Abstract
Aims
To estimate the prospective association of low-density lipoprotein (LDL) cholesterol on cardiovascular disease (CVD) risk among individuals with type 2 diabetes.
Methods
We used extensive literature searching strategies to locate prospective cohort studies that reported LDL cholesterol levels as a risk factor for incidence of cardiovascular events. We conducted meta-analytic procedures for two outcomes: incident CVD and CVD mortality.
Results
A total of 16 studies were included in this analysis with a mean follow-up range of 4.8–11 years. The pooled relative risk associated with a 1 mmol/L increase in LDL cholesterol among patients with type 2 diabetes was 1.30 (95% confidence interval [CI], 1.19 to 1.43) for incident CVD, and 1.50 (95% CI, 1.25 to 1.80) for CVD mortality, respectively. Subgroup analyses showed that for incident CVD, the pooled relative risk was 1.28 (95% CI, 1.17 to 1.41) for 7 studies adjusted for blood pressure and/or glucose concentration (or insulin concentration, glycated hemoglobin) and 1.40 (95% CI, 1.05 to 1.86) for 3 studies that did not adjust for these variables.
Conclusions
Our study demonstrates that LDL cholesterol was associated with an increased risk for cardiovascular outcomes among patients with type 2 diabetes, independently from other conventional risk factors.
Keywords: type 2 diabetes, meta-analysis, low-density lipoprotein cholesterol, cardiovascular outcomes
Introduction
Type 2 diabetes, a common and serious condition associated with reduced life expectancy and considerable morbidity, has posed a great burden on patients, their families, and health care systems.[1] The estimated global diabetes prevalence for 2011 is 8.3%, and it is predicted to be increased to 9.9% by the year 2030. [2] It has been estimated that the global health expenditure on diabetes is at least $376 billion in 2010 and will be $490 billion in 2030.[3] Patients with type 2 diabetes have a 2–4 times higher risk of CVD mortality than those without diabetes.[4, 5] Type 2 diabetes is a major risk factor for cardiovascular disease (CVD) accounting for approximately 70% of deaths among these patients.[6, 7]
Although various cardiovascular risk factors are associated with the excessive CVD risk in diabetic patients, elevated blood pressure, hyperglycemia and lipoprotein abnormalities are key contributors.[8, 9]Furthermore, low-density lipoprotein (LDL), the major cholesterol-bearing lipoprotein, is associated with these key contributors.[10–12] LDL cholesterol is widely considered to be the principal atherogenic lipoprotein and a key predictor for CVD risk in patients with diabetes. The positive effects of controlling LDL cholesterol for preventing or slowing CVD development in diabetes has been documented.[13, 14] Therefore, LDL reduction remains the primary target for lipid-lowering therapy, and plasma LDL cholesterol concentration guides intervention strategies for lipid abnormalities in various guidelines.[1, 15–17]
It is hard for single cohort study to adequately quantify the magnitude of the risk due to the limited sample size, while meta-analysis has higher statistical power to detect an effect than individual studies and is a generalization to the population of studies.[18] Two recent meta-analyses evaluating the association of LDL cholesterol level with CVD risk among cohort studies conducted in the general population and both studies reporting an increased risk of CVD events along with increased LDL cholesterol concentrations.[19, 20] To our knowledge, no previous meta-analysis has been reported for estimating the size of LDL cholesterol levels on CVD risk among individuals with type 2 diabetes in prospective studies. To investigate whether long-term LDL cholesterol concentration can reduce the risks for cardiovascular outcomes, we performed a systematic review and meta-analysis on prospective cohort studies to evaluate the association of LDL cholesterol level with the risks of fatal and non-fatal CVD outcomes in individuals with type 2 diabetes.
Methods
Data sources and searches
We searched the MEDLINE database for articles published in English from January 1974 to June 2012 by using Medical Subject Heading (MeSH) terms cardiovascular diseases; coronary heart disease; heart failure; stroke; and diabetes mellitus, type 2, as well as hyperlipidemia and lipoproteins, LDL or cholesterol, LDL. We also performed a manual search of references cited by original studies and relevant review articles and queried experts to identify any additional studies. This search provided 581 articles, which were further screened for inclusion from abstracts or full texts.
Study selection
We selected the studies based on the following conditions: 1) study design: prospective cohort studies; 2) study population: patients with type 2 diabetes; 3) studies reported at least one of the outcomes of interest: cardiovascular outcomes (CVD, CVD mortality, coronary heart disease [CHD], fatal CHD, heart failure, and stroke; 4) studies reported a measurement of LDL cholesterol; and 5) studies had follow-up duration ≥ 1 years. We first identified 33 full-text articles and then excluded some if they 1) had no original data (review, editorials, meta-analyses), 2) involved non prospective analysis (e.g., case–control studies), 3) included patients with type 2 diabetes receiving lipid lowering medication at baseline, or 4) were duplicate publications. If separate articles from the same study were published, the article with the most updated data was selected for use in this study. In the case of duplicate publications, only one publication was included.
