Abstract
Exposure to early life stress dramatically impacts adult behavior, physiology, and neuroendocrine function. Using rats bred for novelty-seeking differences and known to display divergent anxiety, depression, and stress vulnerability, we examined the interaction between early life adversity and genetic predisposition for high- versus low-emotional reactivity. Thus, bred Low Novelty Responder (bLR) rats, which naturally exhibit high anxiety- and depression-like behavior, and bred High Novelty Responder (bHR) rats, which show low anxiety/depression together with elevated aggression, impulsivity, and addictive behavior, were subjected to daily 3 h maternal separation (MS) stress postnatal days 1–14. We hypothesized that MS stress would differentially impact adult bHR/bLR behavior, physiology (stress-induced defecation), and neuroendocrine reactivity. While MS stress did not impact bHR and bLR anxiety-like behavior in the open field test and elevated plus maze, it exacerbated bLRs’ already high physiological response to stress – stress-induced defecation. In both tests, MS bLR adult offspring showed exaggerated stress-induced defecation compared to bLR controls while bHR offspring were unaffected. MS also selectively impacted bLRs’ (but not bHRs’) neuroendocrine stress reactivity, producing an exaggerated corticosterone acute stress response in MS bLR versus control bLR rats. These findings highlight how genetic predisposition shapes individuals’ response to early life stress. Future work will explore neural mechanisms underlying the distinct behavioral and neuroendocrine consequences of MS in bHR/bLR animals.
Keywords: bred High Responder (bHR), bred Low Responder (bLR), stress-induced defecation, maternal separation stress, corticosterone
1. INTRODUCTION
Evidence in humans and animals shows that early life adversity negatively impacts neurodevelopment and produces lifelong changes in stress reactivity, emotional behavior, and vulnerability to psychopathology (Ladd et al., 2000, Heim et al., 2004). The deleterious effects of early life stress are particularly pronounced in individuals genetically predisposed to psychiatric illness (Caspi et al., 2003, Caspi et al., 2002), although there is much to learn about how gene-environment interactions shape brain development, emotional behavior, and vulnerability to disease.
Animal models offer useful tools to examine how early life adversity impacts brain and behavior. One popular neonatal stress paradigm is the rodent maternal separation (MS) stress model, where pups are deprived of maternal contact for 3-h periods during the first weeks of life (Lehmann et al., 2000, Sanchez et al., 2001). MS elicits myriad negative effects, including increased anxiety (Roth et al., 2009, Huot et al., 2001), depression-like behavior (Franklin et al., 2010, Aisa et al., 2009, Ladd et al., 2000), and exaggerated hypothalamic-pituitary adrenal (HPA) axis stress responses (Holmes et al., 2005, Plotsky and Meaney, 1993, Plotsky et al., 1998, Ladd et al., 2000). The MS literature is somewhat inconsistent, with certain studies unable to confirm such behavioral and neuroendocrine findings; however, the conflicting results likely stem from a host of factors, including varied experimental procedures, gender, and rat strain (Lehmann and Feldon, 2000, Hulshof et al., 2011, Sterley et al., 2011). Importantly, rodents, like humans, exhibit a range of early life stress vulnerability, with some individuals particularly susceptible to its effects and others resilient (Lehmann and Feldon, 2000).
In recent years, our laboratory developed bred lines of Sprague-Dawley rats that display differences in novelty exploration and a host of emotional behaviors (Stead et al., 2006). Rats bred for high response to novelty (bred High Responders, bHRs) exhibit increased aggression (Kerman et al., 2011), impulsivity (Flagel et al., 2010), and proclivity to addictive behavior (Cummings et al., 2011, Davis et al., 2008, Flagel et al., 2010) compared to low novelty reactive rats (bred Low Responders, bLRs), which show high levels of anxiety- and depressive-like behavior (Stedenfeld et al., 2011, Stead et al., 2006, Perez et al., 2009, Clinton et al., 2011b, Clinton et al., 2008). The distinct bHR/bLR phenotypes are likely driven by several factors, including distinct ontogeny of hippocampal circuits (Clinton et al., 2011b), HPA axis reactivity (Clinton et al., 2008, Kerman et al., 2012), and the Fibroblast Growth Factor (FGF) system (Perez et al., 2009, Turner et al., 2011). The bHR/bLR phenotyopes are highly heritable (Stead et al., 2006), although some traits are influenced by environmental factors such as maternal style (Clinton et al., 2007, Kristiansen et al., 2007) and chronic stress (Clinton et al., 2008, Stedenfeld et al., 2011).
Considering bLRs’ anxious/depressive-like phenotype coupled with their chronic stress vulnerability, we hypothesized that bLRs would be susceptible to the effects of MS stress, while bHRs would be resilient. Thus, the present study subjected bHR/bLR pups to daily 3 h MS (or raised under normal conditions) during the first two postnatal weeks. Anxiety- and depression-like behavior and neuroendocrine stress responsivity were assessed in adult offspring.
2. MATERIALS & METHODS
2.1 Animals
Animals were acquired from the University of Michigan in-house bHR/bLR colony. We previously published our breeding strategy and initial behavioral characterization of the bHR/bLR lines (Stead et al., 2006), and continue to examine the bHR-bLR behavioral phenotypes (Flagel et al., 2010, Kerman et al., 2011, Stedenfeld et al., 2011). Animals for the present experiments were taken from the 10th generation of the colony.
Males to be used for mating as well as male offspring used for later experiments were kept on a 12:12 light-dark cycle (lights on at 6 a.m.) with food and water available ad libitum. Female rats (as well as male-female mating pairs) were housed in a separate room kept on a 14:10 light-dark cycle to promote regular estrous cycles and fertility (Everett and Sawyer, 1949, Ying et al., 1973). The animal housing rooms and testing facilities are kept at 21–23 °C at 50–55% humidity. Litters were weaned on postnatal day 21 (P21). Only weaned male offspring were housed 3–4 per cage and kept for subsequent experiments. Procedures were conducted in accordance with the principles and procedures outlined in the National Institutes of Health guidelines for the care and use of animals and approved by the University of Michigan University Committee on the Use and Care of Animals.
2.2 Separation Procedure
Male and female rats (N=10 bHR male/female pairs, and N=10 bLR male/female pairs) were housed together for 14 days; male partners were then removed and females remained singly housed until giving birth. All litters were born within 4 days of one another, and all pups within a litter were treated in similarly (that is, all pups within a ‘maternal separation’ experimental litter were removed from the mother daily 3-h per day from P1–14 and all pups from a control litter remained with their mother P1–14). At birth (P0), bHR/bLR litters were culled to 12 pups (6 males/6 females). Litters remained with their biological mothers and were randomly assigned to experimental groups: (a) MS stress or (b) non-separated control (n=5 litters per bHR/bLR phenotype per stress treatment group). The following day, we initiated a MS stress procedure modeled after Plotsky and Meaney (Plotsky and Meaney, 1993) and Card et al (Card et al., 2005). Non-separated control litters remained with their biological mothers continuously except for once weekly cage changes. Beginning on P1 (and continuing through P14), MS litters were separated from their dam for 3 h. An entire litter was moved with a handful of home bedding to a small box on a heating pad. The temperature of the nest was around 37 °C when the litter was transferred to the cage over the heating pad. The separation cages remained in the same room approximately 1.2 m from the home cage, where the dam remained. Littermates remained in close contact with one another throughout the MS period; at the conclusion of the MS period, pups and bedding were returned to the home cage. Pups were weaned on P21 and only males were chosen for subsequent tests performed in adulthood (75+ days). Each experimental group (n=12/phenotype/treatment) consisted of no more than 3 littermates.
