Abstract
Glucagon-like peptide-1 (GLP-1) agonists are a class of drugs used for the treatment of type 2 diabetes mellitus. GLP-1 is released in response to meal intake; these classes of drugs enhance glucose-dependent insulin secretion and exhibit other antihyperglycemic effects following their release into the circulation from the gut. Psoriasis is a chronic skin condition affecting approximately 2% of the Western population. It is considered to be an autoimmune disease that involves the Th1 pathway and is associated with metabolic syndrome and its components, such as obesity, diabetes, and hypertension. We have reviewed reports in the literature that indicate a beneficial anti-inflammatory effect of GLP-1 in patients with diabetes or who have insulin resistance and psoriasis.
Keywords: dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1, glycemic control, incretin, liraglutide, metabolic syndrome, obesity, psoriasis, type 2 diabetes
Introduction
Psoriasis is the most prevalent immune-mediated chronic skin disease associated with metabolic disorders such as obesity, diabetes, and dyslipidemia, which are all also characterized by enhanced local and/or systemic inflammation [Schön and Boehncke, 2005], affecting 2–5% of the world’s population. According to the National Institutes of Health (Bethesda, MA, USA), approximately 7.5 million Americans have psoriasis [Raychaudhuri et al. 2014]. The exact cause of psoriasis is still unknown, however genetics and the immune system play a major role [Schön and Boehncke, 2005]. There is no cure for the spectrum of psoriatic diseases that comprises various different subtypes of psoriasis and psoriatic arthritis.
Patients with psoriasis are likely to be obese and it is well known that obesity, as well as other metabolic disorders such as diabetes, are more prevalent in those with severe rather than mild psoriasis [Raychaudhuri et al. 2014]. These conditions are associated with chronic systemic inflammatory activation and an increased risk of cardiovascular morbidity and mortality [Raychaudhuri et al. 2014]. Studies have found an association between obesity and chronic inflammation, which could contribute to the development or aggravation of psoriasis. Furthermore, obese patients with psoriasis are more difficult to treat and are at increased risk for dyslipidemia, hypertension, diabetes, and cardiovascular disease. Obesity thus represents a serious problem in the treatment of psoriasis.
Glucagon-like pepide-1 (GLP-1) analogue therapy used in the treatment of type 2 diabetes brings about a considerable reduction in weight and hyperglycemia. It was found that in patients with psoriasis who received GLP-1 agonist for concomitant type 2 diabetes, a marked improvement of psoriasis severity was experienced that interestingly was initiated immediately after the start of treatment before achieving glycemic control or a reduction in weight. This could possibly be due to its immunomodulatory effect. Moreover, the high concentration of dipeptidyl peptidase-4 (DDP-4) in epidermal cells of the skin suggests a possible role of DDP-4 inhibitors in improving psoriasis severity [Nishioka et al. 2012].
Relevant English-language articles were reviewed through searches of Medline, Google scholars using the keywords ‘GLP-1 agonists’, ’psoriasis’, ’obesity’, and ‘type 2 diabetes mellitus’. Since GLP-1 agonists are a novel treatment modality, few articles were found in the review of literature from years 2011 up to the present, and studies were mainly conducted on small populations.
Immunologic importance of GLP-1
Emerging data suggested that GLP-1 has immunological effects, which brought it back to the center of attention. Studies have demonstrated that treatment with the GLP-1 agonist liraglutide results in anti-inflammatory actions demonstrated by a decreased expression of cytokines induced by tumor necrosis factor-α (TNF-α). This is important since studies have shown the crucial role of TNF-α in inflammatory diseases including psoriasis. TNF-α enhances inflammation through the prosurvival transcription factor nuclear factor-kappa B (NF-κB), and studies have shown NF-κB activation to be inhibited in response to GLP-1 [Hogan et al. 2011; Buysschaert et al. 2012] (Figure 1).
Figure 1.
