Introduction
On the global scale, primary liver cancer is a major contributor to both cancer incidence and mortality. It is the sixth most commonly occurring cancer in the world and the third largest cause of cancer mortality.1 The most common histologic type of primary liver cancer, hepatocellular carcinoma (HCC), is a malignant tumor arising from hepatocytes, the liver’s parenchymal cells. HCCs have not been reported in autopsies of well preserved Egyptian mummies2, though Zimmerman and Auferheide found evidence of cirrhosis of the liver. By the 19th century, HCCs were accurately described in European pathology journals, but they were thought to be uncommon.3 In agreement, in the early twentieth century, William Osler in the United States also reported that primary liver cancer was rare.4 These views probably reflected the pronounced geographic disparity in incidence, as HCCs are common in Asia and Africa, but are uncommon in Europe and North America.
Incidence and Mortality
As diagnostic confirmation of HCC is not routine worldwide, it is easier to examine incidence rates of primary liver cancer than incidence rates of HCC. Because HCC is the most common histology in the majority of countries, however, primary liver cancer rates are a close approximation of HCC rates. An exception is northeast Thailand, which has very high rates of primary liver cancer (male incidence = 88/100,000; female incidence=35/100,000)5 due to the exceptionally high incidence of intrahepatic cholangiocarcinoma.
The highest liver cancer incidence rates in the world are reported by registries in Asia and Africa. Approximately 85% of all liver cancers occur in these areas, with Chinese registries alone, reporting over 50%.5 In addition to registries in China (e.g., Hong Kong, Shanghai), other Asian registries with rates greater than 20/100,000 persons include those of Seoul, Korea and Osaka, Japan (Figure 1). Registries in Africa that report incidence rates greater than 20/100,000 include those of Harare, Zimbabwe (African ancestry) and Gharbiah, Egypt. In contrast to these high rate HCC areas, low rate areas include northern Europe as well as North and South America. Registries in these areas, in general, report incidence rates of less than 10/100,000 (Figure 1). Intermediate rate HCC areas, where the incidence rates are typically between 10/100,000 and 20/100,000, are principally located in central Europe (e.g., Italy, France, Switzerland, Greece). Regardless of the magnitude of the incidence rate, almost all areas report rates in males that are two- to three-fold higher than rates in females (Figure 1). Notable exceptions to this gender disparity are the relatively equal incidence rates reported by registries in Harare, Zimbabwe (African ancestry), Costa Rica, Cali, Colombia and South Karachi, Pakistan. The greatest gender disparity in incidence is reported by registries in central Europe, where the male:female ratio is greater than four. Examples of this male:female disparity occur in the registries of Varese Province, Italy (M:F ratio=4.4), Geneva, Switzerland (5.6) and Bas-Rhin, France (6.0).5 The reasons that males have higher rates of liver cancer than females are not completely understood, but may be partly explained by the sex-specific prevalence of risk factors. Males are more likely to be chronically infected with hepatitis B virus (HBV) and hepatitis C virus (HCV), consume alcohol, smoke cigarettes, and have increased iron stores. Males have also been reported to have higher levels of aflatoxin markers in their blood than do females.6 Whether androgenic hormones or increased genetic susceptibility also predispose males to the development of liver cancer is not clear.
Figure 1.
Age-adjusted incidence rates per 100,000 of liver cancer among males by region. Cancer Incidence in 5 Continents. Age adjusted to world standard.
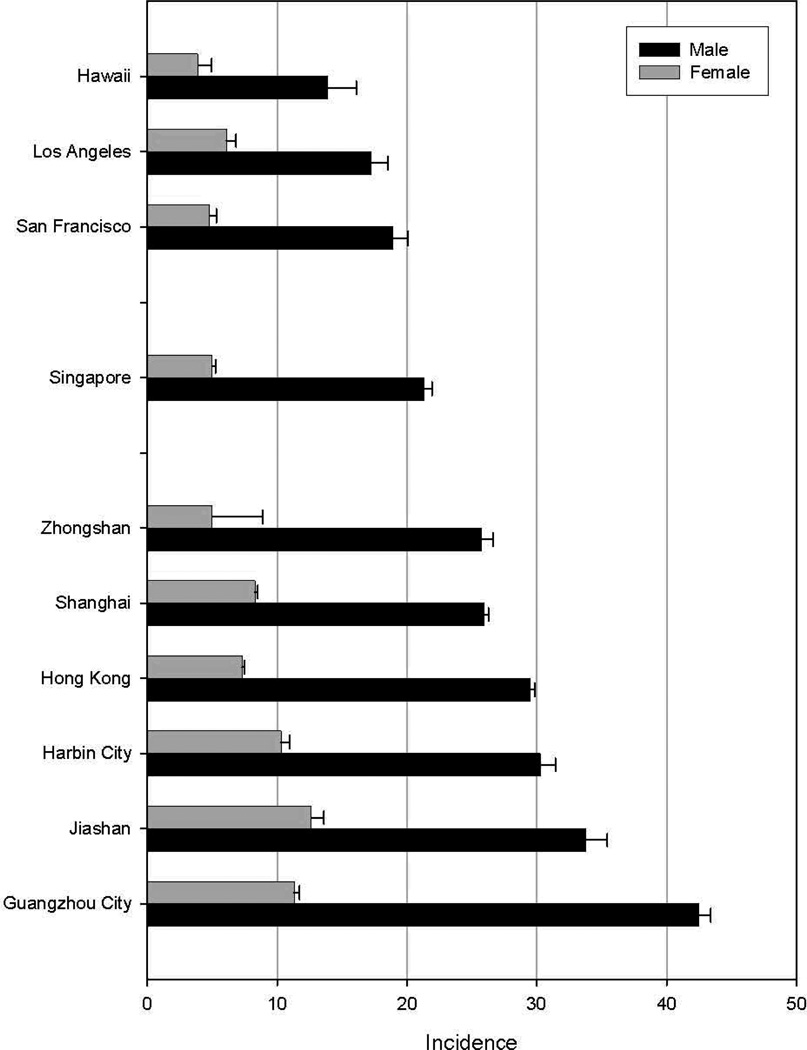
In addition to variability by gender, many areas report incidence rate disparities by race/ethnicity. In the United States, for example, HCC incidence is highest among Asians/Pacific Islanders (11.7/100,000) and lowest among white persons (3.9/100,000).7 Intermediate to these rates are those of Hispanics (8.0/100,000), black persons (7.0/100,000) and American Indians/Alaska Natives (6.6/100,000).7 Just as divergent as the rates among various ethnic groups residing in one area are the rates among members of a single ethnic group living in various locations. For example, incidence rates among Chinese populations are notably lower in the U.S. than they are in either China or in Singapore (Figure 2). As with gender differences, race/ethnic differences in risk are likely to be related to the prevalence of major risk factors in each groups.
Figure 2.
Age-adjusted incidence rates per 100,000 of liver cancer among selected Chinese populations. Cancer Incidence in 5 Continents. Age adjusted to world standard.