2.3 Behavioral Analyses MS and control bHR/bLR Offspring
When MS and control bHR/bLR male offspring reached adulthood (P75), they were subjected to a test battery to assess novelty-induced locomotion, anxiety- and depression-like behaviors (n=12/phenotype/treatment as noted above). The order of the behavioral tests was as follows: 1) the locomotor response to novelty test; 2) the Open Field Test; 3) the Light-Dark Box Test; 4) the Elevated Plus Maze Test; and 5) the Forced Swim Test. Rats were given 2–3 days rest between each test to minimize the carry-over effect on behavior from test to test. Prior experiments with the Selectively-Bred bLR/bHR animals used a similar strategy, assessing bLR and bHR rats in multiple tests over a period of days to weeks. These prior studies demonstrated that bLR/bHR rats (a) exhibit reliable behavioral differences across several behavior tests and (b) behave consistently on a particular test (such as the Light-Dark Box) whether they are exposed only to that single test, or subjected to it after completing a series of other behavior tests over time (Supplementary Figure 1). It took approximately 10 days to complete this test battery, so rats were P85 on the final test day.
2.4 Locomotor Response to Novelty
Rats were screened to assess novelty-induced locomotion as previously described (Stead et al., 2006). Rats were individually placed in standard clear acrylic cages (43 × 21.5 × 25.5 cm high) equipped with infrared photocell emitters mounted 2.3 and 6.5 cm above the floor to record horizontal and rearing movement, respectively. Test chambers were located in a room separate from housing quarters, and the rats were exposed to the test room for the first time on the test day. A computer monitored horizontal and rearing movements in 5-min intervals over 60-min. Testing was performed between 8 a.m. and 11 a.m. Total locomotor scores for each rat were calculated by adding the number of horizontal and rearing movements over the 60-min test period.
2.5 Anxiety Behavior
Rats’ anxiety-like behavior was assessed using three classic rodent behavioral tests: the Open Field (OF) test, Light-Dark Box (LDB) test, and Elevated Plus Maze (EPM) test. Each test assessed novelty-induced locomotor activity, time spent in anxiogenic portions of the test apparatus (center of OF; light compartment in the LDB test, and open arms of the EPM), and latency to initially enter anxiogenic regions of the test apparatus. All testing was performed between 8:00–11:30 a.m.
Open field test
The Open field apparatus was a 100 × 100 × 50 cm white Plexiglas box with black Plexiglas floor, and testing was conducted under dim light (30 lux). Behavior was recorded using a computerized videotracking system (Noldus Ethovision, Leesburg, VA). The experiment began by placing the rat into a corner of the open field. The tracking system recorded the latency to first enter the center of the open field, the amount of time spent in the center, periphery, or corner of the apparatus, and the total distance traveled during the 5-min test.
Light-Dark Box test
The test apparatus was a 30 × 60 × 30 cm Plexiglas shuttle-box divided into two equal-sized compartments by a wall with a 12-cm-wide open door. One compartment was white and brightly illuminated (100 lux), and the other compartment was black and dimly lit (5 lux). The experiment began by placing the rat into the dark compartment and the door between the two compartments was removed. Rows of photocells located 2.5 cm above the stainless steel grid floor monitored beam breaks (indicating locomotor activity) and time spent in each compartment. A microprocessor recorded the latency to first exit the dark compartment, the number of photocell beam breaks and time spent in each compartment during the 5-min test.
Elevated Plus Maze test
The apparatus was constructed of black Plexiglas, with four elevated arms (70 cm from the floor, 45 cm long, and 12 cm wide) arranged in a cross. Two opposite arms were enclosed by 45-cm-high walls (lighting approximately 3–5 lux) and the other two arms were open (lighting approximately 30 lux). A central square platform at the intersection of the open and closed arms provided access to all arms. The test room was dimly lit (approximately 30 lux), and behavior was monitored using a computerized videotracking system (Noldus Ethovision, Leesburg, VA). At the beginning of the 5-min test, each rat was placed in the central square facing a closed arm. The tracking system recorded latency to first enter the open arm, the amount of time spent in the open arms, closed arms, or center square, and the total distance traveled over the course of the 5-min test.
2.6 Depression-like Behavior
Rats’ depression-like behavior was assessed with Porsolt’s Forced Swim Test (FST) using the procedure described by Cryan et al (Cryan et al., 2005). The water chambers were Plexiglas cylinders (40cm high×40 cm diameter) containing 30 cm deep water (25°C). On FST day 1, rats were placed (one rat/cylinder) in the water for 15-min (pretest phase); 24 h later the rats returned to the water-filled cylinders and videotaped for 5-min (test phase). Water was changed after every swim session so that every rat was swimming in clean water. Each rat’s FST day 2 behavior was scored with a computerized program (Noldus Observer 5.0, Leesburg, VA), to assess the duration and frequency of events from the videotape. The person scoring videotapes was blind to experimental treatments and trained to observe: swimming, climbing, and immobility/floating during the test phase. Data are presented as percent time spent immobile, climbing or swimming during the 5-min Day 2 test.
2.7 Neuroendocrine Studies
To assess impact of MS on bHR/bLR adult offsprings’ HPA axis, we measured adrenocorticotrophic hormone (ACTH) and corticosterone (CORT) secretion following the LDB anxiety test. Baseline blood samples (100 μl) were collected in EDTA-coated tubes via tail vein nick one day before LDB testing between 8:00–10:00 a.m. Animals were removed from their home cage, immobilized lightly, and a lateral tail vein was punctured with the corner of a razor blade to collect blood. Rats were then returned to their home cages. The next day, blood samples were similarly collected 5-, 20-, and 60-min after the beginning of the LDB test. Blood samples were separated by centrifugation (1000 × g, 10 min at 4 °C), and plasma was removed, frozen and stored at −80 °C until assay. Plasma ACTH and CORT were measured using commercially available radioimmunoassay kits (MP Biomedicals, Solon, OH) according to package instructions. The sensitivity of the CORT assay was 12.5 ng/ml and sensitivity of the ACTH assay was 15 pg/ml; intra- and inter-assay coefficients of variation were less than 5%.
2.8 Statistical analysis
Behavioral data were analyzed with 2-way ANOVA (bHR/bLR phenotype × MS condition as independent factors). Radioimmunoassay data were analyzed by 3-way ANOVA (bHR/bLR phenotype × MS group x blood collection timepoint as independent factors). ANOVAs were followed by Fisher’s post-hoc comparisons when necessary. Data were analyzed using SPSS for Windows, and for all tests α =0.05.
