Abstract
Despite the fact that most breast cancer patients have estrogen receptor (ER) α-positive tumors, up to 50% of the patients are or soon develop resistance to endocrine therapy. It is recognized that HER2 activation is one of the major mechanisms contributing to endocrine resistance. In this study, we report that the ER coactivator MED1 is a novel cross-talk point for the HER2 and ERα pathways. Tissue microarray analysis of human breast cancers revealed that MED1 expression positively correlates most strongly with HER2 status of the tumors. MED1 was highly phosphorylated, in a HER2-dependent manner, at the site known to be critical for its activation. Importantly, RNAi-mediated attenuation of MED1 sensitized HER2-overexpressing cells to tamoxifen treatment. MED1 and its phosphorylated form, but not the corepressors N-CoR and SMRT, were recruited to the ERα target gene promoter by tamoxifen in HER2-overexpressing cells. Significantly, MED1 attenuation or mutation of MED1 phosphorylation sites was sufficient to restore the promoter recruitment of N-CoR and SMRT. Notably, we found that MED1 is required for the expression of not only traditional E2-ERα target genes but also the newly described EGF-ERα target genes. Our results additionally indicated that MED1 is recruited to the HER2 gene and required for its expression. Taken together, these findings support a key role for MED1 in HER2-mediated tamoxifen resistance and suggest its potential usage as a therapeutic target to simultaneously block both ERα and HER2 pathways for the treatment of this type of endocrine resistant breast cancer.
Introduction
Estrogen receptor (ER) α is the key mediator of estrogen functions in the breast and plays prominent roles in breast cancer (1–5). In fact, about 70% of all breast cancer patients have ER-positive tumors, whereas selective estrogen receptor modulators such as tamoxifen have been widely used in the treatment of these patients. Unfortunately, up to half of all ER-positive tumors either do not respond to this endocrine therapy or, after initial successful treatment, the tumors recur as endocrine-resistant breast cancer (6–10). It has been recognized that activation of the tyrosine kinase ErbB-2/HER2 is one of the major mechanisms contributing to the endocrine resistance (10–12). However, although blockage of HER2 with the monoclonal antibody trastuzumab (Herceptin) has been successfully used as a second-line treatment, again, resistance to this therapy is quite high. Hence, further development of novel strategies to selectively block the activities of these pathways remains a major challenge for the treatment of human breast cancer.
HER2 is amplified and overexpressed in 20% to 30% of invasive breast cancers and has been implicated as a major player in both de novo and acquired tamoxifen resistance (7, 12–18). Several clinical studies have also indicated that HER2 overexpression is associated with a poor outcome in tamoxifen-treated patients. It was found that ectopic over-expression of HER2 in MCF-7 cells is sufficient to confer these cells with tamoxifen resistance (19). Further studies showed that mitogen-activated protein kinase (MAPK) activated by HER2 signaling can phosphorylate both ERα and its cofactors to enhance their activities (11, 16, 20–22). Significantly, this cross-talk between HER2 and ERα pathways has now been recognized as one of the key mechanisms that confers endocrine therapy resistance to human breast cancers.
Recent studies have established mediator subunit 1 (MED1) as a key ERα coactivator both in vitro and in vivo (23–30). It has been shown that ectopic MED1 expression is able to markedly enhance ERα functions, whereas knockdown of MED1 impairs both ERα-regulated transcription and estrogen-dependent growth of breast cancer cells. MED1 directly interacts with ERα through its 2 classical L××LL motifs on its central region, whereas its C-terminus is likely to play important regulatory roles, as recent studies have shown that MED1 can be phosphorylated and activated by the MAP kinase pathway on threonines at the C-terminal 1,032 and 1,457 sites (31–34). Interestingly, further biochemical analyses indicated that MED1 exists only in a subpopulation of the Mediator complex with distinct subunit compositions (29). Importantly, most recent animal studies further revealed that MED1 is expressed only in selected cell types and plays rather cell-, tissue-, and gene-specific roles in mediating estrogen functions in vivo (30). Significantly, MED1 has been reported to be overexpressed in a high percentage (40%–60%) of human breast cancer cell lines and primary breast cancers (25, 35–37).
Consistent with previous reports that the MED1 gene is located within the HER2 amplicon on the chromosome 17q12 region and often coamplifies with HER2 (25, 35), here we provided further evidence showing that MED1 protein expression levels correlate most strongly with the HER2 status of human breast cancer by tissue microarray (TMA) analyses. Significantly, our studies showed that MED1 is phosphorylated at the above-mentioned key MAP kinase sites by HER2 activation. Subsequent mechanistic studies supported a key role for MED1 and its cross-talk with HER2 in tamoxifen-induced coactivator/corepressor switch on the ERα target gene promoter. Moreover, we went on to further explore the underlying molecular mechanisms of MED1 in HER2-mediated tamoxifen resistance and found that MED1 is not only required for the expression of both E2-ERα and EGF-ERα target genes, but also for the optimal expression of HER2 itself.
Materials and Methods
Cell culture
The breast cancer cell lines MCF-7 and BT474 were purchased from American type culture collection and MCF-7/ HER2 cells were described previously (38). Cell lines were authenticated on the basis of viability, recovery, growth, and morphology. The expression status of ERα, HER2, and MED1 was further confirmed by Western blot before they were used in the experiments. All cells were cultured in Dulbecco's Modified Eagle's Media (DMEM) medium containing 10% FBS (Hyclone) at 37°C with 5% CO2 in tissue culture incubators. The MAP Kinase inhibitor PD95089 and HER2 inhibitor AG825 were purchased from Calbiochem and EMD chemicals, respectively. 17β-estradiol (E2) and 4-hydroxytamoxifen (TAM) were purchased from Sigma. For experiments involving E2 or TAM treatments, cells were routinely cultured in phenol red-free DMEM plus 10% charcoal-stripped FBS for at least 3 days before the treatments.
Plasmids and lentiviral vector preparation
The plasmid pERE-TK-Luc (ERE, estrogen response element), pRL-CMV, pcDNA3.1-MED1, pLKO.1-MED1 short hairpin RNA (shRNA), and pLKO.1-scramble shRNA were described previously (29). To generate lentiviral constructs expressing double-phosphomutant MED1 (T1032A and T1457A, termed DM MED1), PCR-mediated mutagenesis was conducted using pcDNA3.1-MED1 as a template. The PCR products were then cloned into lentiviral vector pCDH-CMV (System Biosciences) and the sequence confirmed by sequencing (Genewiz). High titer lentiviruses were generated by transient transfection of 293T cells vector only, pCDH-CMV-WT MED1 or pCDH-CMV-DM MED1 with packaging constructs according to the manufacturer's instructions (System Biosciences).