Data extraction and quality assessment
Data were extracted by two independent reviewers (YW and GH) using standardized data abstraction forms. Disagreements between reviewers were resolved by repeated examination of the original articles and discussion until consensus was achieved. Information on surname of the first author, year of publication, country of origin, mean age, sample size, percentage of male of study participants, number of study participants included in the final analysis, duration of follow-up, outcomes, estimate of the risk of association, variables adjusted in the analyses, and LDL cholesterol measurement method was extracted. For assessment of study quality, we evaluated 6 major items of each study: 1) Was the method for measuring LDL cholesterol validated? 2) Did LDL cholesterol allow quantification as both continuous and categorized variables? 3) Were the outcomes determined by the specified criteria (i.e., medical record) or physician’s or patient’s judgments such as registry, death certificate, questionnaire, and patients’ self-report? 4) Was the total follow-up duration ≥ 5 years? 5) Were major CVD risk factors in the statistical analyses, such as age, sex, blood pressure (hypertension), glucose level (or insulin level, glycated hemoglobin [HbA1c]), smoking, duration of diabetes, treatment, albuminuria, etc.? and 6) Were subjects lost to follow up excluded from the analysis?
During data extraction, we abstracted adjusted relative risk (RR) for the association between LDL cholesterol concentration either as a continuous or a categorical variable and the major outcomes. Standard errors for the estimates were abstracted or derived by using data reported in the original studies. When necessary, the original authors were contacted for additional information.
Reviewers recorded the following as the major outcomes of interest: incident CVD (non-fatal myocardial infarction, non-fatal stroke, and fatal CVD), CVD mortality, incident CHD (non-fatal myocardial infarction and fatal CHD), CHD mortality, heart failure (non-fatal and fatal heart failure), and incident stroke (non-fatal and fatal stroke).
Data synthesis and analysis
Separate meta-analyses of the prospective cohort studies were carried out for two combined outcomes: CVD and CVD mortality due to limited existing studies. All RR estimates included in the pooled analyses were from the most fully adjusted multivariate models.
Most of the studies included in the present analysis reported the RRs of per 1 unit (i.e., 1 mmol/l) change of LDL cholesterol level; therefore, we converted studies that used different units in their original analyses to RRs based on the method previously published.[21] For example, there was 1 study [22] that compared the reported RR of above and below the median value of LDL cholesterol. In order to make these results comparable to the rest of studies, we assumed that there was a normal distribution for LDL cholesterol and used the reported mean and standard deviation to estimate the 25th and 75th percentiles of LDL cholesterol. Then, we divided the log RRs by the difference of these 2 values to estimate the effect of per 1 mmol/L (39 mg/dl) change in LDL cholesterol.[23]
After the RR estimate for each cohort study was converted to reflect per 1 mmol/L increase in LDL cholesterol, the pooled RRs and 95% confidence intervals (CIs) were calculated using the random-effects model. Statistical heterogeneity was assessed using the DerSimonian and Laird’s Q statistic and I2 statistic. The Q test provided information about the presence or absence of between-study heterogeneity, whereas the I2 statistic quantified the degree of heterogeneity and could be interpretable as the percentage of the total association that may be due to heterogeneity between studies (I2 >50% was considered a meaningful level of heterogeneity). We also conducted a sensitivity analysis in which each prospective cohort study was excluded in turn to evaluate the influence of that prospective cohort study on the overall estimate. Publication bias was examined using Begg’s test.[24] A meta-regression analysis was conducted to explore the sources of statistical heterogeneity in the meta-analyses. Subgroup analyses were conducted by stratifying the analysis according to studies that in different areas. All analyses were conducted using STATA 12.0 (Stata Corporation, College Station, TX).
Results
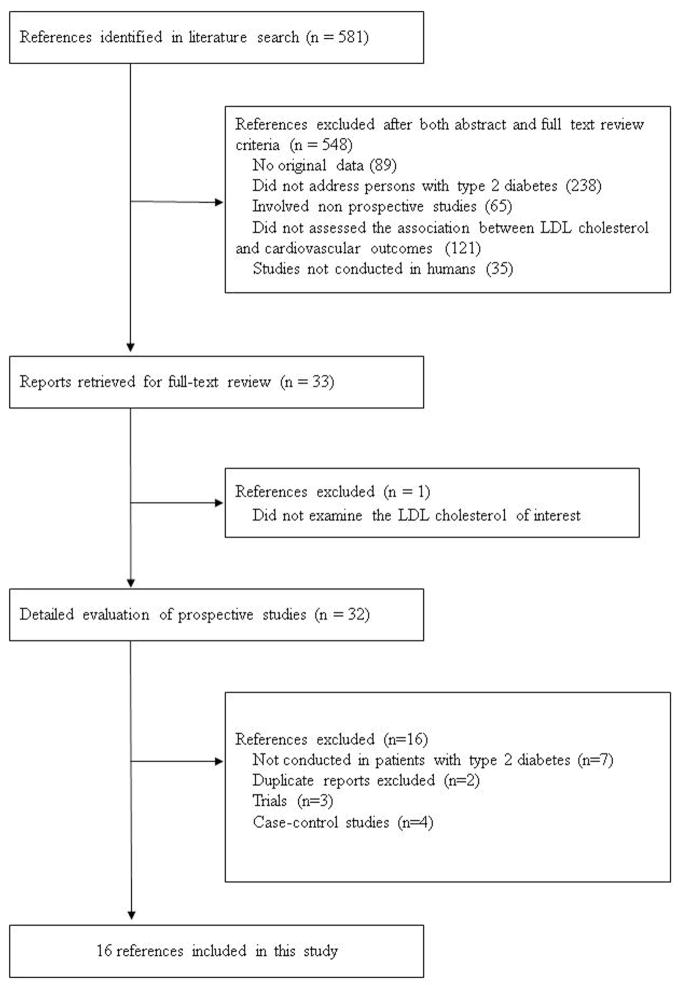
Of 581 articles that were identified from the literature search, 548 were excluded after an abstract or full-text review (Figure 1). Of the 33 articles for further review, 16 articles [8, 22, 25–38] from 15 independent prospective cohorts were included in the present meta-analysis.