3. RESULTS
3.1 Early life MS stress does not impact novelty-induced locomotion in bHR/bLR rats
MS did not affect bHR and bLR offsprings’ adult locomotor response to novelty. Control bHRs (1083± SEM 44 activity counts) and MS bHRs (1263± SEM 42 activity counts) were dramatically more active in the novel environment compared to both bLR controls (185± SEM 16 activity counts) and bLR MS rats (252± SEM 13 counts; main effect of phenotype [F(1,121)=903.36, p<0.0001]). There was no effect of MS, and no MS x phenotype interaction. We observed the same effect in the other behavioral tests, with bHRs consistently being more active than bLRs in the OF test (main effect of phenotype [F(1,153)=132.42, p<0.0001]), and LDB (main effect of phenotype [F(1,90)=113.07, p<0.0001]; data not shown).
3.2 Early life MS stress does not impact typical bHR/bLR anxiety behavior differences
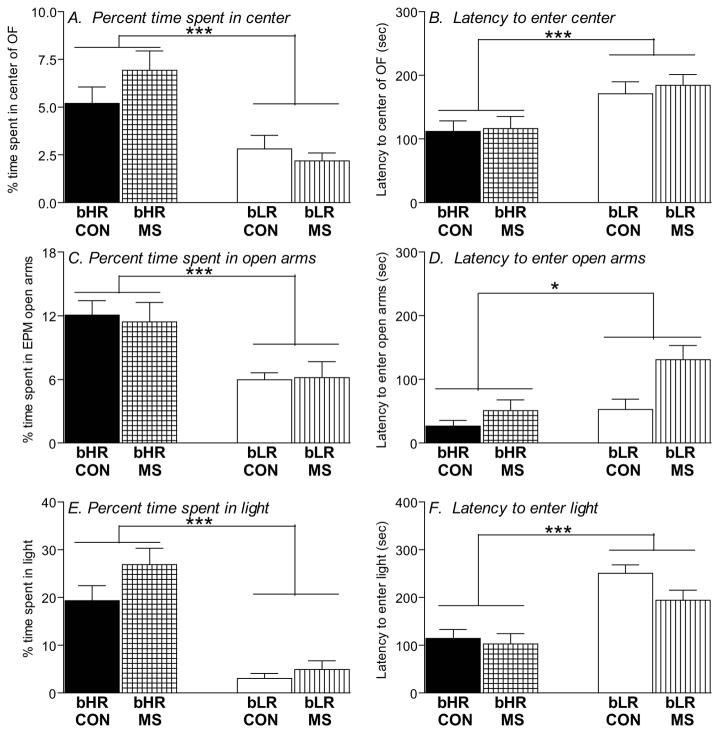
MS and control bLRs showed typically high levels of anxiety-like behavior across all three behavior tests – the OF test, the EPM and LDB. In the OF test, bLRs spent less time in the center of the OF (main effect of phenotype [F(1,153)=23.24, p<0.0001]; Fig. 1A), and showed greater latency to enter the center (main effect of phenotype [F(1,153)=12.12, p<0.001]; Fig. 1B) compared to bHRs. In the EPM, bLRs spent less time in the open arms (main effect of phenotype [F(1,91)=309.37, p<0.001]; Fig. 1C) and showed greater latency to enter the open arms (main effect of phenotype [F(1,91)=6.32, p<0.05]; Fig. 1D) relative to bHRs. Likewise, in the LDB test, bLRs spent less time in the light box (main effect of phenotype [F(1,90)=61.66, p<0.0001]; Fig 1E) and longer latency to enter the light (main effect of phenotype [F(1,90)=36.14, p<0.0001]; Fig. 1F) compared to bHRs. A history of MS stress did not impact any of these measures (time spent in the anxiogenic portion of the test apparatus or the latency to enter that region) in either phenotype (no main effects of MS stress and no MS x phenotype interactions).
Figure 1. Impact of MS stress on adult offspring’s anxiety-like behavior.
Adult bHR and bLR offspring that were maternally separated (MS) or remained undisturbed (CON) in early life were tested in the Open Field (OF), the Elevated Plus Maze (EPM), and the Light Dark Box (LDB). bLRs consistently showed greater anxiety compared to bHRs, spending less time in the center of the OF (A), less time in the open arms of the EPM (C), and less time in the light compartment in the LDB (E). bLRs also showed greater latency to initially enter these anxiogenic regions compared to bHRs (B, D, F). A history of MS had no impact on any of these measures. Groups were compared using a 2-way ANOVA and Fisher’s post hoc comparisons where necessary; * indicates p<0.05; ***indicates p<0.0001
3.3 MS stress leads bLRs to exhibit exaggerated stress-induced defecation in adulthood
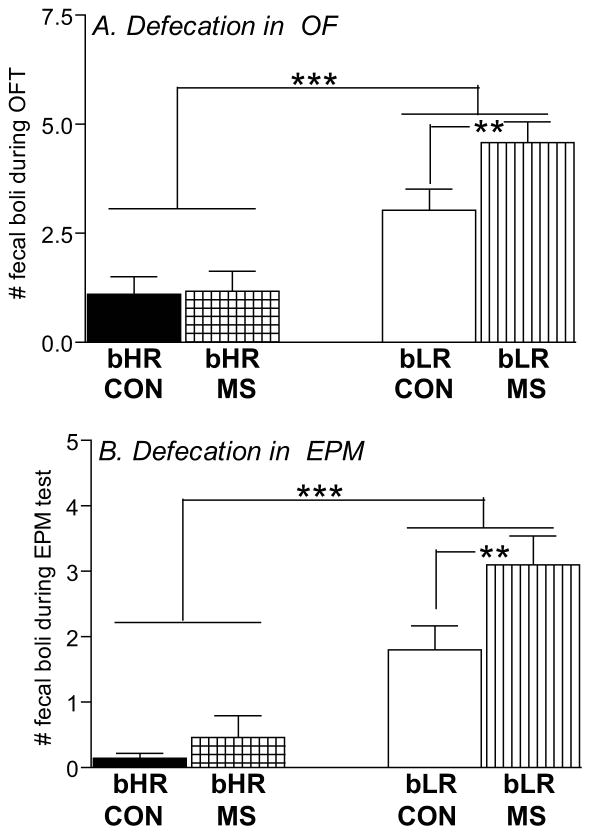
As an additional index of anxiety-like behavior, we also counted the number of fecal boli following each OF and EPM test. Interestingly, this analysis revealed marked differences between bHR/bLR rats as well as an effect of MS stress (Fig. 2). bLR rats generally produced more fecal boli than bHRs in both the OF test (main effect of phenotype [F(1,116)=242.81, p<0.0001]; Fig. 2A) and the EPM (main effect of phenotype [F(1,116)=41.88, p<0.0001]; Fig. 2B). A history of MS stress also increased defecation in the OF and EPM (main effect of MS in the OF ([F(1,116)=4.59, p<0.05]) and a stress x phenotype interaction in the OF ([F(1,116)=4.26, p<0.05]) and EPM ([F(1,116)=5.46, p<0.05]). Post hoc analysis showed that MS specifically impacted bLRs, leading MS-bLR adults to exhibit greater stress-induced defecation compared to control bLRs in the OF and EPM. MS and control bHR rats showed similarly low levels of stress-induced defecation in these tests (Fig. 2).