During the interval between 1983–1987 and 1998–2002, liver cancer incidence increased in many areas of the world. Increases were notable in Northern Europe, North and South America, Oceania, as well as in most countries of Southern Europe, India and Israel (Figure 3).5 In contrast, incidence rates declined in most far east Asian countries and in Spain. Although the reasons for the increase in incidence in some areas are not entirely clear, factors such as HCV infection, increasing rates of obesity and diabetes and improved survival from cirrhosis are likely to be related. The reasons for the declining rates in some far east Asian countries are likely to be several. In Japan, the cohort of individuals infected with HCV in the 1930s and 1940s is becoming ever smaller and thus the rate of HCV-related HCC is declining.8 The declining HCC rates in China and Singapore, areas where HBV is the major risk factor, are more likely due to the elimination of other HCC co-factors. Although HBV vaccination began in these areas in the mid-1980s, the vaccine was given to newborns. Thus, the vaccinated population is only in their mid-twenties at the present time and would have contributed very little to the current HCC incidence in the population.
Figure 3.
Global prevalence of chronic infection with hepatitis B virus, 2006. Centers for Disease Control: http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-5/hepatitis-c.aspx
The prognosis of liver cancer, even in developed countries, is very unfavorable. In the U.S., the one-year survival is less than 50%, and the five-year survival only 10%.7 Survival is even less favorable in developing countries. As a result, mortality rates in all locations are roughly equivalent to, or sometimes higher than, the incidence rates. Higher mortality rates than incidence rates can appear to exist as the liver is a preferred site of metastasis for many cancers, and it is not always easy to distinguish these secondary liver cancers from primary liver cancers.
Risk Factors
At least 80% of HCCs are associated with chronic infection with either of two viruses, hepatitis B virus (HBV) and hepatitis C virus (HCV). Of these, HBV infections account for 75 to 80 percent of virus-associated HCCs, with HCV responsible for 10 to 20 percent.9 Other risk factors include consumption of aflatoxin B1 (AFB1) contaminated foodstuffs, excessive consumption of alcohol, diabetes/obesity and certain rare metabolic disorders, such as hemochromatosis, α-1 antitrypsin deficiency, tyrosinemia and several porphyrias. Although persons with these rare metabolic disorders have elevated risks of HCC, the rarity of the disorders in the population results in the disorders not being major risk factors. The dominant risk factors tend to vary in high-risk and low-risk HCC regions. In most high-risk countries of Asian and Africa, chronic HBV infection and AFB1 exposure are the major risk factors. In contrast, HCV infection, excessive alcohol consumption, and diabetes/obesity play more important roles in low risk HCC areas. Exceptions to these patterns, however, are seen in Japan and Egypt where the dominant risk factor is HCV infection.
The global pattern of HCC incidence is related to the history of these major HCC risk factors and the length of time the factors have been present in human populations. Although fossils of hepadnaviruses in avian genomes date back at least 19 million years10, evidence suggests that HBV has been in human populations for about 6000 years based on the divergence of human HBV from other primate HBVs.11 In contrast, hepatitis C virus dates back less than 1000 years and only became widely dispersed around the world during the twentieth century.12 Alcohol consumption has been a common exposure among humans during all recorded history while high rates of obesity and diabetes are phenomena of the late twentieth century.
Hepatitis B Virus
Several pathologists proposed in the 1950’s that chronic virus infections of the liver could lead to liver cancer.13–15 They also noted the strong association between cirrhosis and liver cancer, as at least 80% of liver cancers occurred in cirrhotic livers. The cirrhosis-liver cancer link was suggested to be stronger in Asia and Africa, where 5% to 50% of men with cirrhosis developed liver cancer, while the percentage in the United States and Western Europe was 3% to 10%.15 In most high-risk HCC regions, HBV infection is associated with most cases of cirrhosis and at least 80% of the cases of HCC.
In 1994, the International Agency for Research on Cancer classified HBV, a member of the Hepadnavirus family, as carcinogenic to humans.16 Currently, about 5% of the world’s population (350 million people) is chronically infected with HBV. The evidence supporting the causal association of HBV with HCC is substantive. Areas of the world with high incidence and mortality rates for HCC have high prevalences of chronic HBV infection (Figure 3). The reverse is also true. Countries with prevalences of chronic HBV infection of greater than 2% have increased incidence and mortality rates of HCC.17–19 Case-control studies in all regions of the world have consistently shown that chronic HBV infection (seropositivity for hepatitis B surface antigen, HBsAg) is significantly more common among cases than controls. Odds ratios have ranged from 5:1 to 65:1.16 Prospective studies of persons chronically infected with HBV have consistently demonstrated high relative risks for HCC, ranging from 5 to 103.16, 20–24 In a seminal study of male government workers in Taiwan, the age adjusted annual incidence of HCC was 474 per 100,000 in HBsAg (+) men compared with 6 per 100,000 in HBsAg(−) men.20 Similarly, in an 8-year follow-up of a very high-risk cohort in China, the cumulative risks of HCC mortality was 8% in HBsAg(+) men and 0.5% in HBsAg(−). Among women, the cumulative risks were 2.0% in HBsAg(+) persons and 0.1% among HBsAg(−) persons.22
In areas of the world with high incidences of HCC and high prevalences of chronic HBV infection, approximately 70 percent of HBV infections are acquired in the perinatal period or in early childhood.19, 25–27 Thus, among HBV carriers in endemic areas, those born to HBsAg(+) mothers are likely to have been infected longest and are at higher risk of HCC than are HBV carriers with HBsAg(−) mothers.28–30 HBV DNA is integrated into the genome of liver tissues in almost all HCC cases from patients with HBsAg in their serum. Investigators have also detected HBV DNA sequences in 10% to 20% of HCC tumors from patients who were seronegative for HBsAg, but positive for antibodies to HBsAg or HB core antigen.31–33
Among individuals with chronic HBV infections, risks of HCC vary by several factors, the major one being HBV DNA levels (viral load).23, 34–35 Although there is no discrete cut-off level, having >105/ml viral copies confers a 2.5 to 3 fold greater risk of HCC over an 8–10 year follow-up period, than does having a lower viral load.