Transient transfection and reporter gene assays
MCF-7 and MCF-7/HER2 cells were first plated in 24-well plates containing phenol red-free DMEM medium supplemented with 10% charcoal-stripped FBS. Control vector only (pcDNA3.1) or pcDNA3.1-MED1 was cotransfected with plasmids expressing ERE-TK-Luc reporter gene by using Lipofectamine 2000 (Invitrogen). pRL-CMV plasmids were also cotransfected to serve as a transfection efficiency control. Following the transfection, the cells were treated with TAM for 24 hours before harvest. A dual luciferase reporter assay system (Promega) was used to measure the luciferase activity.
Real-time reverse transcription PCR
Total RNA was isolated using RNeasy Mini Kits (Qiagen) and reverse transcribed using a SuperScript III first strand synthesis system (Invitrogen). Real-time PCR was then conducted using SYBR Green PCR Master Mix reagents (Roche) on an ABI Prism 7700 Sequence Detection System (Applied Biosystems) using the following primers: cyclin D1: 5′-CGCCCCACCCCTCCAG-3′ and 5′-CCGCCCAGACCCTCAGACT-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-CGGAGTCAACGGATTTGGTCGTA-3′ and 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. The primers used to detect TFF1, Myc, ACP6, and LIF genes were as described (39, 40).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) analysis was conducted as described previously (29, 41). In brief, MCF-7 and MCF-7/HER2 cells were treated for 45 minutes with vehicle (ethanol), 100 nmol/L E2 or 1 umol/L TAM and immediately fixed by adding formaldehyde to the medium to a final concentration of 1%. After PBS washing, cells were harvested and the nuclear lysates were sonicated to generate an average DNA size of 0.5 to 1 kb. Immunoprecipitation experiments were then conducted with antibodies against MED1 (29), p-MED1 (34), NCoR, and SMRT (Santa Cruz Biotechnology). Real-time PCR amplifications were conducted after reverse cross-linking and extraction of DNA from immunoprecipitated chromatin. The primers for TFF1 promoter, ERα-binding site on HER2 gene, ACP6 promoter, and LIF enhancer2 all have been previously described (40, 42).
MTT assay
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, Sigma) assays were conducted as described previously with minor modifications (39). Two thousand cells per well were seeded in 96-well plates and treated with vehicle or tamoxifen at indicated concentrations in regular DMEM supplemented with 10% FBS for 7 days. MTT was added to the medium to a final concentration of 0.5 mg/mL and incubated for 30 minutes at room temperature. The medium was then removed and 0.2 mL DMSO was added. The absorbance of the converted dye was measured at 570 nm using a Synergy spectrophotometer (Biotek).
Immunohistochemistry staining
Immunohistochemistry (IHC) staining of human breast cancer TMA was carried out essentially as previously described (30). In brief, the slide was first deparaffinized and subjected to heat-induced antigen retrieval using citrate buffer. The tissue sections were then incubated with primary antibodies against MED1 overnight at 4°C, followed by extensive washes. The slide was subsequently treated with biotinylated anti-rabbit secondary antibody and then developed using avidin-conjugated horseradish peroxidase with diaminiobenzidine as the substrate (VECTASTAIN Elite ABC kit, Vectorlab). Hematoxylin was used for counterstaining and the images were visualized and captured using axioplan imaging 2e microscope (Zeiss).
Statistical analysis of the data
All experiments were repeated at least 3 to 5 times and data expressed as average ±SD. Statistical analyses of the data were conducted by pairwise Student's t test. Differences are considered statistically significant (*) if P ≤ 0.05 and very significant (**) if P ≤ 0.01. For TMA analyses, the staining intensity of MED1 in tumor cells was scored by a pathologist using a scale of 0 to 3 and analyzed by age-adjusted spearman correlation. The information on age, TNM, tumor grade, and HER2 status were provided by the manufacturer (BR962, 48 cases/96 cores, US Biomax).
Results
MED1 expression levels strongly correlate with HER2 status in human breast cancer
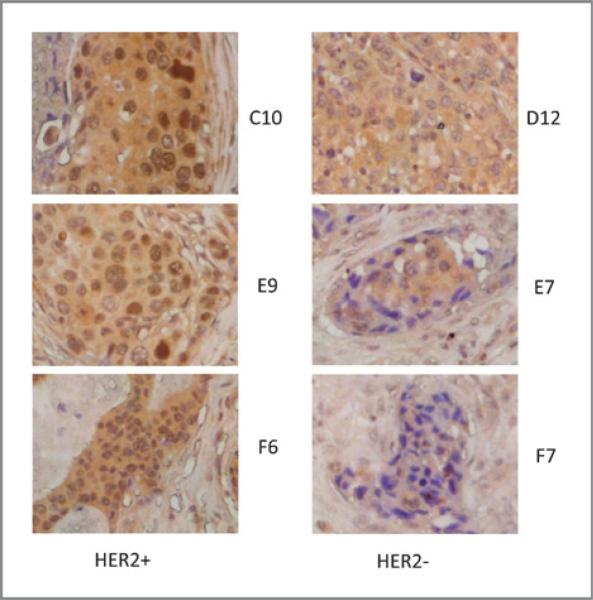
We have previously shown that MED1 is required for ERα-mediated transcription and breast cancer cell growth (29). MED1 has also been reported to be overexpressed in a high percentage of human breast cancer cell lines (25, 35–37). To further explore the role of MED1 in breast cancer, we decided to assess the MED1 protein expression in human breast cancer by using TMA analysis. To achieve that, we carried out immunohistochemical staining of the human breast cancer TMA BR962 (US Biomax) using anti-MED1 antibodies (Fig. 1). The staining intensity of both nuclear and cytosolic MED1 in these tumor sections was scored using a scale of 0 to 3. By using age-adjusted spearman correlation, we found that nuclear, but not cytoplasmic, MED1 expression was highly correlated with HER2 status, but not with tumor grade or stage (TNM) of the human breast cancer tissue samples (Table 1).
Figure 1.
MED1 protein expression highly correlates with HER2 status of human breast cancer. Representative images of MED1 IHC staining of HER2-positive(+) and negative(−) human breast cancer samples are shown.
Table 1.