Figure 2. Impact of MS on adult offspring’s stress-induced defecation.
Anxiety was also assessed via the amount of stress-induced defecation in bHR/bLR maternally separated (MS) and undisturbed (CON) offspring following the Open Field (OF; A); and the Elevated Plus Maze (EPM; B) tests. In general, bLRs exhibited far greater stress-induced defecation compared to bHRs in both tests. Interestingly, this difference was further magnified by a history of MS, as MS-bLR animals showed greater defecation in both tests compared to control bLRs. Groups were compared using a 2-way ANOVA and Fisher’s post hoc comparisons where necessary; ** indicates p<0.01; *** <0.0001
3.4 Early life MS does not impact typical bHR/bLR depression-like behavior differences in the Forced Swim Test
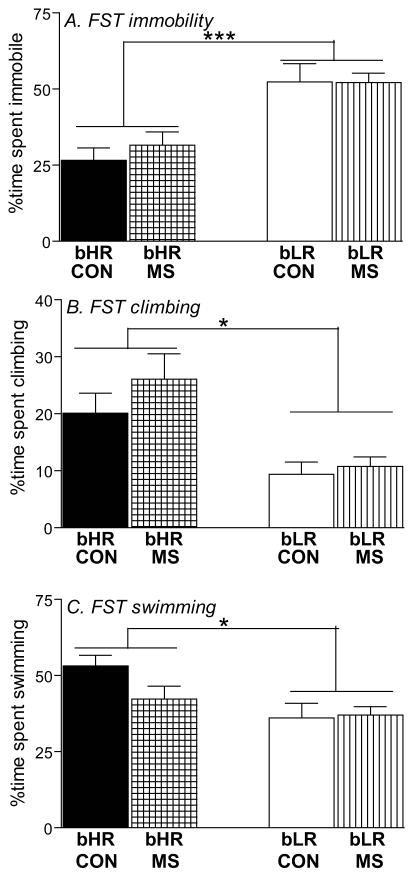
We used Porsolt’s FST to assess depression-like behavior in bHR/bLR MS and control offspring. Both MS and control bLRs showed a high level of immobility (main effect of phenotype [F(1,119)=28.38, p<0.0001]; Fig. 3A), and less climbing (main effect of phenotype [F(1,119)=18.35, p<0.0001]; Fig. 3B) and swimming (main effect of phenotype [F(1,119)=8.69, p<0.01]; Fig. 3C) compared to bHRs. There were no effects of MS on any measure, and no stress x phenotype interactions.
Figure 3. Impact of MS stress on adult offspring’s depression-like behavior.
Adult bHR and bLR offspring that were maternally separated (MS) or remained undisturbed (CON) in early life were subjected to the Forced Swim Test (FST) to assess depression-like behavior. bLRs exhibited greater depression-like behavior in the FST compared to bHRs, showing greater immobility (A), and less climbing (B) and swimming (C) during the 5-min test. A history of MS had no impact on any of these measures. Groups were compared using a 2-way ANOVA and Fisher’s post hoc comparisons where necessary; * indicates p<0.05; ***indicates p<0.0001
3.5 MS stress produces exaggerated neuroendocrine reactivity in bLR offspring
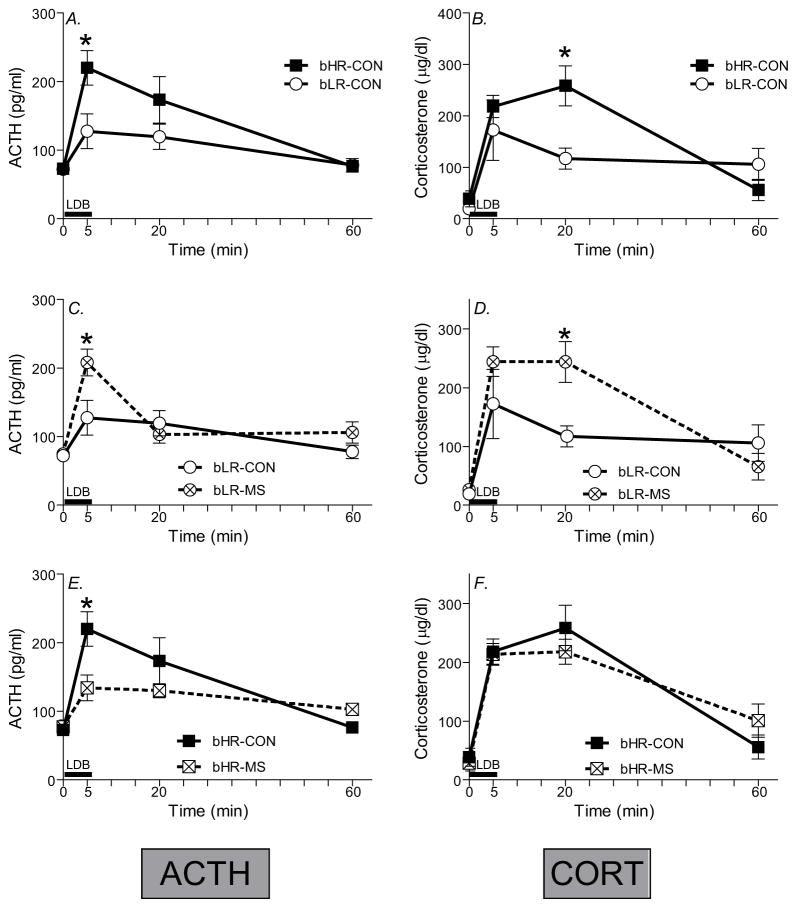
We examined the effect of MS on HPA axis reactivity in bHR/bLR offspring by measuring ACTH and CORT secretion following the LDB test. Across all groups, ACTH and CORT levels sharply increased following the LDB, and then gradually decreased back to near-baseline by 60-min post-test (main effect of time for ACTH [F(1,108)=24.20, p<0.0001] and CORT [F(1,108)=43.22, p<0.0001]; Fig. 4). For ACTH levels, there were no main effects of bHR/bLR phenotype or MS exposure, but there was a timepoint x phenotype x MS exposure interaction [F(1,108)=5.19, p<0.001]. For CORT levels, there were main effects of bHR/bLR phenotype [F(1,108)=5.55, p<0.05] and stress group [F(1,108)=6.39, p<0.05] as well as a timepoint x phenotype x MS exposure interaction [F(1,108)=6.32, p<0.001].
Figure 4. Impact of MS stress on novelty-induced adrenocorticotropin (ACTH) and corticosterone secretion.