Genotypes have been defined as HBV genomes that differ from each other in whole genome sequencing by 8% or more. By those criteria, eight genotypes, A through H, have been identified; sub-genotypes, differing by 4–8% within genotypes have also been reported.36 Genotype distribution varies by geography, response to treatment and HCC risk. In multiple population based studies, genotype C has been associated with a higher risk of HCC than genotypes A2, Ba, Bj, and D. Genotype C is also associated with delayed clearance of hepatitis virus e antigen (HBeAg), a marker of infectivity.37 In studies that controlled for genotype, double mutations in the basal core promoter (BCP) of the HBV genome were independent predictors of increased risk of HCC. Mutations in the precore (PC) region of the viral genome have also been associated, although inconsistently, with increased risks of cirrhosis and HCC.38
The lifetime risk of HCC among HBV carriers is estimated to be 10% to 25%. The World Health Organization and the Centers for Disease Control and Prevention project that, annually, some 600,000 chronically infected people die from HCC and chronic liver disease and, eventually, 35 to 87 million of the 350 million prevalent global HBV carriers will die of HCC.39
Prevention of chronic infection with HBV via vaccination drastically reduces the risk of subsequent HCC, although the vaccine is ineffective in 5–10% of individuals. On the population level, it is anticipated that the widespread neonatal vaccination in many countries that started in the mid-1980s will result in notable decreases in the incidence of HBV-related HCC. In Taiwan, 20 years after the initiation of universal newborn vaccination, HBsAg seropositivity rates in persons younger than 20 years have fallen from 10–17% to 0.7–1.7%.40 Currently, 92% of all countries have integrated newborn hepatitis B vaccination into their routine vaccination programs and 70% are now delivering 3 immunization doses.39 Unfortunately, vaccination is not routine in all high-risk countries, particularly those in sub-Saharan Africa. In these areas, control of aflatoxin is critically important as there is a synergistic effect of aflatoxin consumption and HBV infection on risk of HCC.
Hepatitis C Virus (HCV)
The hepatitis C virus (HCV), an RNA virus of the Flaviviridae family, was identified in 1989.41 Reliable serologic tests for antibody to HCV (anti-HCV) became available in 1990, and in 1994 the International Agency for Research on Cancer (IARC) classified HCV as carcinogenic to humans.{IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 1994 #39 Unlike the hepatitis B virus, HCV has not been demonstrated to infect non-human hosts in the wild.
Phylogenetic analysis of HCV has identified at least six major genotypes (numbered from 1 to 6) and numerous subtypes (denoted by lowercase letters).42–43 Particular genotype-subtype combinations are more common in certain geographic areas and are associated with the mode of viral transmission. By location, genotype 1a is the most common type among HCV-infected persons in the U.S., while 1b is the most common in Japan and 4a is the most common in Egypt. By mode of transmission, in Europe, genotypes 1b and 2 are more common in older persons, while genotypes 1a and 3a are more common among injecting drug users.12 Coalescent theory studies of HCV genotypes have determined that genotypes 1a and 1b originated approximately 100 years ago, while genotypes 4 (found predominantly in Africa and the Middle East) and 6 (found predominantly in Southeast Asia) arose 350 and 700 years ago, respectively.44 Evidence indicates that HCV existed as a long-term, low-level, endemic virus prior to the 20th century, but spread worldwide starting around 1900 via a number of transmission routes including pooling of blood products, widespread blood transfusion and injection drug use.45 How HCV was maintained as an endemic infection prior to the twentieth century is not well understood at present.46
As seen on the map shown in Figure 4, the highest rates of chronic HCV infection in the world occur in northern Africa, particularly Egypt, where the rate has been estimated at 18%.47 In Asia, Mongolia reports rates (10%) considerably higher than those of Vietnam (6%), Cambodia (4%), China (3–4%) or Japan (2%).47 European rates of 0.5–2.5% are similar to the U.S. rate of 1.8%, but higher than the Canadian rate of 0.1–0.8%, which is one of the lowest in the world.
Figure 4.
Global prevalence of hepatitis C infection. Centers for Disease Control: http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-5/hepatitis-c.aspx
Using genetic evolutionary analysis of HCV in Japan, molecular clock studies have suggested that HCV first appeared in that country in the early 1880s and became more widely disseminated throughout the population in the 1930s and 1940s.48 The population dispersal times are consistent with the introduction of anti-schistosomal therapy using intravenous antimony sodium tartrate which began in the 1920s.48–49 HCV infection may have become more widespread during World War II due to the use intravenous stimulants48,the liberal use of blood transfusions to treat anemias50 and the use of blood from paid donors.51 The spread of HCV 1b in Japan, however, began to decline around 1995.52
Molecular clock studies have also examined HCV in Egypt, a country with very high rates of chronic HCV infection. As in Japan, evidence suggests that HCV was spread in the population by the use of intravenous anti-schistosomal therapy.53 Though the anti-schistosomal campaigns began in the 1920’s, they were particularly widespread between 1961 and 1986.54 In addition to spreading HCV, the campaigns were also likely to have spread HBV. The risk of an adult becoming a chronic HBV carrier after infection is fairly low (~10%), however, in comparison with the risk of an adult becoming a chronic HCV carrier after infection (~80%).
Molecular analysis of HCV genotype 1a in the U.S. suggests that the virus first entered the population around 1910 and became more widely disseminated in the 1960s.48 The introduction may have come as a result of U.S. soldiers becoming infected while abroad during the Spanish-American War.55 The reason for dissemination of HCV more widely in the 1960s is less clear, but the timing of the dissemination is consistent with the estimates derived from mathematical modeling56–57. Using NHANES III HCV prevalence data, it was estimated that HCV infection rates rapidly increased from the late 1960s to the early 1980s, then began to decline sharply in the early 1990s.56 Another modeling effort reached similar conclusions, noting an increase in HCV infection starting in the mid-1960s that hit a peak in the mid-1980s, before starting to decline.57 Implications for the future incidence of HCC in the U.S. are not entirely certain. Although several models suggest that HCC incidence could hit the very high levels seen earlier in Japan, other studies suggest that the long-term risk of HCC among HCV-infected Americans is low compared to HCV-infected Japanese.58 As HCV circulated in the US blood supply for fewer years than it did in Japan, and newer anti-viral agents are being developed to treat HCV infection, the long-term effect of HCV on HCC rates may be less dramatic in the US than in Japan.
Aflatoxin
Aflatoxin, a mycotoxin produced by molds of the Aspergillus species (Aspergillus flavus and Aspergillus parasiticus), contaminates maize, groundnuts and tree nuts in warm, humid environments and is a well established hepatic carcinogen.59 Aflatoxin exposure has likely been prevalent in human populations throughout history. There are four principal aflatoxins, B1, B2, G1, and G2, of which, aflatoxin B1 (AFB1) has been demonstrated to be the most potent in animal studies.59 Based largely on the indisputable animal data, IARC determined that there was sufficient evidence to classify aflatoxin as a group 1 human carcinogen.59
Many ecological studies of AFB1 contamination of food stores conducted in the 1970’s and 1980’s were compatible with a role for the carcinogen in human HCC. Person-specific epidemiological studies performed subsequently provided strong evidence that AFB1 was an etiologic factor or co-factor in the development of HCC. These studies were made possible by the development of assays for aflatoxin metabolites in urine, AFB1-albumin adducts in serum, and detection of a signature aflatoxin DNA mutation in tissues. The mutation occurs in a hotspot region of the p53 cancer suppressor gene at the third base of codon 249 (p53 249ser mutation). The G-to-T transversion, observed in 30 to 60% of tumors arising in persons living in aflatoxin rich environments60–62 is postulated to result from the reaction of the 8,9 epoxide activated form of AFB1 with the N-7 guanine in DNA.