Age-adjusted spearman analyses of MED1 staining with HER2 status, tumor grade and stage (TNM) using human breast cancer TMA
| Nuclear MED1 |
Cytosolic MED1 |
|||
|---|---|---|---|---|
| R | P | R | P | |
| Grade | 0.005 | 1 | 0.03 | 0.8 |
| TNM | –0.15 | 0.3 | 0.06 | 0.7 |
| HER2 | 0.35 | 0.007 | 0.02 | 0.9 |
HER2 overexpression enhances MED1 phosphorylation via activation of MAP kinase pathway
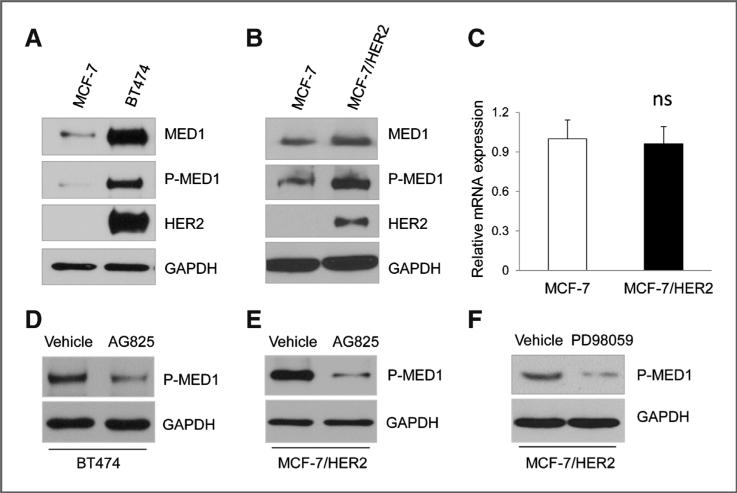
Previous studies have shown that MED1 can be phosphor-ylated and activated by the MAP kinase pathway (31–33). It is also known that the MAP kinase pathway is a key downstream pathway activated by HER2 amplification (15). Therefore, we decided to carry out experiments to determine whether HER2 activation could lead to MED1 phosphorylation. For this, we used an antibody generated specifically against phosphopep-tides containing the phosphorylation site of MED1 (Threonine 1023; ref. 34). We first compared phosphor-MED1 levels using MCF-7 cells and BT474 cells that have HER2 amplification, and found that phosphorylated MED1 were present at significantly higher levels in BT474 cells (Fig. 2A). As BT474 cells may contain additional alterations that can also affect MED1 phosphorylation, we further used MCF-7/HER2 cells to determine whether HER2 overexpression alone is sufficient to enhance MED1 phosphorylation. Consistent with the results above, we found that ectopic overexpression of HER2 in MCF-7 cells was sufficient to induce MED1 phosphorylation at this MAPK site (Fig. 2B). Consistent with previous reports that MED1 phosphorylation could also increase its stability, we also found a slight increase of total MED1 protein levels in MCF-7/ HER2 cells, whereas the mRNA levels of MED1 was not affected (Fig. 2C). Importantly, AG825, a specific inhibitor for HER2, significantly inhibited MED1 phosphorylation in both BT474 and MCF-7/HER2 cells (Fig. 2D and E). Moreover, we found that treatment of MCF-7/HER2 cells with MAP kinase inhibitor PD98059 significantly decreased MED1 phosphorylation levels (Fig. 2F). Taken together, these data support a key role for HER2 overexpression in mediating MED1 phosphorylation through the MAP kinase pathway.
Figure 2.
HER2 overexpression regulates MED1 phosphorylation through MAP kinase pathway. A and B, whole-cell extracts from MCF-7, BT474, and MCF-7/HER2 cells were prepared and subjected to Western blot analyses with anti-MED1, p-MED1, HER2, or GAPDH antibodies. C, total RNA was extracted from MCF-7 and MCF-7/ HER2 cells, followed by real-time RT-PCR analyses of MED1 expression and normalization to GAPDH levels. ns, not significant. D and E, Western blot analyses of p-MED1 levels in BT474 (D) and MCF-7/HER2 (E) cells after control vehicle or 50 μmol/L AG825 treatment. F, MCF-7/HER2 cells were treated with 25 μmol/L PD98059 or vehicle for 24 hours, and subjected to Western blot analyses with anti–p-MED1 and anti-GAPDH (control) antibodies.
Knockdown of MED1 sensitizes HER2 overexpression cells to tamoxifen treatment
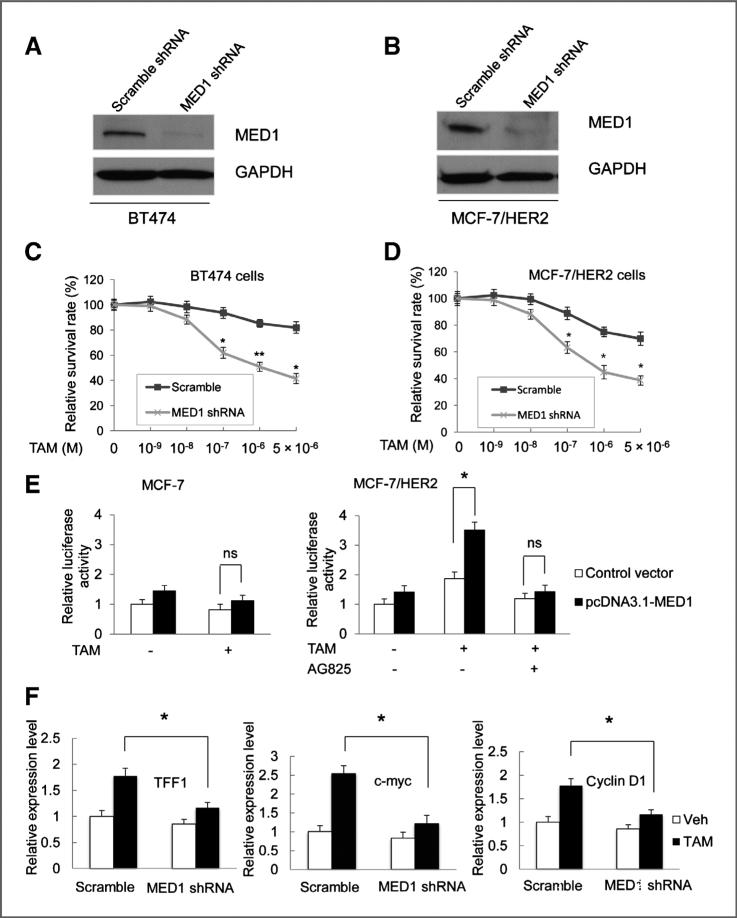
HER2 overexpression has been recognized as a key mechanism in conferring endocrine resistance in human breast cancer. Our data above, indicating both coexpression and cross-talk between HER2 and MED1, led us to further test whether MED1 plays a role in the tamoxifen resistance of HER2-overexpressing cells. To achieve that, we infected the BT474 and MCF-7/HER2 cells with lentivirus expressing control scramble or MED1 shRNA and measured cell proliferation by MTT assay after control vehicle or tamoxifen treatments. As shown by Western blot analyses (Fig. 3A and B), MED1 shRNA treatment successfully knocked down MED1 protein expression in both cells. Importantly, we found that MED1 knockdown significantly sensitized these HER2-overexpressing cells to tamoxifen treatment, as compared with control scramble shRNA-treated cells (Fig. 3C and D Supplementary Fig S1A). As a control, we also conducted the same experiments using above-mentioned MCF-7 cells that do not express high levels of HER2. We found that these cells are already highly sensitive to tamoxifen treatment, as previously reported, and that MED1 knockdown does not significantly alter their sensitivity to tamoxifen (Supplementary Fig. S1B and S1C). These data support a key role for MED1 in mediating the tamoxifen resistance of HER2-overexpressing cells.