Basal blood samples were collected 24 h prior to the Light Dark Box (LDB) test, and additional blood samples were taken 5-, 20-, and 60-min after the beginning of the test. Black bar indicates the 5-min LDB test period. bLR controls, compared to bHR controls, exhibited a blunted stress-induced ACTH (A) and corticosterone (B) secretion following the LDB test. A history of MS shifted animals’ neuroendocrine responsivity, with the most striking effects in bLR. Maternally separated (MS) bLRs exhibited a distinctly “bHR-like” neuroendocrine response, showing enhanced ACTH (C) and corticosterone (D) secretion compared to bLR controls. MS only subtly influenced bHRs’ hormonal stress response, producing a dampened peak ACTH response (E), with no apparent impact on corticosterone (F). Groups were compared using a 3-way ANOVA and Fisher’s post hoc comparisons where necessary;* indicates p<0.05 a significant difference between experimental groups at a particular timepoint.
Post hoc analyses showed that bHR controls exhibited exaggerated ACTH and CORT secretion following the LDB test compared to bLR controls (Fig. 4A-B), which parallels our previous work in the bred lines (e.g. (Clinton et al., 2008)) and other studies in commercially purchased HR/LR rats (e.g.(Kabbaj et al., 2000, Rouge-Pont et al., 1998, Piazza et al., 1993, Piazza et al., 1991). Specifically, bHR controls had greater ACTH levels at the 5-min timepoint (Fig. 4A) and greater CORT levels at the 20-min timepoint compared to bLR controls (Fig. 4B; p<0.05 for both).
To illustrate the impact of MS on bHR and bLR neuroendocrine reactivity, we separately present data for bLR and bHR rats in Fig. 4C-D and Fig 4E-F, respectively. MS markedly changed bLRs’ HPA axis output, leading to enhanced ACTH secretion at the 5-min timepoint (p<0.05) and increased CORT secretion at the 20-min timepoint compared to bLR controls (Fig. 4C-D). On the other hand, bHR’s stress reactivity was only subtly impacted by MS, with MS-bHR animals showing reduced ACTH secretion at the 5-min timepoint (p<0.05) (a pattern that was indistinguishable from control bLRs), but no CORT differences compared to bHR controls (Fig. 4E-F).
4. DISCUSSION
Early life adversity has well-known deleterious effects on brain development, emotional behavior, and stress reactivity (Sanchez et al., 2001, Bos et al., 2011, Sheridan et al., 2012), although certain individuals appear to be particularly vulnerable to these effects (Caspi et al., 2003, Veenstra-Vanderweele et al., 2004, Petersen et al., 2012). The present study shows that rats genetically predisposed to a high anxiety/depressive-like phenotype (bLRs) are vulnerable to early life MS stress, leading to enhanced physiological responses to stress: a) exaggerated HPA axis stress responsiveness, and b) greater stress-induced defecation. bHR rats, on the other hand – naturally prone to high novelty-seeking, aggression, impulsivity, addiction, low anxiety and depression – are largely unaffected by the early-life MS experience.
4.1 Individual differences in MS stress vulnerability
Our findings support previous reports of bLRs’ vulnerability to chronic stress. A 4-week chronic mild stress paradigm enhanced bLRs’ anxiety- and depressive-like behavior and elicited cardiovascular abnormalities reminiscent of those observed in depressed humans (Stedenfeld et al., 2011). Likewise, prenatal stress exacerbated bLRs’ anxiety-like behavior and increased their HPA axis responsiveness (Clinton et al., 2008). In both cases, bHRs were minimally affected by chronic stress. Here we found that early-life MS selectively impacted physiological parameters in bLR offspring (i.e., increasing stress-induced defecation and HPA stress axis reactivity) without impacting classic anxiety- and depression-like behavioral measures. It is important to note that while we examined bHR/bLR MS and control animals in multiple tests of anxiety-like behavior (i.e. the OF test, EPM, and LDB), we only examined possible depression-like behavior effects in the FST, which may have limited our ability to detect effects of MS on depression-like measures. Indeed, inclusion of tests to examine additional depression-like behavioral parameters, such as learned helplessness, novelty-suppressed feeding, sucrose preference or sexual interest (Cryan and Leonard, 2010), may have yielded additional findings and should be considered in future studies.
Our results are also consistent with prior studies evaluating the effects of MS in other rodent models of a high- or low-anxiety phenotype (Neumann et al., 2005, Sterley et al., 2011). For example, MS stress had opposing effects on Wistar rats selectively-bred for either high anxiety behavior (HAB) or low anxiety behavior (LAB) in the EPM. MS stress reduced HAB rats’ anxiety behavior and neuroendocrine stress responsiveness, but increased anxiety in LAB rats (Neumann et al., 2005). Another study found that Wistar-Kyoto rats – a common rat depression model (Pare, 1989, Redei et al., 1994, Dugovic et al., 2000, Pardon et al., 2002, Braw et al., 2006)) – were susceptible to MS while control animals, Spontaneously-Hypertensive (SHR) rats, were resistant (Sterley et al., 2011).
As noted above, MS stress selectively impacted bLRs’ physiological responses to stress while failing to change bLR or bHR behavior. Regardless of MS condition, bHRs consistently showed high novelty exploration, low anxiety/depression-like behavior relative to bLR groups. This is not surprising considering the strong genetic underpinning of the bHR/bLR traits (Stead et al., 2006). Moreover, considering bLR-control animals’ already high levels of anxiety-like behavior (i.e. spending little time in the center of the Open Field, open arms of the EPM or light compartment of the LDB), there may be a floor-effect that obscures our ability to see a possible exacerbation of bLRs’ anxiety-like behavior following MS stress exposure (as in (Roth et al., 2009, Huot et al., 2001, Franklin et al., 2010, Aisa et al., 2009, Ladd et al., 2000)). Modification of our test conditions (or perhaps selection of another anxiety test) may have offered a milder anxiogenic stimulus that could potentially enable us to see an effect of MS on bLRs’ behavior. Nevertheless, the fecal boli data from both the Open Field Test and Elevated Plus maze indicate that MS enhances bLRs’ physiological response to stress above and beyond the already high levels of stress-induced defecation exhibited by bLR controls.
4.2 MS enhanced stress-induced defecation in bLR offspring
The MS paradigm offers a tool to explore functional interactions between the brain, HPA axis, and gut, and has therefore become a popular model for gastric disorders like Irritable Bowel Syndrome (Schmidt et al., 2011). Some previous studies (Daniels et al., 2004, Georgel et al., 2003), but not all (Young et al., 2005, Savignac et al., 2011), report exaggerated stress-induced defecation in adult animals subjected to MS. Our findings support this notion, showing that MS enhances defecation particularly in individuals genetically predisposed to high anxiety (i.e. bLRs). Indeed – even at baseline, control bLRs exhibited far greater stress-induced defecation than bHRs, suggesting that relative to bHR, they already have aberrant gut activity in the absence of MS stress. These findings are consistent with a fairly robust literature showing that exaggerated stress-induced defecation is a common feature in animal models of high anxiety/depression (e.g., the Maudsley Reactive rat (Paterson et al., 2001, Berrettini et al., 1994, Abel et al., 1992, Fournier et al., 2002); Roman High Avoidance Rat (Ferre et al., 1995); the WKY rat (Broide et al., 2007); and others (Hashimoto et al., 2009, Beneyto and Meador-Woodruff, 2008, Beneyto et al., 2007)). Furthermore, this work is further supported by a longstanding theory that defecation in the OF and other behavioral tests is an important indicator of emotionality, with greater defecation correlating with higher levels of emotional distress (Archer, 1973, Walsh and Cummins, 1976). Descending pre-autonomic circuits control visceral motor outflow, including gastrointestinal responses to stress, and a recent study showed that daily 15-min MS enhances gastric preautonomic circuits originating in the paraventricular nucleus of the hypothalamus (PVN) – a key node in the HPA stress axis (Banihashemi and Rinaman 2010). This suggests that 180-min daily MS may also re-wire brain-gut circuits to elicit gastric dysfunction (Coutinho et al., 2002, Gareau et al., 2007, Schwetz et al., 2005, Soderholm et al., 2002). It would be interesting to interrogate these circuits in bHR/bLR rats at baseline and following 3-h MS stress given that the groups differ in both conditions.