The regions of the world with the highest levels of aflatoxin exposure are sub-Saharan Africa, Southeast Asia and China. Within these areas, higher levels are found among rural populations than among urban populations63, among males rather than females6, 64 and among persons chronically infected with HBV.6
The synergistic effect of AFB1 and chronic HBV infection on HCC risk was revealed in short-term prospective studies in Shanghai, China. Compared to persons without aflatoxin or HBV exposure, the risk of HCC was 4-fold greater among persons with elevated levels of aflatoxin metabolites in urine, 7-fold greater among persons chronically infected with HBV and 60-fold greater among individuals with both risk factors.65–66 More current evidence suggests that there is also a synergistic effect between AFB1 and HCV infection.67 AFB1 contamination, however, is more common in areas where HBV is the dominant virus. Using data on aflatoxin levels in food, consumption of aflatoxin-contaminated foods and prevalence of chronic infection with HBV, a recent risk assessment found that aflatoxin is associated with between 4.6% and 28.2% of HCCs worldwide.68
In general, in areas of the world where AFB1 exposure is high, chronic HBV infection is highly prevalent. As little can be done to alter the HBV chronic infection state, eradicating AFB1 from the food supply would be one way to bring down the HCC incidence rate.69 Unfortunately, simply avoiding AFB1 contaminated foods is not a practical solution in areas afflicted with chronic malnutrition.
Alcohol
In 1988, IARC concluded that there was a causal relationship between alcohol consumption and liver cancer.70 In 2007, the World Cancer Research Fund and American Institute for Cancer Research, in a review of diet and physical activity studies, concluded that alcohol consumption was probably a direct cause of liver cancer.71
Most studies in low-risk HCC populations have found alcohol to be a significant risk factor72–80, while the evidence from earlier studies in high-risk populations has been more equivocal.81–86 The disparity between low and high-risk regions may have been due to lower mean alcohol consumption in high-risk populations and/or difference in the interaction between alcohol with HBV and HCV and/or other risk factors. Evidence suggests, however, that both HBV and HCV, in conjunction with alcohol, have synergistic effects on HCC risk.87–89 In addition, the same studies find that alcohol consumption is significantly associated with HCC in the absence of viral infection (odds ratios between 2.4 and 7.0), though higher levels of alcohol consumption are likely required to increase risk in the absence of viral infection. Whether risk is increased with low or moderate levels of alcohol consumption in the absence of other factors is not well understood.
Whether alcohol is more strongly associated with HCC in women than in men has been difficult to study given that women are less likely to be heavy drinkers and less likely to develop HCC than men. A greater effect of alcohol on women has been hypothesized based on differences in alcohol dehydrogenase activity90 and evidence of a greater association between alcohol and cirrhosis among women.91–92 No substantial gender difference in risk of HCC with alcohol consumption, however, was reported by at least one study.87 Results of some studies have suggested that alcohol in combination with smoking, may be more risk-producing than alcohol alone93 and that women may be particularly affected by the combination.94 Recent evidence also suggests that there is a synergistic effect of alcohol consumption and obesity on HCC.95 While women are as likely as men to be obese96, they are less likely than men to either drink or smoke at high levels.
The mechanism by which alcohol increases HCC risk is not entirely clear. Animal and human studies provide little evidence that ethanol is a carcinogen.97 Some of the mechanisms by which alcohol might increase risk include the production of acetaldehyde and free radicals during alcohol metabolism, cytochrome P4502E1 induction, modulation of cell regeneration, promotion or exacerbation of nutritional deficiencies and alterations of the immune system.98 It is certain that alcohol induces cirrhosis and cirrhosis is a factor in 60–90% of HCCs. Whether alcohol is related to HCC independent of cirrhosis is less clear.
Worldwide, alcohol consumption is highest in European countries and lowest in Eastern Mediterranean countries.99 Between 1960 and 2000, however, per capita consumption declined in European, North American and African countries after reaching peak levels in the early 1980s. During the same interval, consumption levels increased in Southeast Asian and, even more notably, in Western Pacific countries. Eastern Mediterranean countries, during the same period, maintained very low levels of consumption levels. As excessive alcohol consumption has historically been a more important HCC risk factor in low-risk HCC areas such as Europe and North America, downward trends in consumption suggest a favorable effect on HCC rates in those areas. Increasing consumption in Southeast Asia and the Western Pacific countries, areas with already high rates of HCC, may be a future concern, however.
Obesity, Diabetes Mellitus and Nonalcoholic Steatohepatitis (NASH)
A significant relationship between diabetes and liver cancer was first reported in 1986.100 While several early epidemiology studies101–102 did not confirm the relationship, most later studies, with a few exceptions103–104, were confirmatory. The bulk of the literature, summarized in systematic reviews105–106 and meta-analyses107, now provides strong evidence from low, intermediate and high-risk countries, that HCC and diabetes are significantly associated.
Many of the studies in individuals with diabetes also noted a relationship between diabetes and cirrhosis.108–111 As insulin resistance is known to be associated with cirrhosis, it is possible that the diabetes-cirrhosis and diabetes-HCC relationships are a consequence of the cirrhotic process. Cohort studies, which have found increased risks of HCC among diabetics and persons with hyperinsulinemia, suggest however, that diabetes usually precedes the development of cirrhosis and HCC.112–114 In support of these observations are results of studies demonstrating that hepatic steatosis is common among persons with type II diabetes.115 Similarly, it has been suggested that the diabetes-HCC relationship is a result of HCV infection116 due to impaired glucose and insulin metabolism.117 HCV status has been determined in several studies that examined the diabetes-HCC relationship. While some of these studies reported that the diabetes’ effect was dependent on HCV infection103–104, others found that the diabetes’ effect was independent.118–119
Obesity is a major risk factor for the development of type II diabetes. In a recent analysis of data from the U.S. National Health and Nutrition Examination Survey (NHANES), it was reported that 80.3% of NHANES participants with diabetes were overweight (body mass index, BMI ≥25) and conversely, the prevalence of diabetes rose linearly with weight class from 8% of persons with normal BMI to 43% of persons with obese BMI.120 A number of studies have reported that obesity is related to liver cancer, as summarized in a recent review.121 In comparing normal weight persons with overweight and obese persons, a meta-analysis of 11 cohort studies found the risk of liver cancer was 1.17 (95%CI=1.0–1.3) in overweight persons and 1.87 (95%CI=1.5–2.4) in obese persons.122 Whether obesity is an independent risk factor, however, is not yet clear. At the present time, there are more studies from low-risk than high-risk populations and many studies have not adjusted their analyses for other known risk factors. One study that did examine the joint effects of obesity and alcohol consumption on risk of liver diseases, including cancer, however, found a synergistic effect on risk.95
With relative risks of approximately 2.5 for diabetes and approximately 1.5 for obesity, neither factor is associated with HCC as strongly as are HCV or HBV. It is worth noting, however the prevalence of diabetes and obesity in developed countries are far greater than HCV and HBV and the prevalence of diabetes in developing countries is growing much faster than it is in developed countries.123 It has been estimated that there are currently 285 million persons in the world, or 6.4% of the global population, with diabetes.123 Further, the prevalence is projected to increase by 69% in developing countries, and 20% in developed countries, by the year 2030. Similarly, increases in BMI have been documented in many countries since 1980.124 In the United States, the prevalence of obesity was fairly stable between 1960 and 1980.125 During the interval 1976–1980 to 1988–1994, however, the prevalence of obesity increased approximately 8%, then further increased during the interval 1988–1994 and 1999–2000.126 More encouraging results came from a comparison of rates between 1999–2000 and 2007–2008, however.96 During the most recent 10-year period, obesity prevalence did not increase among U.S. women. While prevalence did increase among men, the most recent data were flat, suggesting that the prevalence of obesity may not be continuing to increase at the same rate as previously.