Figure 3.
Knockdown of MED1 sensitizes HER2 overexpression cells to tamoxifen treatment. A and B, Western blot analyses of MED1 levels in BT474 and MCF-7/HER2 cells after control scramble or MED1 shRNA treatments. C and D, control or MED1 shRNA knockdown BT474 cells (C) or MCF-7/HER2 cells (D) were treated with vehicle (Veh) or indicated amount of TAM for 7 days. Cells were then harvested and assessed for cell proliferation by MTT assays. E, MCF-7 and MCF-7/HER2 cells were transfected with vector control pcDNA3.1 or pcDNA3.1-MED1, along with plasmids expressing ERE-TK-LUC reporter and PRL-TK (internal control), followed by vehicle (ethanol) or 1 μmol/L TAM treatment for 24 hours. The relative luciferase values are expressed as mean ±SE. F, real-time RT-PCR was conducted to determine TFF1, Myc, and cyclin D1 mRNA levels in control scramble or MED1 shRNA knockdown MCF-7/ HER2 cells after normalization to that of GAPDH. (* , P < 0.05; ** , P < 0.01).
Previous studies have shown that tamoxifen plays an agonist role on ERα-mediated transcription in HER2-overexpressing cells (14, 16). Thus, we carried out to determine the effect of MED1 on ERα transcriptional activity and estrogen-responsive gene expression in response to tamoxifen. In agreement with previous studies, we found that tamoxifen could induce ERα transcriptional activity in MCF-7/HER2 cells but not in MCF-7 cells (Fig. 3E). Interestingly, we found that transient expression of MED1 could further enhance ERE-reporter expression by tamoxifen treatment in MCF-7/HER2 cells. Importantly, AG825 could totally abolish this tamoxifen induced ERE-reporter gene activation by MED1, indicating an HER2 dependency. In addition, we have also examined the requirement of MED1 for tamoxifen-induced expression of several well-known endogenous ERα target genes (TFF1, cyclinD1, and c-Myc) in these cells. As shown in Fig. 3F, our results indicate that MED1 is indeed required for the expression of tamoxifen-induced expression of these endogenous ERα target genes. Moreover, we also examined the potential effect of MED1 on the transcription of the ERα gene and found that MED1 knockdown does not affect the ERα mRNA level in MCF-7/HER2 cells (Supplementary Fig. S2).
MED1 inhibits the recruitment of N-CoR and SMRT to TFF1 promoter by tamoxifen in HER2-overexpressing cells
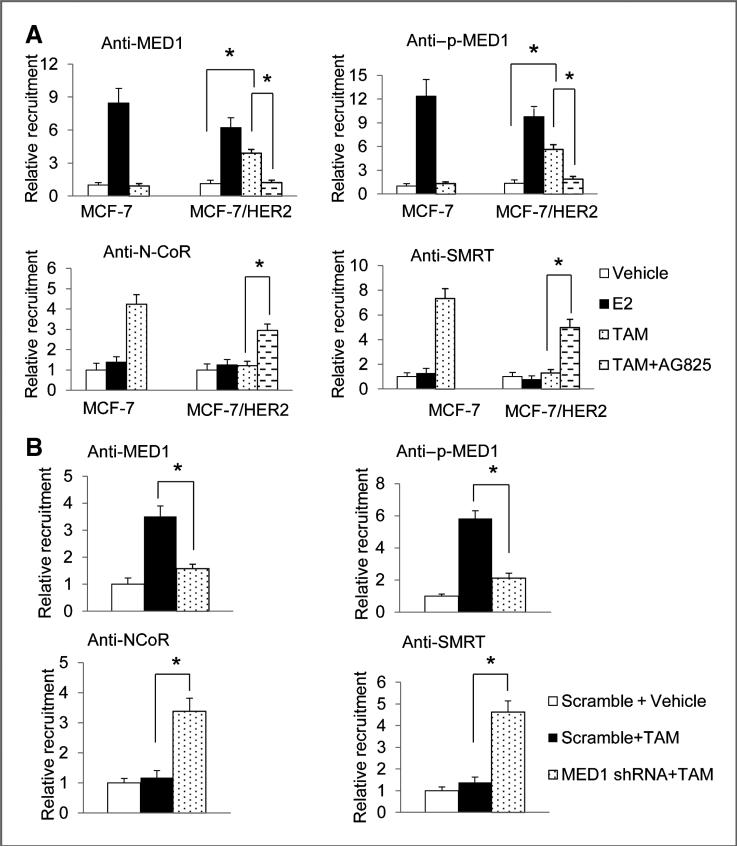
Transcriptional corepressors N-CoR and SMRT have been shown to play important roles in the antiproliferative action of tamoxifen in breast cancer cells through repression of ERα target genes in HER2-overexpressing cells (43–45). To gain a better understanding of the mechanism of how activation of MED1 phosphorylation by HER2 may lead to endocrine resistance, we conducted ChIP assays to examine the role of MED1 on the recruitments of N-CoR and SMRT on the promoter of above TFF1 gene, the most widely used and best-characterized estrogen-responsive gene. As expected, we found tamoxifen-induced promoter occupancy of N-CoR and SMRT but not MED1 or p-MED1 on the TFF1 promoter in MCF-7 cells (Fig. 4A). However, we observed instead an increased occupancy of MED1 and p-MED1, but not N-CoR or SMRT on TFF1 gene promoter in MCF-7/HER2 cell in the presence of tamoxifen. Importantly, treatment with HER2 inhibitor AG825 effectively restored the recruitment of N-CoR and SMRT to TFF1 gene promoter by tamoxifen. Furthermore, we found that knockdown of MED1 by shRNA in these cells can also block this tamoxifen-induced occupancy of MED1 and p-MED1, and also restore tamoxifen-induced recruitment of N-CoR and SMRT (Fig. 4B). Moreover, we have carried out re-ChIP assay and confirmed that MED1 and ERα are acting on the same TFF1 promoter in MCF-7/HER2 cells by tamoxifen (Supplementary Fig. S3A). Finally, re-ChIP assays again supported that gain of the interactions between N-CoR/SMRT and ERα occurred on the same TFF1 promoter in a MED1-dependent manner (Supplementary Fig. S3B). These results are consistent with the hypothesis that MED1 phosphorylation by HER2 plays key roles in preventing tamoxifen-induced recruitment of N-CoR and SMRT to the promoter of ERα target genes in HER2-overexpressing cells. In addition, we found that knockdown of MED1 also increases tamoxifen-induced recruitment of ERα corepressor HDAC1 but has no effect on the recruitment of either ERα itself or ERα coactivator CBP (Supplementary Fig. S4).