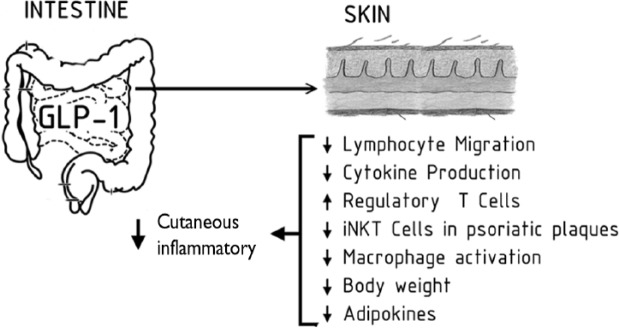
GLP-1 action on the skin. GLP-1, glucagon-like pepide.
GLP-1 receptor signaling may regulate lymphocyte proliferation and maintenance of peripheral regulatory T cells [Drucker and Rosen, 2011] (Figure 1).
Studies showed that GLP-1 inhibits chemokine-induced CD4-positive lymphocyte migration in vitro by inhibition of the phosphatidylinositol-4,5-bisphosphate 3-kinase pathway. Moreover, anti-inflammatory actions of GLP-1 in adipocytes and mesenteric endothelium have been found [Hogan et al.2011; Drucker and Rosen, 2011].
Although data on the immunologic effects of GLP-1 are still scarce and primarily based on in vitro and animal models, a few case reports have shown a remission of psoriasis in obese patients following bypass bariatric surgery with an improvement noticed immediately after the operation, which coincides with an up to 20-fold increase in plasma GLP-1 levels. These effects may result from a direct immune action of GLP-1, in particular by a reduction of dermal γδ T cells and interleukin (IL)-17 expression [Faurschou et al. 2011].
GLP-1 could however also directly affect the skin as one study demonstrated the expression of the GLP-1 receptor and proglucagon in the skin of newborn mice with a higher level noticed around the hair follicles. The GLP-1 receptor was similarly found in cultured skin cells [Raychaudhuri et al. 2014].
Case reports and studies about GLP-1 analogues and psoriasis
The literature reports that obesity is associated with innate immune cell dysfunction, which is a key feature of psoriasis [Raychaudhuri et al. 2014]. One study by Hogan and colleagues demonstrated that, compared with non-obese controls, obese patients have a reduction in both the number and function of circulating natural killer cells, as well as a lower accumulation of invariant natural killer T (iNKT) cells in the omentum [Hogan et al. 2011]. iNKT cells are a rare subset of innate T cells that exert multiple immunoregulatory functions. When stimulating iNKT cells, they can rapidly produce multiple cytokines that direct the immune response towards an either pro-inflammatory (Th1 or Th17) or an anti-inflammatory (Th2) bias. iNKT cells are known to be implicated in the pathogenesis of various obesity-related diseases, including psoriasis, cancer, and arthritis [Hogan et al. 20011; Faurschou et al. 2013].
Another study by Hogan and colleagues showed that the co-existing psoriasis in an obese patient with type 2 diabetes improved unexpectedly on GLP-1 therapy. According to the study, this improvement in psoriasis symptoms was seen immediately before observing improvements in glycemic control or weight loss. This has led to the hypothesis that GLP-1 may influence the severity of psoriasis by interacting directly with the innate immune system, in particular the iNKT cells [Drucker and Rosen, 2011; Faurschou et al. 2013] (Figure 2).
Figure 2.