In 1980, Ludwig et al. coined the term nonalcoholic steatohepatitis (NASH) to describe a condition among non-drinkers, characterized by morphologic evidence of fatty changes in the liver with lobular hepatitis.127 Though subsequent definitions have varied, Brunt et al.128 proposed that NASH be defined by the presence of steatosis, inflammation, hepatocellular degenerative changes and variable fibrosis. Now recognized as the most severe form of nonalcoholic fatty liver disease (NAFLD), NASH is estimated to be the third most common liver disorder in North America129, and the most common in Australia and New Zealand.130
While the majority of the patients described in the initial report of Ludwig et al.127 were female, subsequent reports have found that NASH occurs equally among males and females.131 Conditions frequently found in association with NASH include insulin resistance, impaired glucose tolerance, type II diabetes mellitus, hypertriglyceridemia, age greater than 45 years and obesity; particularly central obesity.130 In addition, elevated body iron stores have been reported to be common among NASH patients131–132 and may be related to mutations in the hemochromatosis gene.133–134 Evidence for a possible genetic component to risk has come from a family study that found an unexpectedly high occurrence of NASH-related conditions in relatives of NASH probands.135
Although some early reports suggested that NASH was a non-progressive disorder, it is now recognized that severe fibrosis occurs in 15%–50% of NASH patients and cirrhosis in 7%–25%.136 It has also been suggested that ‘burned out’ NASH is the cause of many cases of cryptogenic cirrhosis because many of the same co-morbid conditions are equally present in NASH and cryptogenic cirrhosis.137–139 The incidence of HCC is increased in most forms of cirrhosis140, and NASH is proving to be no exception.136, 141–145 A recent analysis of risk factors for HCC in the US between 2002 and 2008 reported that non-alcoholic fatty liver disease/NASH is already the most common risk factor (59%), followed by diabetes (36%).146
Summary
The global risk of HCC has been largely driven by HBV infection for the past century. Contributions to risk have also been made by other factors, including HCV, aflatoxin, excessive alcohol consumption and obesity/diabetes. The dominant effect of HBV on global HCC risk should decline in future generations as the population vaccinated against HBV advances in years. Infection with HCV should also decline as a major cause of HCC in future generations as HCV was removed from the blood supply of most countries in the early 1990s. Although projections of HCV-related HCC rates have suggested high rates for another 30 years, the projections may be overly pessimistic. Declining levels of alcohol consumption in many areas also suggest that alcohol may be less of a factor in HCC in coming years. Unfortunately, high global prevalence rates of obesity and diabetes may ensure that they become even much more important risk factors for HCC as the prevalence of other risk factors declines.
Acknowledgments
This work was supported by funding of the NCI Intramural Research Program.
Footnotes
The authors have nothing to disclose.
References
- 1.Ferlay J, Shin H, Bray F, D F, C M, Parkin DM GLOBOCAN. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] 2008 http://globocan.iarc.fr. [Google Scholar]
- 2.Zimmerman M, Aufderheide AC. Paleopath Newsletter. 2010;150:16–23. [Google Scholar]
- 3.Frerichs FT. A clinical treatise on diseases of the liver. New York: Wood; 1879. [Google Scholar]
- 4.Osler W, McCrae T. The principles and practice of medicine: designed for the use of practitioners and students of medicine. Eighth ed. New York: D. Appleton and Company; 1912. [Google Scholar]
- 5.Ferlay J, Parkin DM, Curado MP, et al. Cancer Incidence in Five Continents, Volumes I to IX: IARC CancerBase No. 9 [Internet] http://ci5.iarc.fr. [Google Scholar]
- 6.Sun CA, Wu DM, Wang LY, Chen CJ, You SL, Santella RM. Determinants of formation of aflatoxin-albumin adducts: a seven-township study in Taiwan. Br J Cancer. 2002;87:966–970. doi: 10.1038/sj.bjc.6600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka H, Imai Y, Hiramatsu N, et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med. 2008;148:820–826. doi: 10.7326/0003-4819-148-11-200806030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert C, Feschotte C. Genomic fossils calibrate the long-term evolution of hepadnaviruses. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fares MA, Holmes EC. A revised evolutionary history of hepatitis B virus (HBV) J Mol Evol. 2002;54:807–814. doi: 10.1007/s00239-001-0084-z. [DOI] [PubMed] [Google Scholar]
- 12.Simmonds P. Reconstructing the origins of human hepatitis viruses. Philos Trans R Soc Lond B Biol Sci. 2001;356:1013–1026. doi: 10.1098/rstb.2001.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Higginson J, Grobbelaar BG, Walker ARP. Hepatic fibrosis and cirrhosis in man in relation to malnutrition. Am J Pathol. 1957;33:29–44. [PMC free article] [PubMed] [Google Scholar]
- 15.Edmondson HA. Tumors of the liver and intrahepatic bile duct. Washington, D.C.: Armed Forces Institute of Pathology; 1958. [Google Scholar]
- 16.Hepatitis viruses. Lyon, France: International Agency for Research on Cancer; 1994. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer. [Google Scholar]
- 17.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 18.Schutte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis. 2009;27:80–92. doi: 10.1159/000218339. [DOI] [PubMed] [Google Scholar]
- 19.CDC. Hepatitis B. http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/hepatitis-b.aspx.
