34(2):353–361. doi: 10.1099/0022-1317-34-2-353. [DOI] [PubMed] [Google Scholar]