4.3 MS enhanced HPA axis function in bLRs
Numerous studies show that MS stress produces lifelong changes in the HPA axis, including exaggerated neuroendocrine response to acute stress (Rosenfeld et al., 1992, Plotsky and Meaney, 1993, Huot et al., 2004, Waterland, 2006, Daniels et al., 2009, Smith et al., 2011, Bird, 2002), altered CRF and glucocorticoid receptor expression (Bravo et al., 2011, Meaney et al., 1996), and increased noradrenergic input to the PVN (Wang et al., 2010). Neuroendocrine responsivity in bHR rats was only subtly affected by MS, with MS-exposed bHRs showing diminished ACTH secretion following the LDB test compared to control bHRs and no differences in CORT secretion. On the other hand, MS-bLRs exhibited HPA axis abnormalities reminiscent of previously published results, with MS-bLRs showing exaggerated ACTH and CORT secretion following the LBD test. These disparate findings are not altogether surprising considering bHR/bLR baseline HPA axis reactivity differences (Fig. 4A-B; (Clinton et al., 2008, Kabbaj et al., 2000, Rouge-Pont et al., 1998, Piazza et al., 1993, Piazza et al., 1991)). Compared to bLRs, bHRs exhibit an exaggerated HPA axis response to acute stress (as in Fig. 4A). Furthermore, earlier work showed that high novelty-seeking rats find elevated corticosterone levels rewarding, which may contribute to their “thrill seeking”/risk-taking temperament (Piazza et al., 1993). Several neurobiological factors likely contribute to these effects, including different expression levels of glucocorticoid receptors and corticotrophic releasing hormone (CRH) (Kabbaj et al., 2000, Rouge-Pont et al., 1998), and altered mineralocorticoid/glucocorticoid receptor balance (Kerman et al., 2012). If bHR/bLR rats exhibit fundamental differences in structure and function of the HPA axis, it reasonably follows that MS may differentially impact the stress circuit in each phenotype (Veenstra-VanderWeele and Cook, 2004, Biagini et al., 1998).
4.4 Considering the effect of MS on maternal care in bHR/bLR dams
The quality and quantity of maternal care critically shapes rodent neurodevelopment, emotional behavior, and hormonal stress responsivity (Caldji et al., 1998, Francis et al., 1999, Wang et al., 2010, Liu et al., 2000, Liu et al., 1997, Meaney, 2001). Moreover, MS (like other postnatal manipulations) influences maternal care, suggesting that MS stress exerts its effect, in part, via subtle behavioral or stress hormone changes in dams when they reunite with litters (Macri et al., 2011, Huot et al., 2004). We previously reported that bLR mothers are more maternal (showing more licking, grooming, arched back nursing) with pups compared to bHR mothers. Although bHR dams exhibited less maternal behavior than bLRs during the dark/active phase, they were very attentive to pups during the light phase, spending greater time passive nursing and in pup contact compared to bLR dams (Kristiansen et al., 2007, Clinton et al., 2007). These findings concur with observed HAB/LAB maternal differences, where highly anxious HAB mothers attend more to pups compared to LAB dams (Bosch, 2011, Bosch and Neumann, 2008). One caveat of the present study was that we did not evaluate bHR/bLR maternal behavior in MS and control litters. Neumann et. al. reported that HAB/LAB maternal differences persisted throughout the MS process, and that the two lines acted similarly upon reunion with their litters (Neumann et al., 2005). It would be quite interesting to know a) whether bHR and bLR mothers differentially react to the MS procedure; and b) to what extent their reactions may contribute to MS effects in adult offspring (which could potentially be explored in a cross-fostering experiment). Future experiments are required to explore these new questions.
4.6 Conclusions
We believe that the bLR rat represents a useful new rodent model of co-morbid anxiety- and depression given their naturally high levels of anxiety- and depressive-like behavior (Kabbaj et al., 2000, White et al., 2007, Flagel et al., 2010, Clinton et al., 2011a, Perez et al., 2009, Clinton et al., 2008, Stedenfeld et al., 2011, Garcia-Fuster et al., 2012), diminished aggression (Kerman et al., 2011) and sexual motivation (McCullumsmith et al., 2007), and reduced responsivity to reward (Kabbaj, 2004, Piazza et al., 1989, Flagel et al., 2011, Davis et al., 2008, Garcia-Fuster et al., 2010, Cummings et al., 2011). The present study together with our earlier work (Clinton et al., 2008, Stedenfeld et al., 2011) also demonstrates bLRs’ high vulnerability to chronic stress whereas bHRs are fairly resilient. MS during the first two postnatal weeks appears to exacerbate adult bLRs’ physiological response to stress — increasing their already high levels of stress-induced defecation and also enhancing HPA axis responsivity. Our previous studies point to differences in the ontogeny of hippocampal circuits in bLR versus bHR rats as a possible neurobiological underpinning for their marked behavioral and neuroendocrine phenotypes (Clinton et al., 2011b). Ongoing studies will determine whether the deleterious effects of MS on bLR offspring are associated with changes within the hippocampus and other limbic regions.
Supplementary Material
Our studies with bHR and bLR animals routinely involve behavioral test batteries comprised of multiple tests of environmental reactivity and other emotional behavior. (A) bHR rats (compared to bLRs) consistently exhibit exaggerated locomotor response to a novelty when exposed to a variety of novel test environments. For example, bHRs show significantly greater beam breaks during a 1-h novel cage exposure (left panel), traverse greater distance during a 5-min Open Field (OF) test (middle panel), and are more active in the 5-min Light-Dark Box (LDB) test (right panel) compared to bLRs (n=12–15 per group). (B) bHR and bLR behavioral differences are also apparent whether rats are exposed to a single behavioral test, or if a given test occurs following multiple other tests. For example, separate groups of bHR and bLR rats were examined either in a single LDB test (Experiment 1), or in a LDB that followed a behavioral test battery like the one described in the current study (Experiment 2; n=12–15 per group). Across experiments, we found clear bHR/bLR differences, with bLRs showing significantly less locomotor activity (left panel), greater latency to enter the light compartment (middle panel), and spending less time in the light compartment (right panel) compared to bHR rats. * indicates p<0.05 bHR vs. bLR
Acknowledgments
We are very grateful to Sue Miller, Antony Abraham, and Tracy Bedrosian for excellent technical assistance.