- 20.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 21.Heyward WL, Lanier AP, McMahon BJ, Fitzgerald MA, Kilkenny S, Paprocki TR. Early detection of primary hepatocellular carcinoma. Screening for primary hepatocellular carcinoma among persons infected with hepatitis B virus. JAMA. 1985;254:3052–3054. [PubMed] [Google Scholar]
- 22.Evans AA, Chen G, Ross EA, Shen FM, Lin WY, London WT. Eight-year follow-up of the 90,000-person Haimen City cohort: I. Hepatocellular carcinoma mortality, risk factors, and gender differences. Cancer Epidemiol Biomarkers Prev. 2002;11:369–376. [PubMed] [Google Scholar]
- 23.Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11:797–816. doi: 10.1016/j.cld.2007.08.005. viii. [DOI] [PubMed] [Google Scholar]
- 24.Ijima T, Saitoh N, Nobutomo K, Nambu M, Sakuma K. A prospective cohort study of hepatitis B surface antigen carriers in a working population. Gann. 1984;75:571–573. [PubMed] [Google Scholar]
- 25.Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771–774. doi: 10.1056/NEJM197504102921503. [DOI] [PubMed] [Google Scholar]
- 26.Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med. 1976;294:746–749. doi: 10.1056/NEJM197604012941402. [DOI] [PubMed] [Google Scholar]
- 27.Marinier E, Barrois V, Larouze B, et al. Lack of perinatal transmission of hepatitis B virus infection in Senegal, West Africa. Journal of Pediatrics. 1985;106:843–849. doi: 10.1016/s0022-3476(85)80371-1. [DOI] [PubMed] [Google Scholar]
- 28.Larouze B, Saimot G, Lustbader ED, London WT, Werner BG, Payet M. Host responses to hepatitis-B infection in patients with primary hepatic carcinoma and their familie. A case/control study in Senegal, West Africa. Lancet. 1976;2:534–538. doi: 10.1016/s0140-6736(76)91792-x. [DOI] [PubMed] [Google Scholar]
- 29.Hann HW, Kim CY, London WT, Whitford P, Blumberg BS. Hepatitis B virus and primary hepatocellular carcinoma: family studies in Korea. International Journal of Cancer. 1982;30:47–51. doi: 10.1002/ijc.2910300109. [DOI] [PubMed] [Google Scholar]
- 30.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Shafritz DA, Shouval D, Sherman HI, Hadziyannis SJ, Kew MC. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981;305:1067–1073. doi: 10.1056/NEJM198110293051807. [DOI] [PubMed] [Google Scholar]
- 32.Brechot C, Degos F, Lugassy C, et al. Hepatitis B virus DNA in patients with chronic liver disease and negative tests for hepatitis B surface antigen. N Engl J Med. 1985;312:270–276. doi: 10.1056/NEJM198501313120503. [DOI] [PubMed] [Google Scholar]
- 33.Ming L, Thorgeirsson SS, Gail MH, et al. Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology. 2002;36:1214–1220. doi: 10.1053/jhep.2002.36366. [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol. 2006;101:1797–1803. doi: 10.1111/j.1572-0241.2006.00647.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen CJ, Yang HI, Iloeje UH. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009;49:S72–S84. doi: 10.1002/hep.22884. [DOI] [PubMed] [Google Scholar]
- 36.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–S55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 37.McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis. 2010;14:381–396. doi: 10.1016/j.cld.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Sumi H, Yokosuka O, Seki N, et al. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37:19–26. doi: 10.1053/jhep.2003.50036. [DOI] [PubMed] [Google Scholar]
- 39.WHO. Hepatitis B. http://www.who.int/immunization/topics/hepatits_b/en/index.html. [Google Scholar]
- 40.Chang MH, You SL, Chen CJ, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 41.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 42.Simmonds P. Genetic diversity and evolution of hepatitis C virus--15 years on. J Gen Virol. 2004;85:3173–3188. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- 43.Simmonds P, Holmes EC, Cha TA, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74(Pt 11):2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 44.Pybus OG, Charleston MA, Gupta S, Rambaut A, Holmes EC, Harvey PH. The epidemic behavior of the hepatitis C virus. Science. 2001;292:2323–2325. doi: 10.1126/science.1058321. [DOI] [PubMed] [Google Scholar]
- 45.Pybus OG, Barnes E, Taggart R, et al. Genetic history of hepatitis C virus in East Asia. J Virol. 2009;83:1071–1082. doi: 10.1128/JVI.01501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pybus OG, Markov PV, Wu A, Tatem AJ. Investigating the endemic transmission of the hepatitis C virus. Int J Parasitol. 2007;37:839–849. doi: 10.1016/j.ijpara.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Bostan N, Mahmood T. An overview about hepatitis C: a devastating virus. Crit Rev Microbiol. 2010;36:91–133. doi: 10.3109/10408410903357455. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka Y, Hanada K, Mizokami M, et al. Inaugural Article: A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci U S A. 2002;99:15584–15589. doi: 10.1073/pnas.242608099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iida F, Iida R, Kamijo H, et al. Chronic Japanese schistosomiasis and hepatocellular carcinoma: ten years of follow-up in Yamanashi Prefecture, Japan. Bull World Health Organ. 1999;77:573–581. [PMC free article] [PubMed] [Google Scholar]
- 50.Primary liver cancer in Japan. The Liver Cancer Study Group of Japan. Cancer. 1984;54:1747–1755. doi: 10.1002/1097-0142(19841015)54:8<1747::aid-cncr2820540846>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 51.Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology. 2010;53:39–43. doi: 10.1159/000252782. [DOI] [PubMed] [Google Scholar]
- 52.Moriya T, Koyama T, Tanaka J, Mishiro S, Yoshizawa H. Epidemiology of hepatitis C virus in japan. Intervirology. 1999;42:153–158. doi: 10.1159/000024974. [DOI] [PubMed] [Google Scholar]
- 53.Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka Y, Agha S, Saudy N, et al. Exponential spread of hepatitis C virus genotype 4a in Egypt. J Mol Evol. 2004;58:191–195. doi: 10.1007/s00239-003-2541-3. [DOI] [PubMed] [Google Scholar]
- 55.Mizokami M, Tanaka Y. Tracing the evolution of hepatitis C virus in the United States, Japan, and Egypt by using the molecular clock. Clin Gastroenterol Hepatol. 2005;3:S82–S85. doi: 10.1016/s1542-3565(05)00705-6. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31:777–782. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 57.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156:761–773. doi: 10.1093/aje/kwf100. [DOI] [PubMed] [Google Scholar]
- 58.Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med. 2000;132:105–111. doi: 10.7326/0003-4819-132-2-200001180-00003. [DOI] [PubMed] [Google Scholar]
- 59.Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. Vol. 2010. Lyon, France: International Agency for Research on Cancer; 2002. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer. [PMC free article] [PubMed] [Google Scholar]
- 60.Ozturk M. p53 mutation in hepatocellular carcinoma after aflatoxin exposure. Lancet. 1991;338:1356–1359. doi: 10.1016/0140-6736(91)92236-u. [DOI] [PubMed] [Google Scholar]