Figure 1.
Flow diagram of studies assessed and included.
Table 1 summarizes the characteristics of the studies included in the present analysis. The sample size ranged from 133 to 18,673 participants, four studies (25%) had more than 3,000 patients with type 2 diabetes. The mean follow-up time in studies ranged from 4.8 to 11 years. The studies included were geographically heterogeneous: three were conducted in the United States (US), two in the United Kingdom (UK), three in Finland, one in The Netherlands, one in Sweden, one in Iran, one in Italy, two in Japan, and two in China. Most studies had primary care or clinic-based patient populations. Both men and women were included in 15 of the 16 studies, and the remaining study included only women.[31]
Table 1.
Design characteristics of prospective cohort studies on the association between low-density lipoprotein cholesterol and cardiovascular outcomes, 1993–2012.*
| Study name | Country | Project | No. of subjects |
Age (y) | Men (%) |
Outcome | Follow- up time, y |
LDL-cholesterol variable |
LDL cholesterol measurement |
Adjusted covariates |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Continuous | Category | ||||||||||
| Laakso et al., 1993 [25] | Finland | Central register, Kuopio University Hospital district | 313 | 56 (men)/58(women) | 48.9 | CHD mortality, CHD | 7.2 | √ | Calculated by subtraction HDL cholesterol from the bottom fraction of ultra-centrifuged serum | Age | |
| Niskanen et al., 1996 [26] | Finland | Population register, Kuopio | 133 | 45–64 | 52.6 | CVD mortality | 5 | √ | measure | Age, sex, ischemic electrocardiogram, HDL cholesterol, triglycerides, albuminuria, HbA1c, hypertension, smoking history, log fasting insulin, and BMI | |
| Lehto et al., 1997 [27] | Finland | Central register, Kuopio University Hospital district; Turku University Central Hospital district | 1059 | 45–64 | 54.9 | CHD mortality, CHD | 7 | √ | Calculated by Friedewald formula | Age, sex, area, previous MI, total cholesterol, log triglycerides, HDL cholesterol, FPG, and duration of diabetes | |
| Turner et al., 1998 [28] | United Kingdom | UKPDS 23 | 3,055 | 25–65 | 58.1 | CHD | 7.9 | √ | measure | Age, sex, HDL cholesterol, HbA1c, SBP, and smoking | |
| Mattock et al., 1998 [29] | United Kingdom | Diabetes clinic at Lewisham Hospital | 146 | 59 | 56 | CHD mortality, all-cause mortality | 7 | √ | measure | Age | |
| Lu et al., 2003 [8] | United States | The Strong Heart Study | 2,108 | 45–74 | 36.6 | CHD, MI, Stroke, CVD | 9 | √ | measure | Age, sex, BMI, smoking status, study center, SBP, HbA1c, fibrinogen, insulin, and albumin to creatinine ratio | |
| Jiang et al., 2004 [30] | United States | The Health Professionals’ Follow-up Study | 746 | 46–81 | 100 | CVD | 6 | √ | measure | Age, BMI, family history of myocardial infarction, physical activity, smoking, alcohol consumption, history of hypertension, aspirin use and HbA1c | |
| Schulze et al., 2004 [31] | United States | The Nurses’ Health Study | 921 | 30–55 | - | CHD | 10 | √ | measure | Age, physical activity, alcohol intake, parental history of CHD, history of high blood pressure, aspirin use, postmenopausal hormone use and BMI | |
| Bruno et al., 2006 [32] | Italy | The Casale Monferrato Study | 1,565 | 68.9 | 43.4 | CVD mortality | 11 | √ | Calculated by Friedewald formula | Age, sex, hypertension, smoking, CHD, AER, fibrinogen, cumulative average individual HbA1c and referring physician | |
| Yang et al., 2008 [33] | China | The Hong Kong Diabetes Registry | 3,456 | 57 | 45.2 | HF | 5.52 | √ | Calculated by Friedewald formula | None | |
| Van Hateren et al., 2009 [34] | Netherlands | The ZODIAC-13 Study (primary care) | 881 | ≥60 | 39.5 | CVD mortality, all-cause mortality | 9.8 | √ | Calculated by Friedewald formula | Unknown selected confounder | |
| Ting et al., 2010 [22] | China | The Hong Kong Diabetes Registry | 4,521 | 54 (non-CVD)/64(CVD) | 46.2 | CVD | 4.9 | √ | Calculated by Friedewald formula | Age, smoking status, duration of diabetes, HbA1c, ln (ACR +1), use of statins, fibrates, gliclazide and rosiglitazone during follow-up, and years of enrolment | |
| Tohidi et al., 2010 [35] | Iran | The TLGS Study | 1,021 | 54.8 | 40.5 | CVD | 8.6 | √ | Calculated by Friedewald formula | Age, SBP, FPG, lipid lowering use (men) /SBP, FPG, waist to hip ratio, lipid lowering drug use, menopause status and family history of premature CVD (women) | |