Footnotes
Declaration of Interest: The study was funded by NIMH R00 MH085859-03 (SMC), Office of Naval Research ONR-N00014-09-1-0598 (HA and SJW), NIDA PPG 5P01DA021633-02 (HA and SJW) and a Hope for Depression Research Foundation (HDRF) grant (HA). There are no biomedical financial interests or conflicts of interest for any of the authors.
References
- Abel EL, Altman HJ, Commissaris RL. Maudsley reactive and nonreactive rats in the forced swim test: comparison in fresh water and soiled water. Physiology & behavior. 1992;52:1117–9. doi: 10.1016/0031-9384(92)90469-i. [DOI] [PubMed] [Google Scholar]
- Aisa B, Elizalde N, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: implications for spatial memory. Hippocampus. 2009;19:1222–31. doi: 10.1002/hipo.20586. [DOI] [PubMed] [Google Scholar]
- Archer J. Tests for emotionality in rats and mice: a review. Animal behaviour. 1973;21:205–35. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, Mccullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:1888–902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2175–86. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Harris N, Ferraro TN, Vogel WH. Maudsley reactive and non-reactive rats differ in exploratory behavior but not in learning. Psychiatric genetics. 1994;4:91–4. doi: 10.1097/00041444-199422000-00005. [DOI] [PubMed] [Google Scholar]
- Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. International Journal of Devevelopmental Neuroscience. 1998;16:187–97. doi: 10.1016/s0736-5748(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes & development. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Bos K, Zeanah CH, Fox NA, Drury SS, Mclaughlin KA, Nelson CA. Psychiatric outcomes in young children with a history of institutionalization. Harvard review of psychiatry. 2011;19:15–24. doi: 10.3109/10673229.2011.549773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ. Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Hormones and behavior. 2011;59:202–12. doi: 10.1016/j.yhbeh.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17139–44. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Dinan TG, Cryan JF. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011;14:666–83. doi: 10.1017/S1461145710000994. [DOI] [PubMed] [Google Scholar]
- Braw Y, Malkesman O, Merlender A, Bercovich A, Dagan M, Maayan R, Weizman A, Weller A. Stress hormones and emotion-regulation in two genetic animal models of depression. Psychoneuroendocrinology. 2006;31:1105–16. doi: 10.1016/j.psyneuen.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ. Distribution of histone deacetylases 1–11 in the rat brain. Journal of molecular neuroscience : MN. 2007;31:47–58. doi: 10.1007/BF02686117. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences of the USA. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. Journal of Neuroscience. 2005;25:9102–11. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Mcclay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–4. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Mcclay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Clinton S, Miller S, Watson SJ, Akil H. Prenatal stress does not alter innate novelty-seeking behavioral traits, but differentially affects individual differences in neuroendocrine stress responsivity. Psychoneuroendocrinology. 2008;33:162–77. doi: 10.1016/j.psyneuen.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Kerman IA, Orr HR, Bedrosian TA, Abraham AD, Simpson DN, Watson SJ, Akil H. Pattern of forebrain activation in high novelty-seeking rats following aggressive encounter. Brain Research. 2011a;1422:20–31. doi: 10.1016/j.brainres.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Stead JD, Miller S, Watson SJ, Akil H. Developmental underpinnings of differences in rodent novelty-seeking and emotional reactivity. Eur J Neurosci. 2011b;34:994–1005. doi: 10.1111/j.1460-9568.2011.07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Vazquez DM, Kabbaj M, Kabbaj MH, Watson SJ, Akil H. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Horm Behav. 2007;51:655–64. doi: 10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, Mcroberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. American Journal of Physiology Gastrointestinal and Liver Physiology. 2002;282:G307–16. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Leonard BE. Depression : from psychopathology to pharmacotherapy. Basel; New York: Karger; 2010. [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience and Biobehavioral Reviews. 2005;29:547–69. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2011;2:3. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels WM, Fairbairn LR, Van Tilburg G, Mcevoy CR, Zigmond MJ, Russell VA, Stein DJ. Maternal separation alters nerve growth factor and corticosterone levels but not the DNA methylation status of the exon 1(7) glucocorticoid receptor promoter region. Metabolic brain disease. 2009;24:615–27. doi: 10.1007/s11011-009-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008;90:331–8. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C, Solberg LC, Redei E, Van Reeth O, Turek FW. Sleep in the Wistar-Kyoto rat, a putative genetic animal model for depression. Neuroreport. 2000;11:627–31. doi: 10.1097/00001756-200002280-00038. [DOI] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH. A neural timing factor in the mechanism by which progesterone advances ovulation in the cyclic rat. Endocrinology. 1949;45:581–95. doi: 10.1210/endo-45-6-581. illust. [DOI] [PubMed] [Google Scholar]
- Ferre P, Fernandez-Teruel A, Escorihuela RM, Driscoll P, Corda MG, Giorgi O, Tobena A. Behavior of the Roman/Verh high- and low-avoidance rat lines in anxiety tests: relationship with defecation and self-grooming. Physiology & behavior. 1995;58:1209–13. doi: 10.1016/0031-9384(95)02068-3. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier I, Ploye F, Cottet-Emard JM, Brun J, Claustrat B. Folate deficiency alters melatonin secretion in rats. The Journal of nutrition. 2002;132:2781–4. doi: 10.1093/jn/132.9.2781. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Annals of the New York Academy of Science. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biological psychiatry. 2010;68:408–15. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Parks GS, Clinton SM, Watson SJ, Akil H, Civelli O. The melanin-concentrating hormone (MCH) system in an animal model of depression-like behavior. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2012;22:607–13. doi: 10.1016/j.euroneuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Perez JA, Clinton SM, Watson SJ, Akil H. Impact of cocaine on adult hippocampal neurogenesis in an animal model of differential propensity to drug abuse. Eur J Neurosci. 2010;31:79–89. doi: 10.1111/j.1460-9568.2009.07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Jury J, Macqueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–8. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgel PT, Horowitz-Scherer RA, Adkins N, Woodcock CL, Wade PA, Hansen JC. Chromatin compaction by human MeCP2. Assembly of novel secondary chromatin structures in the absence of DNA methylation. The Journal of biological chemistry. 2003;278:32181–8. doi: 10.1074/jbc.M305308200. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biological Psychiatry. 2009;65:1015–23. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–8. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Holmes A, Le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–46. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Hulshof HJ, Novati A, Sgoifo A, Luiten PG, Den Boer JA, Meerlo P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behavioural brain research. 2011;216:552–60. doi: 10.1016/j.bbr.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Huot RL, Gonzalez ME, Ladd CO, Thrivikraman KV, Plotsky PM. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004;29:279–89. doi: 10.1016/s0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–73. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Kabbaj M. Neurobiological bases of individual differences in emotional and stress responsiveness: high responders-low responders model. Archives of Neurology. 2004;61:1009–1012. doi: 10.1001/archneur.61.7.1009. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. Journal of Neuroscience. 