Figure 4.
MED1 regulates the recruitment of N-CoR and SMRT to TFF1 promoter by tamoxifen. A, MCF-7 and MCF-7/HER2 cells were treated with 100 nmol/L E2, 1 μmol/L TAM or 50 μmol/L AG825 for 45 minutes. The soluble chromatin was then prepared and subjected to immunoprecipitation experiments using control IgG or antibodies against MED1, p-MED1, N-CoR, or SMRT. Immunoprecipitated DNA corresponding to the TFF1 promoter region was amplified and quantified by real-time PCR. B, chromatin immunoprecipitation experiments were carried out as in A by using control scramble or MED1 shRNA treated MCF-7/ HER2 cells.
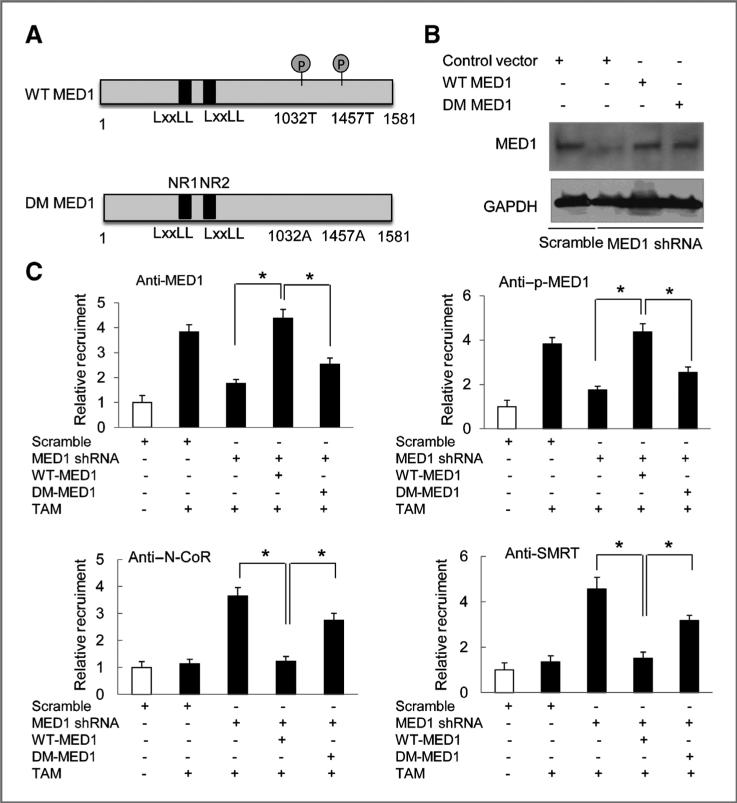
Wild type but not phosphor mutant MED1 prevents tamoxifen-induced N-CoR and SMRT recruitment
To further determine whether MED1 phosphorylation is required for the inhibition of the recruitment of these corepressors (N-CoR and SMRT) to the TFF1 gene promoter in HER2-overexprssing cells, we further generated a double phosphomutant MED1 (DM MED1) with both threonines (T1032 and T1457) known to be activated by the MAP kinase pathway mutated to alanine (Fig. 5A). Lentivirus expressing WT and DM MED1 was then used to infect MCF-7/HER2 cells pretreated with MED1 shRNA for the rescue experiments. We found that although both WT and DM MED1 express at a very similar level as shown by Western blot analyses (Fig. 5B), only WT MED1 but not DM MED1 can effectively restore the tamoxifen-induced recruitment of MED1 and p-MED1 to TFF1 gene promoter (Fig. 5C). We also found that expression of WT MED1 completely abolishes tamoxifen-induced recruitments of N-CoR and SMRT to TFF1 gene promoter. However, in contrast, expression of DM MED1 failed to prevent the promoter recruitments of either N-CoR or SMRT by tamoxifen. Taken together, these findings support the importance of MED1 phosphorylation sites on tamoxifen-induced recruitment of transcriptional corepressors N-CoR and SMRT in HER2-overexpressing cells.
Figure 5.
MED1 phosphorylation is required for the recruitment of NCoR and SMRT to TFF1 promoter by tamoxifen. A, schematic diagram of wild-type (WT) MED1 and T1032A/ T1457A double-mutated (DM) MED1. B, Western blot analyses of MED1 protein levels after infections with lentivirus expressing WT or DM MED1 into MCF-7/HER2 cells pretreated with control scramble or MED1 shRNA. C, cells described in B were further treated with 1 μmol/L TAM or vehicle for 45 minutes and then subjected to ChIP assays using IgG (negative control) or antibodies for MED1, p-MED1, NCoR, or SMRT. (* , P < 0.05).
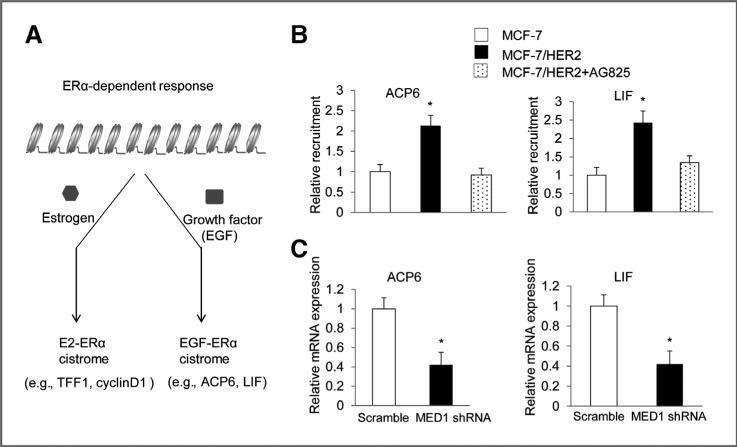
MED1 regulates EGF-induced ERα target genes in MCF-7/HER2 cells
Most recently, a genome-wide ERα-cistrome analysis revealed that growth factors such as EGF can stimulate the binding of ERα to a distinct group of genes (e.g., ACP6 and LIF; Fig. 6A; ref. 40). These genes were named EGF-ERα target genes as they are different from previously described E2- induced ERα target genes (e.g., TFF1 and CyclinD). Importantly, these EGF-ERα target genes are also overexpressed in HER2-positive breast cancers. To determine whether MED1 can also regulate the expression this type of genes in HER2-overexpres-sing cells, we first examine the chromatin occupancy of MED1 on ACP6 and LIF promoters. As shown in Fig. 6B, we found that MED1 is present on the promoter of ACP6 and LIF genes. Importantly, the presence of MED1 on these promoters is significantly enhanced in HER2 overexpressing MCF-7/HER2 cells when compared with that of MCF-7 cells. Significantly, treatment with AG825 again totally abolished this increased recruitment of MED1 to the promoters of ACP6 and LIF genes in MCF-7/HER2 cells. Next, we conducted real-time RT-PCR assays to determine whether MED1 is required for the expression of ACP6 and LIF genes by knocking down MED1 in MCF-7/ HER2 cells. As shown in Fig. 6C, we found MED1 shRNA but not control scramble shRNA treatment significantly inhibited the mRNA levels of both ACP6 and LIF in MCF-7/HER2 cells. These, together with our above data (Fig. 3F), indicate that MED1 plays a key role in controlling the expression of both EGF-ERα target genes and traditional E2-ERα target genes in HER2 overexpression breast cancer cells.