| Eliasson et al., 2011 [36] | Sweden | Swedish NDR | 18,673 | 30–70 | 60.4 | CHD | 4.8 | √ | √ | Calculated by Friedewald formula | Age, sex, diabetes duration, type of hypoglycemic treatment, HbA1c, SBP, smoking, BMI, albuminuria>20 μg/min, and a history of CVD |
| Sone et al., 2012 [37] | Japan | The Japan Diabetes Complications Study | 1,771 | 58.2 (mean) | 53.1 | stroke | 7.86 | √ | Calculated by Friedewald formula | Age, gender, diabetes duration, BMI, SBP, HbA1c, HDL cholesterol, triglycerides, smoking status, and alcohol intake | |
| Sone et al., 2012 [38] | Japan | The Japan Diabetes Complications Study | 1,771 | 40–70 | 53.1 | CHD | 8 | √ | √ | Calculated by Friedewald formula | Age, diabetes duration, BMI, SBP, HbA1c, smoking, and alcohol intake |
CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure; LDL-cholesterol, low-density lipoprotein cholesterol; BMI, body mass index; SBP, systolic blood pressure; FPG, fasting plasma glucose; HDL cholesterol, high-density lipoprotein cholesterol; AER, albumin excretion rate; ACR, urine albumin-creatinine ratio; HbA1c, glycated hemoglobin
Most studies modeled the effect of baseline LDL cholesterol measurements on the risk for CVD outcomes; however, one study[34] used updated mean LDL cholesterol levels and modeled LDL cholesterol as a time-dependent variable in the model.
Quality assessments of the included studies are summarized in Table 2. The overall quality of included studies was good according to our 6-item evaluation criteria. Fourteen of the 16 studies adjusted for major CVD risk factors in the statistical analyses. All had validated methods for measuring LDL cholesterol, and had outcomes determined by specified criteria and excluded participants who were lost during the follow-up. All studies had follow-up time longer than 4 years and only two studies had follow-up of less than 5 years. Nine studies treated LDL cholesterol as continuous variables; two studies treated LDL cholesterol both as continuous and categorized variables; and the remaining five studies treated LDL cholesterol as categorical variables only in the analyses.
Table 2.
Quality assessments on prospective cohort studies on the association between low-density lipoprotein cholesterol and cardiovascular outcomes.*
| Reference | 1. Is the instrument for measuring LDL cholesterol validated? | 2. Does LDL cholesterol allow quantification as both continuous and categorized variables? | 3. Are the outcomes determined by the specified criteria (i.e., medical record) or physician’s or patient’s judgments such as registry, death certificate, questionnaire, and patients’ self-report? | 4. Is the total follow-up duration ≥5 years? | 5. Are major CVD risk factors adjusted for in the statistical analyses?† | 6. Are subjects lost-to-follow up excluded from the analysis? | 7. Overall quality score |
|---|---|---|---|---|---|---|---|
| Laakso et al., 1993 [25] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Niskanen et al., 1996 [26] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Lehto et al., 1997 [27] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Turner et al., 1998 [28] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Mattock et al., 1998 [29] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Lu et al., 2003 [8] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Jiang et al., 2004 [30] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Schulze et al., 2004 [31] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Bruno et al., 2006 [32] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Yang et al., 2008 [33] | 1 | 0 | 1 | 1 | 0 | 1 | 4 |
| Van Hateren et al., 2009 [34] | 1 | 0 | 1 | 1 | 0 | 1 | 4 |
| Ting et al., 2010 [22] | 1 | 0 | 1 | 0 | 1 | 1 | 4 |
| Tohidi et al., 2010 [35] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Eliasson et al., 2011 [36] | 1 | 1 | 1 | 0 | 1 | 1 | 5 |
| Sone et al., 2012 [37] | 1 | 0 | 1 | 1 | 1 | 1 | 5 |
| Sone et al., 2012 [38] | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| % Studies scoring “Yes” | 100.0% | 12.5% | 100.0% | 87.5% | 87.5% | 100.0% | 5 |
Low-density lipoprotein, LDL; CVD, cardiovascular disease
1 = “Yes”, 0 = “No”, “Unable to determine”, or “Not applicable”.
Major CVD risk factors, such as age, sex, blood pressure (hypertension), glucose level (or insulin level, glycated hemoglobin [HbA1c]), smoking, duration of diabetes, treatment, albuminuria, etc., were included in the multivariable analyses.