- 61.Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- 62.Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- 63.Wild CP, Hall AJ. Primary prevention of hepatocellular carcinoma in developing countries. Mutat Res. 2000;462:381–393. doi: 10.1016/s1383-5742(00)00027-2. [DOI] [PubMed] [Google Scholar]
- 64.Plymoth A, Viviani S, Hainaut P. Control of hepatocellular carcinoma through hepatitis B vaccination in areas of high endemicity: perspectives for global liver cancer prevention. Cancer Lett. 2009;286:15–21. doi: 10.1016/j.canlet.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 65.Qian GS, Ross RK, Yu MC, et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3:3–10. [PubMed] [Google Scholar]
- 66.Ross RK, Yuan JM, Yu MC, et al. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 67.Kuang SY, Lekawanvijit S, Maneekarn N, et al. Hepatitis B 1762T/1764A mutations, hepatitis C infection, and codon 249 p53 mutations in hepatocellular carcinomas from Thailand. Cancer Epidemiol Biomarkers Prev. 2005;14:380–384. doi: 10.1158/1055-9965.EPI-04-0380. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118:818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alcohol Drinking. Lyon, France: International Agency for Research on Cancer; 1988. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer. [Google Scholar]
- 71.Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, D.C.: American Institute for Cancer Research; 2007. World Cancer Research Fund/American Institute for Cancer Research. [Google Scholar]
- 72.Aizawa Y, Shibamoto Y, Takagi I, Zeniya M, Toda G. Analysis of factors affecting the appearance of hepatocellular carcinoma in patients with chronic hepatitis C. A long term follow-up study after histologic diagnosis. Cancer. 2000;89:53–59. [PubMed] [Google Scholar]
- 73.Corrao G, Bagnardi V, Zambon A, Arico S. Exploring the dose-response relationship between alcohol consumption and the risk of several alcohol-related conditions: a meta-analysis. Addiction. 1999;94:1551–1573. doi: 10.1046/j.1360-0443.1999.9410155111.x. [DOI] [PubMed] [Google Scholar]
- 74.Kono S, Ikeda M, Tokudome S, Nishizumi M, Kuratsune M. Cigarette smoking, alcohol and cancer mortality: a cohort study of male Japanese physicians. Jpn J Cancer Res. 1987;78:1323–1328. [PubMed] [Google Scholar]
- 75.Hirayama T. A large-scale cohort study on risk factors for primary liver cancer, with special reference to the role of cigarette smoking. Cancer Chemother Pharmacol. 1989;23(Suppl):S114–S117. doi: 10.1007/BF00647254. [DOI] [PubMed] [Google Scholar]
- 76.Roudot-Thoraval F, Bastie A, Pawlotsky JM, Dhumeaux D. Epidemiological factors affecting the severity of hepatitis C virus-related liver disease: a French survey of 6,664 patients. The Study Group for the Prevalence and the Epidemiology of Hepatitis C Virus. Hepatology. 1997;26:485–490. doi: 10.1002/hep.510260233. [DOI] [PubMed] [Google Scholar]
- 77.Shibata A, Hirohata T, Toshima H, Tashiro H. The role of drinking and cigarette smoking in the excess deaths from liver cancer. Jpn J Cancer Res. 1986;77:287–295. [PubMed] [Google Scholar]
- 78.Tanaka K, Hirohata T, Takeshita S. Blood transfusion, alcohol consumption, and cigarette smoking in causation of hepatocellular carcinoma: a case-control study in Fukuoka, Japan. Jpn J Cancer Res. 1988;79:1075–1082. doi: 10.1111/j.1349-7006.1988.tb01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsukuma H, Hiyama T, Oshima A, et al. A case-control study of hepatocellular carcinoma in Osaka, Japan. Int J Cancer. 1990;45:231–236. doi: 10.1002/ijc.2910450205. [DOI] [PubMed] [Google Scholar]
- 80.Ikeda K, Saitoh S, Suzuki Y, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930–938. doi: 10.1016/s0168-8278(98)80339-5. [DOI] [PubMed] [Google Scholar]
- 81.Chen CJ, Liang KY, Chang AS, et al. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991;13:398–406. [PubMed] [Google Scholar]
- 82.Oshima A, Tsukuma H, Hiyama T, Fujimoto I, Yamano H, Tanaka M. Follow-up study of HBs Ag-positive blood donors with special reference to effect of drinking and smoking on development of liver cancer. Int J Cancer. 1984;34:775–779. doi: 10.1002/ijc.2910340607. [DOI] [PubMed] [Google Scholar]
- 83.Lam KC, Yu MC, Leung JW, Henderson BE. Hepatitis B virus and cigarette smoking: risk factors for hepatocellular carcinoma in Hong Kong. Cancer Res. 1982;42:5246–5248. [PubMed] [Google Scholar]
- 84.Lu SN, Lin TM, Chen CJ, et al. A case-control study of primary hepatocellular carcinoma in Taiwan. Cancer. 1988;62:2051–2055. doi: 10.1002/1097-0142(19881101)62:9<2051::aid-cncr2820620930>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 85.Goodman MT, Moriwaki H, Vaeth M, Akiba S, Hayabuchi H, Mabuchi K. Prospective cohort study of risk factors for primary liver cancer in Hiroshima and Nagasaki, Japan. Epidemiology. 1995;6:36–41. doi: 10.1097/00001648-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 86.Yu MW, Hsu FC, Sheen IS, et al. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol. 1997;145:1039–1047. doi: 10.1093/oxfordjournals.aje.a009060. [DOI] [PubMed] [Google Scholar]
- 87.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–331. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 88.Kuper H, Tzonou A, Kaklamani E, et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498–502. [PubMed] [Google Scholar]
- 89.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009–1017. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 90.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 91.Tuyns AJ, Pequignot G. Greater risk of ascitic cirrhosis in females in relation to alcohol consumption. Int J Epidemiol. 1984;13:53–57. doi: 10.1093/ije/13.1.53. [DOI] [PubMed] [Google Scholar]
- 92.Corrao G, Arico S, Zambon A, Torchio P, Di Orio F. Female sex and the risk of liver cirrhosis. Collaborative Groups for the Study of Liver Diseases in Italy. Scand J Gastroenterol. 1997;32:1174–1180. doi: 10.3109/00365529709002999. [DOI] [PubMed] [Google Scholar]
- 93.Mukaiya M, Nishi M, Miyake H, Hirata K. Chronic liver diseases for the risk of hepatocellular carcinoma: a case-control study in Japan. Etiologic association of alcohol consumption, cigarette smoking and the development of chronic liver diseases. Hepatogastroenterology. 1998;45:2328–2332. [PubMed] [Google Scholar]
- 94.Hassan MM, Spitz MR, Thomas MB, et al. Effect of different types of smoking and synergism with hepatitis C virus on risk of hepatocellular carcinoma in American men and women: case-control study. Int J Cancer. 2008;123:1883–1891. doi: 10.1002/ijc.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 97.McKillop IH, Schrum LW. Role of alcohol in liver carcinogenesis. Semin Liver Dis. 2009;29:222–232. doi: 10.1055/s-0029-1214377. [DOI] [PubMed] [Google Scholar]
- 98.Seitz HK, Poschl G, Simanowski UA. Alcohol and cancer. Recent Dev Alcohol. 1998;14:67–95. doi: 10.1007/0-306-47148-5_4. [DOI] [PubMed] [Google Scholar]
- 99.WHO. WHO Global Status Report on Alcohol. 2004 http://www.who.int/substance_abuse/publications/global_status_report_2004_overview.pdf. [Google Scholar]