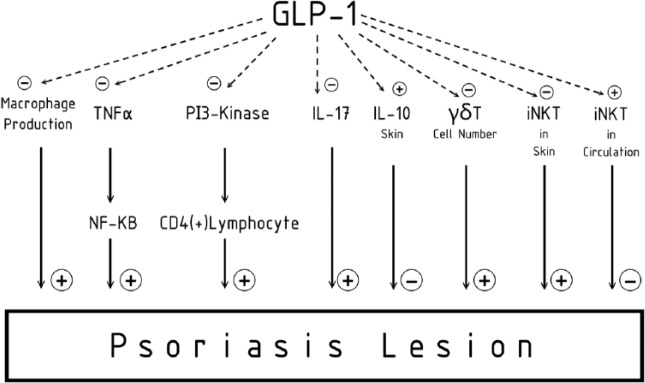
The immunologic role of GLP-1. GLP-1, glucagon-like pepide; IL, interleukin; iNKT, invariant natural killer T cell; NF-κB, nuclear factor-kappa B; PI3-kinase, phosphatidylinositol-4,5-bisphosphate 3-kinase; TNF-α; tumor necrosis factor-α.
In one study of three patients, an improvement in psoriasis following GLP-1 therapy was noticed in one patient and two other participants with obesity and type 2 diabetes. In this study, the presence of iNKT cells in psoriasis plaques was increased with a relative depletion in circulating iNKT cell number, and following 6 weeks of therapy with GLP-1 analogue showed a reversal of this ratio in the two nonindex participants, that is, a reduction in iNKT cell numbers in the psoriatic plaques and an increase in circulating iNKT cell numbers. The GLP-1 receptor was expressed on iNKT cells and modulated cytokine production in vitro following stimulation with native GLP-1 or the GLP-1 analogue, liraglutide. Thus, the improvement in clinical psoriasis observed in all three patients is attributed to the putative immunoregulatory action of GLP-1 on iNKT cells [Drucker and Rosen, 2011].
A prospective cohort study was carried out by Ahern and colleagues in which seven patients had chronic plaque psoriasis and none had concomitant psoriatic arthritis [Ahern et al. 2013]. The median interquartile range (IQR) age was 48 years. Out of the seven patients, five were severely obese with a body mass index (BMI) above 40 kg/m2. None were receiving active topical therapy nor had ever received systemic therapy for psoriasis. The change in the proportion of circulating monocytes that produced cytokines was determined in four patients. There was a significant decrease in psoriasis severity associated with liraglutide, the median IQR for the psoriasis area and severity index (PASI) decreased by 1.8 (p = 0.03). In two patients, there was a greater than 50% reduction in PASI and none experienced a greater than 75% reduction in PASI. Additionally, there was a decrease in the median IQR for the dermatology life quality index (DLQI) from 6 to 2 (p = 0.03). In all patients liraglutide was well tolerated with no serious adverse events throughout the study. Liraglutide also resulted in a median weight loss of 5% (IQR: 1.8–10.2%) (p = 0.06). Median IQR fasting plasma-glucose concentration decreased from 6.1 mmol/ L to 5.8 mmol / L (p = 0.08). Also, an increase of 37.9% was seen in the circulating iNKT cells (p = 0.03), with a decrease in the proportion of monocytes that produced TNF-α, but there were no changes seen in the number of circulating T lymphocytes, B lymphocytes or natural killer cells with liraglutide therapy [Ahern et al. 2013].
In another case report a 59-year-old patient with moderate and stable psoriasis over many years who did not respond well to local treatments had an immediate improvement of psoriasis when given the GLP-1 receptor agonist liraglutide as a treatment for his type 2 diabetes. It was suggested that the rapid improvement of psoriasis was due to the direct anti-inflammatory effect of liraglutide. The patient previously had well-controlled type 2 diabetes, and psoriasis was not improved then, that is why it is unlikely that the immediate effect of liraglutide was due to glycemic control. This is supported by the previously mentioned study by Hogan and colleagues that showed an effect of liraglutide in psoriasis that was independent of glycemic control [Hogan et al. 2011]. Also, the effect on psoriasis was initiated before any weight loss occurred. The weight losses achieved during the period of treatment with liraglutide have reinforced the improvement in psoriasis.