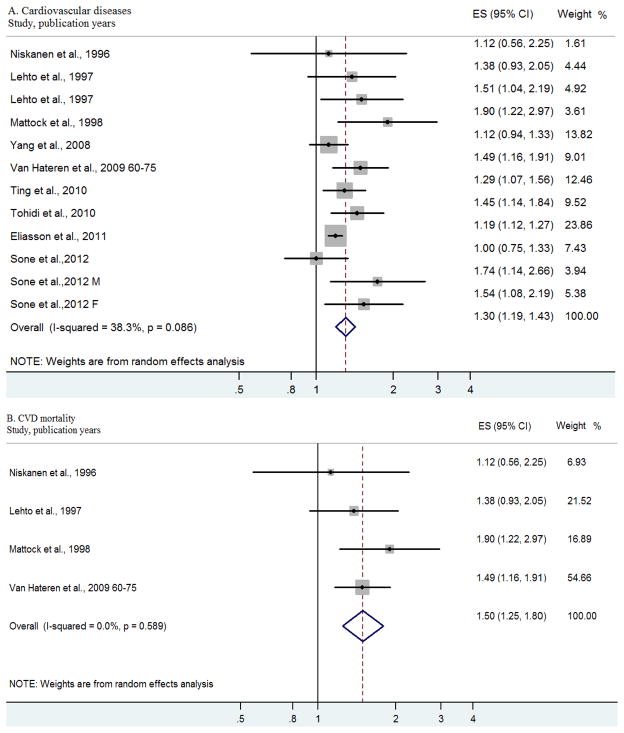
Figure 2 presents the individual and pooled RRs for incident CVD and CVD mortality. The pooled RR associated with per 1 mmol/L increase in LDL cholesterol level among patients with type 2 diabetes was 1.30 (95% CI, 1.19 to 1.43) for incident CVD outcomes in 10 independent studies and 1.50 (95% CI, 1.25 to 1.80) for CVD mortality in four independent studies. Begg’s test suggested that there was no significant potential publication bias for both incident CVD (P = 0.12) and CVD mortality (P = 0.73). In sensitivity analyses, exclusion of any single prospective cohort study from the analysis did not alter the overall findings of a positive association between LDL cholesterol level and cardiovascular outcomes.
Figure 2.
Forest plot of relative risks (RRs) and 95% confidence intervals (CIs) for the association between per 1mmol/l increase in low-density lipoprotein cholesterol and the main study outcomes risks in type 2 diabetes.
We also analyzed the heterogeneity among the studies of cardiovascular outcomes in persons with type 2 diabetes. The I2 statistics (P values) for the Q test in the above analyses were 38.3% (0.09) for incident CVD and 0.0% (0.59) for CVD mortality, respectively, pointing to no statistically significant heterogeneity for incident CVD or for CVD mortality.
To further investigate the potential sources of heterogeneity, we conducted subgroup analyses that compared the RR estimates for studies that adjusted for age, sex, blood pressure, and/or glucose concentration (or insulin concentration, HbA1c) with those did not: for CVD, the only outcome that allowed us to conduct subgroup analysis, the pooled RR was 1.28 (95% CI, 1.17 to 1.41) for 7 studies adjusted for blood pressure and/or glucose concentration (or insulin concentration, HbA1c) and 1.40 (95% CI, 1.05 to 1.86) for 3 studies that did not adjust for blood pressure and/or glucose concentration (or insulin concentration, HbA1c). In addition, we also conducted meta-regression and subgroup analyses to compare the RRs for studies that were conducted in different geographic areas. For CVD, area did not contribute to heterogeneity. (P value = 0.15).
Table 3 shows the individual RRs for cardiovascular outcomes according to categories of LDL cholesterol levels. Five studies which reported on ≥3 categories of LDL cholesterol level were included. Among these, only one study[8] reported on both the number of cases and the total number of cases for each category subgroup; thus it was not possible to determine the dose-response relationship between LDL cholesterol level and the risk of cardiovascular outcomes due to the weight calculation. Based on the five studies, three showed no significant association between LDL cholesterol levels and CVD risk,[8, 30, 32] while a positive association was demonstrated with in the other two studies.[28, 31]
Table 3.
Hazard ratios for cardiovascular outcomes according to low-density lipoprotein cholesterol by different categories*
| Cardiovascular outcomes | Categories | Categories (medians) | Number of events/number of participants | Hazard ratios (95% CI) | P Value for trend | |
|---|---|---|---|---|---|---|
| Turner et al., 1998 [28] | CHD | <3.02 mmol/L | 1 | |||
| 3.02–3.89 mmol/L | 1.41 (1.00–2.00) | |||||
| ≥3.89 mmol/L | 2.11 (1.50–2.95) | |||||
| Lu et al., 2003 [8] | CHD | <91 mg/dl | 57/728 | 1 | ||
| 91–115 mg/dl | 72/683 | 1.29 (0.90–1.85) | ||||
| >115 mg/dl | 103/697 | 1.90 (1.35–2.67) | ||||
| MI | <91 mg/dl | 23/728 | 1 | |||
| 91–115 mg/dl | 27/683 | 1.28 (0.71–2.30) | ||||
| >115 mg/dl | 41/697 | 1.96 (1.14–3.37) | ||||
| Stroke | <91 mg/dl | 30/728 | 1 | |||
| 91–115 mg/dl | 21/683 | 0.66 (0.36–1.19) | ||||
| >115 mg/dl | 37/697 | 1.03 (0.61–1.75) | ||||
| CVD | <91 mg/dl | 146/728 | 1 | |||
| 91–115 mg/dl | 172/683 | 1.28 (1.02–1.62) | ||||
| >115 mg/dl | 203/697 | 1.61 (1.29–2.02) | ||||
| Jiang et al., 2004 [30] | CVD | 11.5–102 | 83.4 | 21/ | 1 | 0.03 |
| 103–126 | 116 | 17/ | 0.84 (0.44–1.59) | |||
| 127–148 | 138 | 31/ | 1.47 (0.85–2.56) | |||
| 149–260 | 166 | 34/ | 1.63 (0.94–2.81) | |||
| Schulze et al., 2004 [31] | CHD | 2.54 | 1 | 0.016 | ||
| 3.31 | 1.34 (0.77–2.35) | |||||
| 3.87 | 1.11 (0.63–1.98) | |||||
| 4.65 | 1.93 (1.15–3.22) | |||||
| Bruno et al., 2006 [32] | CVD mortality | 82/ | 1 | 0.2 | ||
| 84/ | 0.90 (0.63–1.28) | |||||
| 79/ | 0.91 (0.63–1.29) | |||||
| 70/ | 0.77 (0.53–1.12) |
CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure; CI, confidence interval.