Figure 6.
Knockdown of MED1 also inhibits the expression of EGF-ERα target genes. A, diagram for the regulation of ERα-dependent E2-ERα and EGF-ERα target genes by E2 and EGF. B, MCF-7 and MCF-7/ HER2 cells were treated with 50 μmol/L AG825 or vehicle for 1 hour and subject to chromatin IP experiments using control IgG or anti-MED1 antibodies. C, total RNA was extracted from MCF-7/ HER2 cells treated with control scramble or MED1 shRNA, and the expression of EGF-ERα target genes ACP6 and LIF were measured by real-time RT-PCR and normalization to that of GAPDH. (*, P < 0.05).
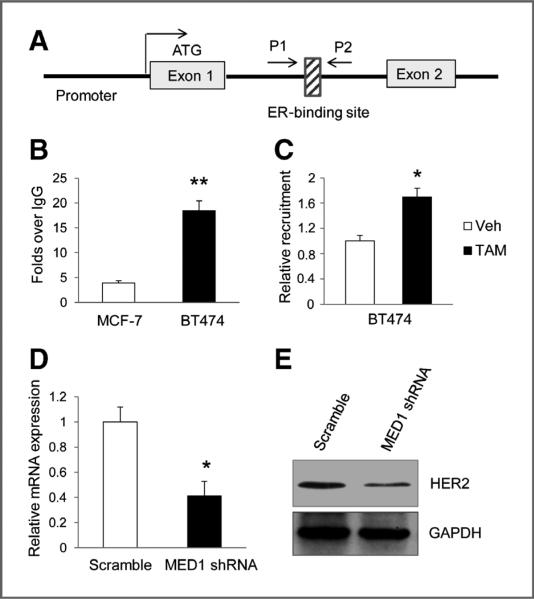
MED1 is required for the optimal expression of HER2 gene
Interestingly, it has been previously reported that ERα is able to directly regulate the HER2 gene expression through its binding site within the HER2 gene (ref. 42; also see diagram in Fig. 7A). As we have shown that MED1 functions as an ERα coactivator in both E2-ERα and EGF-ERα target genes, it raises an important question as to whether MED1 can also regulate the expression of HER2. To determine the role of MED1 in the regulation of HER2 expression, we first conducted ChIP experiments to determine whether MED1 can bind to this ERα-binding region on the HER2 gene. As shown in Fig. 7B, using IgG as a control, we found MED1 does bind to this ERα-binding region in both MCF-7 and BT474 cells. Furthermore, our data indicated that MED1 is present in a significantly higher level at this site in BT474 cells when compared with that of MCF-7 cells. Moreover, we found tamoxifen treatment can further increase the occupancy of MED1 at this ERα-binding site in BT474 cells through ChIP and re-ChIP assays (Fig. 7C, Supplementary Fig. S5). Finally, to determine whether MED1 is required for HER2 gene expression, we conducted further MED1 shRNA knockdown experiments using BT474 cells. The data show that MED1 shRNA, but not control scramble shRNA, effectively inhibits the mRNA and protein expression levels of HER2 (Fig. 7D and E). Collectively, these data support a direct role for MED1 in regulating HER2 gene expression.
Figure 7.
MED1 is present on the ERα-binding site of HER2 gene and required for its expression. A, schematic representation of HER2 gene sequence harboring the ERα-binding site. B, ChIP assays were conducted using MCF-7 and BT474 cells using control IgG or anti-MED1 antibodies. C, BT474 cells were treated with TAM or Veh for 1 hour and then subjected to ChIP assays as in B. D and E, real-time PCR and Western blot analyses for HER2 mRNA and protein expression levels, respectively, by using control scramble or MED1 shRNA-treated BT474 cells. (* , P < 0.05; ** , P < 0.01).
Discussion
Through this study, we have established the cross-talk between HER2 and MED1 and showed its roles in mediating tamoxifen resistance of human breast cancer cells. We found that: (i) MED1 protein expression highly correlates with HER2 status in human breast cancer; (ii) HER2 overexpression induces MED1 phosphorylation and knockdown of MED1 sensitize HER2-overexpressing cells to tamoxifen; (iii) instead of known corepressors N-CoR and SMRT, MED1 and p-MED1 are recruited to ERα target gene promoters by tamoxifen in HER2-overexpressing cells; (iv) knockdown of MED1 or mutation of MED1 phosphorylation sites is able to restore the tamoxifen-induced recruitment of N-CoR and SMRT; (v) MED1 is required for the expression of both E2-ERα and EGF-ERα target genes in HER2-overexpressing cells; and (vi) MED1 is also recruited to the HER2 gene cis-regulatory element and required for its optimal expression. Taken together, these findings support a key role for MED1 in HER2-mediated tamoxifen resistance in human breast cancer.