- 100.Lawson DH, Gray JM, McKillop C, Clarke J, Lee FD, Patrick RS. Diabetes mellitus and primary hepatocellular carcinoma. Q J Med. 1986;61:945–955. [PubMed] [Google Scholar]
- 101.Kessler II. Cancer mortality among diabetics. J Natl Cancer Inst. 1970;44:673–686. [PubMed] [Google Scholar]
- 102.Ragozzino M, Melton LJ, 3rd, Chu CP, Palumbo PJ. Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. J Chronic Dis. 1982;35:13–19. doi: 10.1016/0021-9681(82)90025-x. [DOI] [PubMed] [Google Scholar]
- 103.El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462–2467. doi: 10.1111/j.1572-0241.2001.04054.x. [DOI] [PubMed] [Google Scholar]
- 104.Hadziyannis S, Tabor E, Kaklamani E, et al. A case-control study of hepatitis B and C virus infections in the etiology of hepatocellular carcinoma. Int J Cancer. 1995;60:627–631. doi: 10.1002/ijc.2910600510. [DOI] [PubMed] [Google Scholar]
- 105.Beasley RP. Diabetes and hepatocellular carcinoma. Hepatology. 2006;44:1408–1410. doi: 10.1002/hep.21430. [DOI] [PubMed] [Google Scholar]
- 106.Gao C, Yao SK. Diabetes mellitus: a "true" independent risk factor for hepatocellular carcinoma? Hepatobiliary Pancreat Dis Int. 2009;8:465–473. [PubMed] [Google Scholar]
- 107.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 108.Adami HO, McLaughlin J, Ekbom A, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control. 1991;2:307–314. doi: 10.1007/BF00051670. [DOI] [PubMed] [Google Scholar]
- 109.Koskinen SV, Reunanen AR, Martelin TP, Valkonen T. Mortality in a large population-based cohort of patients with drug-treated diabetes mellitus. Am J Public Health. 1998;88:765–770. doi: 10.2105/ajph.88.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weiderpass E, Gridley G, Nyren O, Pennello G, Landstrom AS, Ekbom A. Cause-specific mortality in a cohort of patients with diabetes mellitus: a population-based study in Sweden. J Clin Epidemiol. 2001;54:802–809. doi: 10.1016/s0895-4356(01)00342-0. [DOI] [PubMed] [Google Scholar]
- 111.Sasaki A, Uehara M, Horiuchi N, Hasegawa K, Shimizu T. A 15-year follow-up study of patients with non-insulin-dependent diabetes mellitus (NIDDM) in Osaka, Japan. Factors predictive of the prognosis of diabetic patients. Diabetes Res Clin Pract. 1997;36:41–47. doi: 10.1016/s0168-8227(97)00026-0. [DOI] [PubMed] [Google Scholar]
- 112.Adami HO, Chow WH, Nyren O, et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472–1477. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 113.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360–1365. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 114.Balkau B, Kahn HS, Courbon D, Eschwege E, Ducimetiere P. Hyperinsulinemia predicts fatal liver cancer but is inversely associated with fatal cancer at some other sites: the Paris Prospective Study. Diabetes Care. 2001;24:843–849. doi: 10.2337/diacare.24.5.843. [DOI] [PubMed] [Google Scholar]
- 115.Clark JM, Diehl AM. Hepatic steatosis and type 2 diabetes mellitus. Curr Diab Rep. 2002;2:210–215. doi: 10.1007/s11892-002-0085-3. [DOI] [PubMed] [Google Scholar]
- 116.Mason AL, Lau JY, Hoang N, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 117.Petrides AS, DeFronzo RA. Glucose and insulin metabolism in cirrhosis. J Hepatol. 1989;8:107–114. doi: 10.1016/0168-8278(89)90169-4. [DOI] [PubMed] [Google Scholar]
- 118.Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 119.Lagiou P, Kuper H, Stuver SO, Tzonou A, Trichopoulos D, Adami HO. Role of diabetes mellitus in the etiology of hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:1096–1099. doi: 10.1093/jnci/92.13.1096. [DOI] [PubMed] [Google Scholar]
- 120.Nguyen NT, Nguyen XM, Lane J, Wang P. Relationship Between Obesity and Diabetes in a US Adult Population: Findings from the National Health and Nutrition Examination Survey, 1999–2006. Obes Surg. 2011;21:351–355. doi: 10.1007/s11695-010-0335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saunders D, Seidel D, Allison M, Lyratzopoulos G. Systematic review: the association between obesity and hepatocellular carcinoma - epidemiological evidence. Aliment Pharmacol Ther. 2010;31:1051–1063. doi: 10.1111/j.1365-2036.2010.04271.x. [DOI] [PubMed] [Google Scholar]
- 122.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 124.James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 125.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 126.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 127.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 128.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 129.Byron D, Minuk GY. Clinical hepatology: profile of an urban, hospital-based practice. Hepatology. 1996;24:813–815. doi: 10.1002/hep.510240410. [DOI] [PubMed] [Google Scholar]
- 130.Farrell GC. Non-alcoholic steatohepatitis: what is it, why is it important in the Asia-Pacific region? J Gastroenterol Hepatol. 2003;18:124–138. doi: 10.1046/j.1440-1746.2003.02989.x. [DOI] [PubMed] [Google Scholar]
- 131.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 132.Ludwig J, McGill DB, Lindor KD. Review: nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 1997;12:398–403. [Google Scholar]
- 133.Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology. 1998;27:1661–1669. doi: 10.1002/hep.510270627. [DOI] [PubMed] [Google Scholar]
- 134.George DK, Goldwurm S, MacDonald GA, et al. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311–318. doi: 10.1016/s0016-5085(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 135.Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am J Med. 2000;108:9–13. doi: 10.1016/s0002-9343(99)00315-0. [DOI] [PubMed] [Google Scholar]
- 136.Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 137.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 138.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689–692. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 139.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 140.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 141.Cotrim HP, Parana R, Braga E, Lyra L. Nonalcoholic steatohepatitis and hepatocellular carcinoma: natural history? Am J Gastroenterol. 2000;95:3018–3019. doi: 10.1111/j.1572-0241.2000.03241.x. [DOI] [PubMed] [Google Scholar]
- 142.Shimada M, Hashimoto E, Taniai M, et al. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002;37:154–160. doi: 10.1016/s0168-8278(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 143.Zen Y, Katayanagi K, Tsuneyama K, Harada K, Araki I, Nakanuma Y. Hepatocellular carcinoma arising in non-alcoholic steatohepatitis. Pathol Int. 2001;51:127–131. doi: 10.1046/j.1440-1827.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 144.Sorensen HT, Mellemkjaer L, Jepsen P, et al. Risk of cancer in patients hospitalized with fatty liver: a Danish cohort study. J Clin Gastroenterol. 2003;36:356–359. doi: 10.1097/00004836-200304000-00015. [DOI] [PubMed] [Google Scholar]
- 145.Ertle J, Dechene A, Sowa JP, et al. Nonalcoholic fatty liver disease progresses to HCC in the absence of apparent cirrhosis. Int J Cancer. 2010 doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 146.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–2191. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]