Discussion
The meta-analysis of data for 16 prospective studies provides evidence that increased LDL cholesterol level was associated with increased risks of cardiovascular outcomes among patients with type 2 diabetes. Herein, we report that the risk of incident CVD increased 30% and the risk of CVD mortality increased 50% along with per 1 mmol/L increase in LDL cholesterol.
Our finding that an increased LDL cholesterol level is associated with an increased risk of cardiovascular outcome is consistent with previous studies in general populations.[19, 20] This effect has been shown to be independent of other cardiovascular risk factors. The Merging Risk Factors Collaboration Group evaluated 68 prospective studies and concluded that per 1 SD increase in LDL cholesterol (approximately 0.85 mmol/L) was associated with a 38% increase in CHD risk among a general population after controlling for potential confounders.[20] These reported effect sizes are similar to those estimated in our present study for CVD risk among type 2 diabetic patients, suggesting that the predictive power of LDL cholesterol in non-diabetic subjects is similar to that in individuals with type 2 diabetes. A more recent meta-analysis that included 12 studies of the general population and which aimed to compare the predictive power of LDL cholesterol, non-high-density lipoprotein (HDL) cholesterol and apolipoprotein B, reported a standardized RR ratio of 1.25 (95% CI 1.18–1.33) for cardiovascular risk associated with 1 SD increased LDL cholesterol.[19] As the investigators employed a different parameter to estimate the risk in the meta-analysis,[19] it is not possible to compare the effect size of that study with our finding. Our estimates of risk from prospective studies are also in agreement with the results from the randomized controlled trials (RCTs). [13, 14] A meta-analysis of 14 RCTs showed a one mmol/L reduction in LDL cholesterol resulted in a 12% reduction in CHD mortality, 13% in all-vascular mortality, and 21% reduction in major vascular events in individuals with diabetes.[14] Another meta-analysis of 12 RCTs reported similar results for a 21% reduction for major coronary events in patients with type 2 diabetes.[13] Both studies[13, 14] estimated the number of diabetic patients who could benefit from lipid-lowering treatment: 27 fewer developing major coronary events over 4.5 years [13] and 42 fewer developing major vascular events per 1000 diabetics over 5 years[14], respectively.
Although the mechanisms underlying the association between LDL cholesterol and the risk of CVD outcomes are incompletely understood, several mechanisms have been proposed.[39, 40] It has been shown that exposure to high LDL levels decreased nitric oxide bioavailability. Nitric oxide is one of several molecules by which endothelium maintain the balance between thrombosis and fibrinolysis, regulating the recruitment of inflammatory cells into the vascular wall. Intimal LDL is regarded as the triggering factor of atherosclerosis because of its effect on endothelial dysfunction.[39, 41] Additionally, LDL particles, especially the modified forms, can precipitate atherosclerotic lesions by affecting the vascular endothelium and directly favoring the entry of monocytes into the vascular wall via a process that may be mediated by CD11 and the protein kinase C pathway.[42] In addition, atherogenic concentrations of LDL contribute to the vulnerability of advanced-staged plaque formation by reducing the migratory capacity of human vascular smooth muscle cells which express a variety of receptors for cholesterol uptake, resulting in the early accumulation of lipids within the plaque.[39, 40, 43, 44] In patients with type 2 diabetes, increased plasma LDL, as a result of reduced turn-over of the LDL particles, would promote cholesterol deposition in the arterial wall.[40, 45] Increased triglyceride-rich lipoprotein level, as observed in type 2 diabetes, promotes the transfer of triglycerides to LDL leading to the formation of small dense triglyceride-rich LDL particles and this is associated with increased cardiovascular risk.[40, 46] Additionally, increased LDL oxidation, as observed in type 2 diabetes, results in rapid uptake of LDL by macrophages and this amplifies the inflammatory atherosclerotic response.[40]
Although extensive evidence from animal studies, clinical trials, and epidemiological studies has confirmed the causal role of LDL cholesterol in atherosclerosis, LDL cholesterol was previously reported to be a predictor of CVD outcomes in patients with diabetes in some,[27, 28, 31] but not all studies. [8, 26, 30, 32] The non-standardized LDL cholesterol measurement may account in part for this discrepancy. In some studies, LDL cholesterol was directly measured,[8, 26, 28–31] while LDL was calculated in others studies.[22, 25, 27, 32–38] The LDL cholesterol direct measurement requires fasting. Previously, LDL cholesterol has been calculated by subtraction HDL cholesterol from the bottom fraction of ultra-centrifuged serum.[25] More recently, LDL cholesterol level is usually calculated using the Friedewald formula based on the measurement of total cholesterol, HDL cholesterol, and triglycerides.