It has been previously reported that the MED1 gene is localized on chromosome 17q12 and often coamplifies with HER2 in human breast cancer cell lines and primary tumors examined (25, 35). Our studies here provided further evidence that MED1 protein expression levels strongly correlate with HER2 status by using human breast cancer TMAs. Importantly, we found that overexpression of HER2 alone is sufficient to induce MED1 phosphorylation at Thr (1032), a key site that is known to be critical for its functions, whereas blockage of HER2 or its downstream MAP kinase diminishes its phosphor-ylation levels in these cells. HER2 overexpression has been reported to be one of the major mechanisms for tamoxifen resistance of ERα-positive breast cancer cells (10, 11, 16, 20– 22). Because our own and others' studies have established MED1 as a key transcriptional coactivator for ERα, we decided to further examine the role of MED1 in tamoxifen resistance of these cells. Indeed, we found that knockdown of MED1 significantly sensitizes the HER2-overexpressing cells to tamoxifen. Although our studies here focus on HER2, we should also mention that tamoxifen resistance has also been linked to a number of other kinases [e.g., epidermal growth factor receptor (EGFR), IGF1R, and c-Src; refs. 9, 46–48]. As they are also known to activate the MAP kinase pathway, it is conceivable that MED1 could also be phosphorylated and play a role in these kinase-mediated tamoxifen resistances.
In the presence of tamoxifen, ERα is known to preferentially bind and recruit transcriptional corepressors N-CoR and SMRT to target gene promoters. Importantly, the antiproliferative effect of tamoxifen critically depends on the recruitment of these corepressors, whereas reducing the levels of NCoR and SMRT can instead convert tamoxifen into an agonist to stimulate endogenous ER target gene expression and cell growth (43–45). Interestingly, in this study, we found MED1 and p-MED1, but not N-CoR and SMRT, are recruited to the ERα target gene promoter by tamoxifen in HER2-overexpres-sing cells. Importantly, blockage of HER2 signaling, knockdown of MED1, or mutations of both phosphorylation sites of MED1 all could effectively prevent the recruitment of MED1 and p-MED1, and restore N-CoR and SMRT to the promoter of ERα target gene. Taken together, these evidences strongly support a key role for HER2/MED1 cross-talk in tamoxifen resistance through affecting the preferential recruitment of MED1/p-MED1 versus N-CoR/SMRT by tamoxifen-bound ERα. However, one important question remaining is exactly how MED1 phosphorylation may lead to its recruitment to ERα target gene promoters by tamoxifen. One possibility is that it could be simply because of an increased MED1 protein level in these cells rendered by its phosphorylation-induced stabilization. Indeed, it has been previously proposed that the overall balance of coactivators and corepressors levels is an important determinant of the agonist or antagonist nature for tamoxifen (43–45). An alternative or maybe even complement possibility to this is that phosphorylation of MED1 may also lead to its conformation changes, which can in turn increase its accessibility or may even render higher affinity to tamoxifen-bound ERα. Nevertheless, future structural studies on ERα interactions with both phosphorylated and unphosphorylated MED1, in the presence of tamoxifen or estrogen, may be required to gain further deep insights into this important question.
Recent genome-wide ChIP-seq studies have found that activation of the growth factor signaling EGFR pathway by EGF could lead to the binding of ERα to EGF-ERα target genes, a distinct group of genes that are different from previously reported E2 induced E2-ERα target genes (40). Importantly, similar phenomena have most recently been further confirmed by using primary breast cancers from patients with different endocrine responses and clinical outcomes (49). It is known that amplified HER2 often forms complex with EGFR to activate its downstream signaling pathways. Importantly, these EGF-ERα target genes have been found to be over-expressed in HER2 overexpressing cells and are proposed to play important roles in mediating endocrine resistance of HER2 positive cells. Significantly, we found MED1 is not only recruited to E2-ERα target genes but also to EGF-ERα target genes, and are required for the expression of both these target genes. These results suggest that MED1 could potentially be used as an advantageous therapeutic target to block both these pathways for the treatment of tamoxifen resistant human breast cancer. Importantly, as MED1 functions at one of the last steps right before transcription starts, targeting MED1 could effectively block these EGF-ERα and E2-ERα target genes, even if other HER2 and ERα downstream components, cofactors, or even ERα itself is misregulated or activated. Furthermore, as we have found that MED1 could also regulate the expression of HER2 itself, targeting MED1 could also simultaneously attenuate the activation of the HER2 pathway in these cells. Moreover, our most recently published data revealed a previously unexpected tissue- and gene-specific role for MED1 in vivo, and showed that disruption of MED1 resulted in an impaired estrogen response in breast, but not in uterus and bone (30). Most importantly, recent studies found that MED1 expression highly correlates with poor clinical outcome of breast cancer patients treated with endocrine therapy (49). Thus, targeting MED1 in a combined therapy with lower doses of tamoxifen may also result in selective inhibition of these pathways in the breast and overcome the severe adverse effects of currently used high dose tamoxifen regimens.
Supplementary Material
Acknowledgments
The authors thank Drs. Sohaib Khan, Caroline Price, Susan Waltz, and their lab members for reagents and advice. The authors also thank Q. Hu, D. Zhang, and M. Leonard for technical and editorial supports.
Grant Support
This study was supported by University of Cincinnati Cancer Center Startup and Pilot Grants, Ride Cincinnati Award, and Susan G. Komen for the Cure Foundation Career Catalyst Grant (to X. Zhang). This project was also supported by Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number UL1RR026314 and in part by PHS Grant DK P30 DK078392.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Authors' Contributions
Conception and design: J. Cui, X. Zhang
Development of methodology: J. Cui
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): J. Cui, J. Wang, S.-C. Wang, Q. Wang
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): T. Wu, X. Zhang
Writing, review, and/or revision of the manuscript: J. Cui, K. Germer, J. Wang, S.-C. Wang, Q. Wang, X. Zhang
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): K. Germer, J. Luo, S.-C. Wang Study supervision: X. Zhang
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 2.Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000;(27):17–37. doi: 10.1093/oxfordjournals.jncimonographs.a024241. [DOI] [PubMed] [Google Scholar]
- 3.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–85. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 4.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–52. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 5.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 6.Clarke R, Skaar TC, Bouker KB, Davis N, Lee YR, Welch JN, et al. Molecular and pharmacological aspects of antiestrogen resistance. J Steroid Biochem Mol Biol. 2001;76:71–84. doi: 10.1016/s0960-0760(00)00193-x. [DOI] [PubMed] [Google Scholar]
- 7.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2:101–12. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 8.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators – mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–29. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 9.Herynk MH, Fuqua SA. Estrogen receptors in resistance to hormone therapy. Adv Exp Med Biol. 2007;608:130–43. doi: 10.1007/978-0-387-74039-3_10. [DOI] [PubMed] [Google Scholar]