Another case report of a 54-year-old obese man (weight 138 kg, BMI 42.1 kg/m2) with extensive plaque psoriasis presented with a bleeding erythematous nodule in his natal cleft. This was excised and histopathology revealed an ulcerated nodular amelanotic malignant melanoma with a Breslow thickness of 5 mm and a high mitotic rate. Sentinel node biopsy and subsequent staging did not show metastatic disease. The patient had received a multiple systemic regimen to treat his psoriasis but none had controlled the disease.The latest treatment was adalimumab (anti-TNF), but was discontinued when a melanoma was diagnosed [Reid et al. 2013].
Acitretin 50 mg daily was commenced but was ineffective, and after 4 months the patient’s PASI was 14.2 and his DLQI was 25. He did not have diabetes, but his fasting insulin was elevated at 24.2 mUI/ml and his homeostasis model of assessment–insulin resistance was 6.02 (normal value < 2.0). It was shown that liraglutide improves insulin resistance and reduces BMI, both of which correlate with psoriasis severity. After 1 year of treatment with liraglutide in combination with acitretin 50 mg daily, the PASI dropped to 7.6 and the DLQI decreased to 12 with a 10 kg loss in weight. Serial imaging showed no evidence of metastatic melanoma. This was the first reported case where a GLP-1 analogue was used successfully in a patient without diabetes in combination with acitretin for the management of severe psoriasis [Reid et al. 2013].
A recent prospective series study by Buysschaert and colleagues is the first to show a slight decrease in epidermis thickness assessed after a few months of exenatide/liraglutide therapy, together with the clinical reduction of infiltration of psoriasis lesions [Buysschaert et al. 2012]. As expected, the incretin-based treatment was associated at the end of the study with weight loss and improvement of glycemic control.
The explanation for this could be based on the anti-inflammatory action by itself of the GLP-1 receptor agonist, which exerts direct and indirect effects on immune functions, independently of changes in weight and glycemic control [Buysschaert et al. 2012].
Another possible immunologic role of the GLP-1 receptor agonist is that γδ T cells as well as IL-17 levels are also increased in psoriatic lesions when compared with control skin in patients with type 2 diabetes. Moreover, these results indicate that an incretin-based therapy was associated, in particular in subjects with diabetes who clinically improved their PASI, with a decreased number of dermal γδ T cells and a reduction of IL-17 mRNA expression. A significant correlation between delta PASI and delta γδ T cells percentage after exenatide/liraglutide treatment in all individuals reinforced the rationale of the above hypothesis [Drucker and Rosen, 2011; Faurschou et al. 2013] (Figure 2).
Taken together, all data clearly suggest that the immunoregulatory action of the GLP-1 receptor agonist could account for, or contribute to, the improvement of psoriasis lesions both clinically and histopathologically, as reported in this cohort, especially by an effect on γδ T cells as well as its main effect to the molecule, IL-17 [Drucker and Rosen, 2011; Faurschou et al. 2013, 2014] (Figure 2).
Nishioka and colleagues also observed an improvement of psoriasis lesions in one patient treated with a DPP-4 inhibitor, possibly following an increase in GLP-1 levels and an inhibited activation of immune T cells by sitagliptin, probably due to the high expression of DDP-4 in the epidermis and on multiple immune cell subtypes [Nishioka et al. 2012]. Yet preclinical studies demonstrating cutaneous vasculitis in nonhuman primates has heightened awareness of the potential consequences of some DPP-4 inhibitors on the skin.
Conclusion
GLP-1 analogue therapy appears to improve slightly clinical and histopathologic psoriasis severity with good tolerance in contrast to the vast majority of established systemic treatments since GLP-1 receptor agonists are not immunosuppressive. These effects may result from a direct immune action of GLP-1, in particular by a reduction of dermal γδ T cells and IL-17 expression. These observations raise the possibility of larger therapeutic applications of GLP-1 in psoriasis, in particular in obese and/or patients with type 2 diabetes.