2000;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman IA, Clinton SM, Bedrosian TA, Abraham AD, Rosenthal DT, Akil H, Watson SJ. High novelty-seeking predicts aggression and gene expression differences within defined serotonergic cell groups. Brain Research. 2011;1419:34–45. doi: 10.1016/j.brainres.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman IA, Clinton SM, Simpson DN, Bedrosian TA, Bernard R, Akil H, Watson SJ. Inborn differences in environmental reactivity predict divergent diurnal behavioral, endocrine, and gene expression rhythms. Psychoneuroendocrinology. 2012;37:256–69. doi: 10.1016/j.psyneuen.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Current opinion in pharmacology. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Progress in Brain Research. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Reviews in Neuroscience. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Stohr T, Feldon J. Long-term effects of prenatal stress experiences and postnatal maternal separation on emotionality and attentional processes. Behavioral Brain Research. 2000;107:133–44. doi: 10.1016/s0166-4328(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Macri S, Zoratto F, Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neuroscience and biobehavioral reviews. 2011;35:1534–43. doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Mccullumsmith RE, Gupta D, Beneyto M, Kreger E, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophrenia research. 2007;90:15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, Laplante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Kromer S, Frank E, Landgraf R, Bosch OJ. Differential effects of periodic maternal separation on adult stress coping in a rat model of extremes in trait anxiety. Neuroscience. 2005;132:867–77. doi: 10.1016/j.neuroscience.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–42. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Pare WP. Stress ulcer susceptibility and depression in Wistar Kyoto (WKY) rats. Physiol Behav. 1989;46:993–8. doi: 10.1016/0031-9384(89)90203-5. [DOI] [PubMed] [Google Scholar]
- Paterson A, Whiting PJ, Gray JA, Flint J, Dawson GR. Lack of consistent behavioural effects of Maudsley reactive and non-reactive rats in a number of animal tests of anxiety and activity. Psychopharmacology. 2001;154:336–42. doi: 10.1007/s002130000640. [DOI] [PubMed] [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–87. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen IT, Bates JE, Goodnight JA, Dodge KA, Lansford JE, Pettit GS, Latendresse SJ, Dick DM. Interaction between serotonin transporter polymorphism (5-HTTLPR) and stressful life events in adolescents’ trajectories of anxious/depressed symptoms. Developmental psychology. 2012;48:1463–75. doi: 10.1037/a0027471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proceedings of the National Academy of Science of the USA. 1993;90:11738–42. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proceedings of the National Academy of Sciences of the USA. 1991;88:2088–92. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatric Clinics of North America. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Redei E, Pare WP, Aird F, Kluczynski J. Strain differences in hypothalamic-pituitary-adrenal activity and stress ulcer. Am J Physiol. 1994;266:R353–60. doi: 10.1152/ajpregu.1994.266.2.R353. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Wetmore JB, Levine S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiology & behavior. 1992;52:787–91. doi: 10.1016/0031-9384(92)90415-x. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–9. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10:3903–7. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Developmental Psychopathology. 2001;13:419–49. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Dinan TG, Cryan JF. Resistance to early-life stress in mice: effects of genetic background and stress duration. Frontiers in behavioral neuroscience. 2011;5:13. doi: 10.3389/fnbeh.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, Tassone F, Hertz-Picciotto I. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. 2011;22:476–85. doi: 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwetz I, Mcroberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M, Million M, Ohning G, Tache Y, Plotsky PM, Mayer EA. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. American journal of physiology Gastrointestinal and liver physiology. 2005;289:G704–12. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, Mclaughlin KA, Nelson CA., 3rd Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12927–32. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Konycheva G, Dziadek MA, Ravelich SR, Patel S, Reddy S, Breier BH, Vickers MH, Owens JA, Ferguson LR. Pre- and postnatal methyl deficiency in the rat differentially alters glucose homeostasis. Journal of nutrigenetics and nutrigenomics. 2011;4:175–91. doi: 10.1159/000330227. [DOI] [PubMed] [Google Scholar]
- Soderholm JD, Yates DA, Gareau MG, Yang PC, Macqueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1257–63. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiology & Behavior. 2011;103:210–6. doi: 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterley TL, Howells FM, Russell VA. Effects of early life trauma are dependent on genetic predisposition: a rat study. Behavioral and brain functions : BBF. 2011;7:11. doi: 10.1186/1744-9081-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8021–5. doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Christian SL, Cook EH., Jr Autism as a paradigmatic complex genetic disorder. Annual review of genomics and human genetics. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. [DOI] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Cook EH., Jr Molecular genetics of autism spectrum disorder. Molecular Psychiatry. 2004;9:819–32. doi: 10.1038/sj.mp.4001505. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychological bulletin. 1976;83:482–504. [PubMed] [Google Scholar]
- Wang Z, Xu L, Zhu X, Cui W, Sun Y, Nishijo H, Peng Y, Li R. Demethylation of specific Wnt/beta-catenin pathway genes and its upregulation in rat brain induced by prenatal valproate exposure. Anatomical record. 2010;293:1947–53. doi: 10.1002/ar.21232. [DOI] [PubMed] [Google Scholar]
- Waterland RA. Assessing the effects of high methionine intake on DNA methylation. The Journal of nutrition. 2006;136:1706S–1710S. doi: 10.1093/jn/136.6.1706S. [DOI] [PubMed] [Google Scholar]
- White DA, Kalinichev M, Holtzman SG. Locomotor response to novelty as a predictor of reactivity to aversive stimuli in the rat. Brain Research. 2007;1149:141–8. doi: 10.1016/j.brainres.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SY, Gove S, Fang VS, Greep RO. Ovulation in postpartum rats. Endocrinology. 1973;92:108–16. doi: 10.1210/endo-92-1-108. [DOI] [PubMed] [Google Scholar]
- Young JI, Hong EP, Castle JC, Crespo-Barreto J, Bowman AB, Rose MF, Kang D, Richman R, Johnson JM, Berget S, Zoghbi HY. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17551–8. doi: 10.1073/pnas.0507856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Our studies with bHR and bLR animals routinely involve behavioral test batteries comprised of multiple tests of environmental reactivity and other emotional behavior. (A) bHR rats (compared to bLRs) consistently exhibit exaggerated locomotor response to a novelty when exposed to a variety of novel test environments. For example, bHRs show significantly greater beam breaks during a 1-h novel cage exposure (left panel), traverse greater distance during a 5-min Open Field (OF) test (middle panel), and are more active in the 5-min Light-Dark Box (LDB) test (right panel) compared to bLRs (n=12–15 per group). (B) bHR and bLR behavioral differences are also apparent whether rats are exposed to a single behavioral test, or if a given test occurs following multiple other tests. For example, separate groups of bHR and bLR rats were examined either in a single LDB test (Experiment 1), or in a LDB that followed a behavioral test battery like the one described in the current study (Experiment 2; n=12–15 per group). Across experiments, we found clear bHR/bLR differences, with bLRs showing significantly less locomotor activity (left panel), greater latency to enter the light compartment (middle panel), and spending less time in the light compartment (right panel) compared to bHR rats. * indicates p<0.05 bHR vs. bLR