- 10.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11:865s–70s. [PubMed] [Google Scholar]
- 12.Gee JM, Howell A, Gullick WJ, Benz CC, Sutherland RL, Santen RJ, et al. Consensus statement. Workshop on therapeutic resistance in breast cancer: impact of growth factor signalling pathways and implications for future treatment. Endocr Relat Cancer. 2005;12(Suppl 1):S1–7. doi: 10.1677/erc.1.01054. [DOI] [PubMed] [Google Scholar]
- 13.Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 14.Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, et al. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-over-expressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–94. [PubMed] [Google Scholar]
- 15.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 16.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–35. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 17.Arpino G, Green SJ, Allred DC, Lew D, Martino S, Osborne CK, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin Cancer Res. 2004;10:5670–6. doi: 10.1158/1078-0432.CCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 18.Witters LM, Kumar R, Chinchilli VM, Lipton A. Enhanced anti-proliferative activity of the combination of tamoxifen plus HER-2-neu antibody. Breast Cancer Res Treat. 1997;42:1–5. doi: 10.1023/a:1005798224288. [DOI] [PubMed] [Google Scholar]
- 19.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 20.Takimoto GS, Graham JD, Jackson TA, Tung L, Powell RL, Horwitz LD, et al. Tamoxifen resistant breast cancer: coregulators determine the direction of transcription by antagonist-occupied steroid receptors. J Steroid Biochem Mol Biol. 1999;69:45–50. doi: 10.1016/s0960-0760(98)00148-4. [DOI] [PubMed] [Google Scholar]
- 21.Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–7. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–61. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 23.Roeder RG. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem Sci. 1996;21:327–35. [PubMed] [Google Scholar]
- 24.Roeder RG. Role of general and gene-specific cofactors in the regulation of eukaryotic transcription. Cold Spring Harb Symp Quant Biol. 1998;63:201–18. doi: 10.1101/sqb.1998.63.201. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Qi C, Jain S, Le Beau MM, Espinosa R, III, Atkins GB, et al. Amplification and overexpression of peroxisome proliferator-activated receptor binding protein (PBP/PPARBP) gene in breast cancer. Proc Natl Acad Sci U S A. 1999;96:10848–53. doi: 10.1073/pnas.96.19.10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burakov D, Wong CW, Rachez C, Cheskis BJ, Freedman LP. Functional interactions between the estrogen receptor and DRIP205, a subunit of the heteromeric DRIP coactivator complex. J Biol Chem. 2000;275:20928–34. doi: 10.1074/jbc.M002013200. [DOI] [PubMed] [Google Scholar]
- 27.Warnmark A, Almlof T, Leers J, Gustafsson JA, Treuter E. Differential recruitment of the mammalian mediator subunit TRAP220 by estrogen receptors ERalpha and ERbeta. J Biol Chem. 2001;276:23397–404. doi: 10.1074/jbc.M011651200. [DOI] [PubMed] [Google Scholar]
- 28.Kang YK, Guermah M, Yuan CX, Roeder RG. The TRAP/Mediator coactivator complex interacts directly with estrogen receptors alpha and beta through the TRAP220 subunit and directly enhances estrogen receptor function in vitro. Proc Natl Acad Sci U S A. 2002;99:2642–7. doi: 10.1073/pnas.261715899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Krutchinsky A, Fukuda A, Chen W, Yamamura S, Chait BT, et al. MED1/TRAP220 exists predominantly in a TRAP/Mediator sub-population enriched in RNA polymerase II and is required for ER-mediated transcription. Mol Cell. 2005;19:89–100. doi: 10.1016/j.molcel.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Jiang P, Hu Q, Ito M, Meyer S, Waltz S, Khan S, et al. Key roles for MED1 LxxLL motifs in pubertal mammary gland development and luminal-cell differentiation. Proc Natl Acad Sci U S A. 2010;107:6765–70. doi: 10.1073/pnas.1001814107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misra P, Owuor ED, Li W, Yu S, Qi C, Meyer K, et al. Phosphorylation of transcriptional coactivator peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP). Stimulation of transcriptional regulation by mitogen-activated protein kinase. J Biol Chem. 2002;277:48745–54. doi: 10.1074/jbc.M208829200. [DOI] [PubMed] [Google Scholar]
- 32.Pandey PK, Udayakumar TS, Lin X, Sharma D, Shapiro PS, Fondell JD. Activation of TRAP/mediator subunit TRAP220/Med1 is regulated by mitogen-activated protein kinase-dependent phosphorylation. Mol Cell Biol. 2005;25:10695–710. doi: 10.1128/MCB.25.24.10695-10710.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belakavadi M, Pandey PK, Vijayvargia R, Fondell JD. MED1 phosphorylation promotes its association with mediator: implications for nuclear receptor signaling. Mol Cell Biol. 2008;28:3932–42. doi: 10.1128/MCB.02191-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, Zhang C, Wu D, Chen H, Rorick A, Zhang X, et al. Phospho-MED1-enhanced UBE2C locus looping drives castration-resistant prostate cancer growth. EMBO J. 2011;30:2405–19. doi: 10.1038/emboj.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luoh SW. Amplification and expression of genes from the 17q11 approximately q12 amplicon in breast cancer cells. Cancer Genet Cytogenet. 2002;136:43–7. doi: 10.1016/s0165-4608(01)00657-4. [DOI] [PubMed] [Google Scholar]
- 36.Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005;102:13550–5. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66:10292–301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 38.Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6:251–61. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Zhang D, Jiang P, Xu Q, Zhang X. ARGLU1 interacts with MED1 and is required for estrogen receptor-mediated gene transcription and breast cancer cell growth. J Biol Chem. 2011;286:17746–54. doi: 10.1074/jbc.M110.206029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lupien M, Meyer CA, Bailey ST, Eeckhoute J, Cook J, Westerling T, et al. Growth factor stimulation induces a distinct ER(alpha) cistrome underlying breast cancer endocrine resistance. Genes Dev. 2011;24:2219–27. doi: 10.1101/gad.1944810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–52. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 42.Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, et al. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456:663–6. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith CL, Nawaz Z, O'Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–66. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 44.Keeton EK, Brown M. Cell cycle progression stimulated by tamoxifen-bound estrogen receptor-alpha and promoter-specific effects in breast cancer cells deficient in N-CoR and SMRT. Mol Endocrinol. 2005;19:1543–54. doi: 10.1210/me.2004-0395. [DOI] [PubMed] [Google Scholar]
- 45.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–8. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 46.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–83. [PMC free article] [PubMed] [Google Scholar]
- 47.Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growthfactor, and insulin-likegrowthfactorsignaling inbreast cancer. Clin Cancer Res. 2001;7:4429s–35s. discussion 11s–12s. [PubMed] [Google Scholar]
- 48.Creighton CJ, Casa A, Lazard Z, Huang S, Tsimelzon A, Hilsenbeck SG, et al. Insulin-like growth factor-I activates gene transcription programs strongly associated with poor breast cancer prognosis. J Clin Oncol. 2008;26:4078–85. doi: 10.1200/JCO.2007.13.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012;481:389–93. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.