There are three ongoing clinical trials on the effect of GLP-1 on psoriasis. One is a double-blinded, randomized placebo-controlled clinical trial to investigate the effect of the GLP-1 analogue (liraglutide) on psoriasis performed on 20 patients [www.clinicaltrials.gov]. The second trial is a randomized, double-blinded trial to examine GLP-1 receptors in the skin of psoriasis patients compared with the skin of humans with no skin disease performed on 12 patients [www.clinicaltrials.gov]. The latter is a single-arm, open-label trial to analyze short and medium efficacy on clinical, immunologic and histopathologic parameters of the GLP-1 receptor agonist on moderate to severe psoriasis plaques in a group of patients with type 2 diabetes carried out on 10 patients. However, prospective controlled trials of GLP-1 therapies (GLP-1 analogues and gliptins), blinded, dose-finding studies, across all weight groups with large population size and for a long period of follow up are needed to confirm and extend this potential beneficial effect of incretin-based treatments on psoriasis in patients with or without diabetes.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that they have no conflict of interest.
Contributor Information
Marwa R. Al-Badri, Department of Internal Medicine, Division of Endocrinology and Metabolism, American University of Beirut- Medical Center, New York, USA
Sami T. Azar, Department of Internal Medicine, Division of Endocrinology and Metabolism, American University of Beirut-Medical Center, 3 Dag Hammarskjold Plaza, 8th floor, New York, NY 10017, USA
References
- Ahern T., Tobin A., Corrigan M., Hogan A., Sweeney C., Kirby B., et al. (2013) Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: A prospective cohort study. J Eur Acad Dermatol Venereol 27: 1440–1443 [DOI] [PubMed] [Google Scholar]
- Buysschaert M., Tennstedt D., Preumont V. (2012) Improvement of psoriasis during exenatide treatment in a patient with diabetes. Diabetes Metab 38: 86–88 [DOI] [PubMed] [Google Scholar]
- Drucker D., Rosen C. (2011) Glucagon-like peptide-1 (GLP-1) receptor agonists, obesity and psoriasis: diabetes meets dermatology. Diabetologia 54: 2741-2744 [DOI] [PubMed] [Google Scholar]
- Faurschou A., Knop F., Thyssen J., Zachariae C., Skov L., Vilsbøll T. (2014) Improvement in psoriasis after treatment with the glucagon-like peptide-1 receptor agonist liraglutide. Acta Diabetol 51: 147–150 [DOI] [PubMed] [Google Scholar]
- Faurschou A., Pedersen J., Gyldenløve M., Poulsen S., Holst J., Thyssen J., et al. (2013) Increased expression of glucagon-like peptide-1 receptors in psoriasis plaques. Exp Dermatol 22: 150–152 [DOI] [PubMed] [Google Scholar]
- Faurschou A., Zachariae C., Skov L., Vilsbøll T., Knop F. (2011) Gastric bypass surgery: improving psoriasis through a GLP-1-dependent mechanism? Med Hypotheses 77: 1098–1101 [DOI] [PubMed] [Google Scholar]
- Hogan A., Tobin A., Ahern T., Corrigan M., Gaoatswe G., Jackson R., et al. (2011) Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia 54: 2745–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka T., Shinohara M., Tanimoto N., Kumagai C., Hashimoto K. (2012) Sitagliptin, a dipeptidyl peptidase-IV inhibitor, improves psoriasis. Dermatology 224: 20–21 [DOI] [PubMed] [Google Scholar]
- Raychaudhuri S., Maverakis E., Raychaudhuri S. (2014) Diagnosis and classification of psoriasis. Autoimmun Rev 13: 490–495 [DOI] [PubMed] [Google Scholar]
- Reid C., Tobin A., Ahern T., O’Shea D., Kirby B. (2013) Liraglutide in combination with acitretin for severe recalcitrant psoriasis. Br J Dermatol 169: 230–231 [DOI] [PubMed] [Google Scholar]
- Schön M., Boehncke W. (2005) Psoriasis. N Engl J Med 352: 1899-1912 [DOI] [PubMed] [Google Scholar]