[22, 27, 32–38] However, the Friedewald formula requires a fasting triglyceride level <400 mg/dl in order to accurately calculate LDL cholesterol. The calculated LDL cholesterol level is likely to be unreliable because the dyslipidemia in type 2 diabetic patients is characterized by elevated triglyceride and decreased HDL cholesterol.[30, 47] Therefore, other lipid or apolipoprotein measurements or calculated ratios, such as non-HDL cholesterol, apolipoprotein B, total cholesterol/HDL cholesterol, and triglyceride/HDL cholesterol, have been proposed to be used for CVD risk prediction besides LDL cholesterol. Among them, non-HDL cholesterol, which provides the total cholesterol content of LDL cholesterol, intermediate-density lipoprotein, and very-low-density lipoprotein, has been recognized as the secondary target of cholesterol-lowering therapy in individuals with type 2 diabetes.[15] It has been also reported that apolipoprotein B, which reflects the total particle number in LDL cholesterol, intermediate-density lipoprotein, and very-low-density lipoprotein, improves the prediction of CVD outcomes.[32, 48] Besides, the Emerging Risk Factor Collaboration group concluded that the measurements of either total and HDL cholesterol levels or apolipoproteins is enough for lipid assessment in vascular disease.[20] This study was conducted on participants without initial vascular disease, the conclusion of which may not be able to be generalized to people with type 2 diabetes. Although these lipid variables for CVD in the general population have been extensively studied,[19, 20] no meta-analysis so far has compared the predictive power of these variables on CVD outcomes in diabetic patients due to lack of data. This topic certainly deserves further investigations.
The strengths of the meta-analysis presented here include the following: we included large studies with a correspondingly high number of incident cases, which improved the statistical power to detect significant differences. Our study was based on a comprehensive literature search. We assumed that the inclusion of large studies with follow-ups over 8 years, such as the Strong Heart Study,[8] the Japan Diabetes Complications Study,[38] and the Casale Monferrato Study,[32] and larger cohort study conducted by Eliasson et al.[36] in Sweden, could potentially make our analysis more reliable. There are several limitations to our review. All studies were observational in nature and residual confounding cannot be totally ruled out. The analysis was based on a single measurement of LDL cholesterol. Due to the lacking data, the dose-response relationships between LDL cholesterol and CVD events and mortality could not be estimated in the current analysis. We were also unable to examine cardiovascular outcomes separately; we used two combined outcomes: incident CVD and CVD mortality. Finally, as with any systematic literature review, a limitation is the potential of publication bias; as going against bias is the finding that estimates in our current study are similar to estimates of previous studies.[13, 14, 19, 20]
In conclusion, our results suggest that increased circulating LDL cholesterol is associated with increased risk for cardiovascular outcomes among patients with type 2 diabetes and independent of other conventional risk factors. Our finding supports the notion that patients with diabetes and with elevated LDL cholesterol should be closely followed due to their higher risks for cardiovascular events. Additionally, our data emphasizes the importance of lowering LDL cholesterol to a target goal in patients with diabetes.
Acknowledgments
This work was supported by Louisiana State University’s Improving Clinical Outcomes Network (LSU ICON). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors have no relevant financial interest to declare.
References
- 1.Amer Diabet A. Standards of Medical Care in Diabetes-2013. Diabetes Care. 2013;36:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, Nichols G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 6.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48:937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 7.Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes. Am J Public Health. 1991;81:1158–1162. doi: 10.2105/ajph.81.9.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu WQ, Resnick HE, Jablonski KA, Jones KL, Jain AK, Howard WJ, Robbins DC, Howard BV. Non-HDL cholesterol as a predictor of cardiovascular disease in type 2 diabetes - The Strong Heart Study. Diabetes Care. 2003;26:16–23. doi: 10.2337/diacare.26.1.16. [DOI] [PubMed] [Google Scholar]
- 9.Pyorala K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes/metabolism reviews. 1987;3:463–524. doi: 10.1002/dmr.5610030206. [DOI] [PubMed] [Google Scholar]
- 10.Nasri H, Yazdani M. The relationship between serum LDL-cholesterol, HDL-cholesterol and systolic blood pressure in patients with type 2 diabetes. Kardiologia polska. 2006;64:1364–1368. discussion 1369–1371. [PubMed] [Google Scholar]
- 11.Ali ZA, Al-Zaidi MS. The association between body mass index, lipid profile and serum estradiol levels in a sample of iraqi diabetic premenopausal women. Oman medical journal. 2011;26:263–266. doi: 10.5001/omj.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Z, Liu Y, Huang H. Association of glycosylated hemoglobin level with lipid ratio and individual lipids in type 2 diabetic patients. Asian Pacific journal of tropical medicine. 2012;5:469–471. doi: 10.1016/S1995-7645(12)60080-7. [DOI] [PubMed] [Google Scholar]
- 13.Costa J, Borges M, David C, Vaz Carneiro A. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ. 2006;332:1115–1124. doi: 10.1136/bmj.38793.468449.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 15.E. Expert Panel on Detection, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Scholte op Reimer W, Weissberg P, Wood D, Yarnell J, Zamorano JL, Walma E, Fitzgerald T, Cooney MT, Dudina A. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts) Eur Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 17.Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jonsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyorala K, Raz I, Schernthaner G, Volpe M, Wood D. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 18.Hannedouche T, Albouze G, Chauveau P, Lacour B, Jungers P. Effects of blood pressure and antihypertensive treatment on progression of advanced chronic renal failure. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1993;21:131–137. doi: 10.1016/0272-6386(93)70104-7. [DOI] [PubMed] [Google Scholar]
- 19.Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, Furberg CD. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circulation Cardiovascular quality and outcomes. 2011;4:337–345. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 20.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 22.Ting RZW, Yang X, Yu LWL, Luk AOY, Kong APS, Tong PCY, So WY, Chan JCN, Ma RCW. Lipid control and use of lipid-regulating drugs for prevention of cardiovascular events in Chinese type 2 diabetic patients: a prospective cohort study. Cardiovascular Diabetology. 2010;9:77. doi: 10.1186/1475-2840-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 24.Begg CB. A measure to aid in the interpretation of published clinical trials. Stat Med. 1985;4:1–9. doi: 10.1002/sim.4780040103. [DOI] [PubMed] [Google Scholar]
- 25.Laakso M, Lehto S, Penttila I, Pyorala K. Lipids and lipoproteins predicting coronary heart disease mortality and morbidity in patients with non-insulin-dependent diabetes. Circulation. 1993;88:1421–1430. doi: 10.1161/01.cir.88.4.1421. [DOI] [PubMed] [Google Scholar]
- 26.Niskanen LK, Penttila I, Parviainen M, Uusitupa MIJ. Evolution, risk factors, and prognostic implications of albuminuria in NIDDM. Diabetes Care. 1996;19:486–493. doi: 10.2337/diacare.19.5.486. [DOI] [PubMed] [Google Scholar]
- 27.Lehto S, Ronnemaa T, Haffner SM, Pyorala K, Kallio V, Laakso M. Dyslipidemia and hyperglycemia predict coronary heart disease events in middle-aged patients with NIDDM. Diabetes. 1997;46:1354–1359. doi: 10.2337/diab.46.8.1354. [DOI] [PubMed] [Google Scholar]
- 28.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattock MB, Barnes DJ, Viberti G, Keen H, Burt D, Hughes JM, Fitzgerald AP, Sandhu B, Jackson PG. Microalbuminuria and coronary heart disease in NIDDM - An incidence study. Diabetes. 1998;47:1786–1792. doi: 10.2337/diabetes.47.11.1786. [DOI] [PubMed] [Google Scholar]
- 30.Jiang R, Schulze MB, Li TC, Rifal N, Stampfer MJ, Rimm EB, Hu FB. Non-HDL cholesterol and apolipoprotein B predict cardiovascular disease events among men with type 2 diabetes. Diabetes Care. 2004;27:1991–1997. doi: 10.2337/diacare.27.8.1991. [DOI] [PubMed] [Google Scholar]
- 31.Schulze MB, Shai I, Manson JE, Li T, Rifai N, Jiang R, Hu FB. Joint role of non-HDL cholesterol and glycated haemoglobin in predicting future coronary heart disease events among women with type 2 diabetes. Diabetologia. 2004;47:2129–2136. doi: 10.1007/s00125-004-1593-2. [DOI] [PubMed] [Google Scholar]
- 32.Bruno G, Merletti F, Biggeri A, Bargero G, Prina-Cerai S, Pagano G, Cavallo-Perin P. Effect of age on the association of non-high-density-lipoprotein cholesterol and apolipoprotein B with cardiovascular mortality in a Mediterranean population with type 2 diabetes: the Casale Monferrato study. Diabetologia. 2006;49:937–944. doi: 10.1007/s00125-006-0195-6. [DOI] [PubMed] [Google Scholar]
- 33.Yang X, Ma RC, So WY, Kong AP, Ko GT, Ho CS, Lam CW, Cockram CS, Tong PC, Chan JC. Development and validation of a risk score for hospitalization for heart failure in patients with Type 2 diabetes mellitus. Cardiovasc Diabetol. 2008;7:9. doi: 10.1186/1475-2840-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Hateren KJJ, Landman GWD, Kleefstra N, Logtenberg SJJ, Groenier KH, Kamper AM, Houweling ST, Bilo HJG. The Lipid Profile and Mortality Risk in Elderly Type 2 Diabetic Patients: A Ten-Year Follow-Up Study (ZODIAC-13) PLoS One. 2009;4:e8464. doi: 10.1371/journal.pone.0008464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tohidi M, Hatami M, Hadaegh F, Safarkhani M, Harati H, Azizi F. Lipid measures for prediction of incident cardiovascular disease in diabetic and nondiabetic adults: results of the 8.6 years follow-up of a population based cohort study. Lipids in Health and Disease. 2010;9:6. doi: 10.1186/1476-511X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eliasson B, Cederholm J, Eeg-Olofsson K, Svensson A-M, Zethelius B, Gudbjornsdottir S, Natl Diabet R. Clinical Usefulness of Different Lipid Measures for Prediction of Coronary Heart Disease in Type 2 Diabetes A report from the Swedish National Diabetes Register. Diabetes Care. 2011;34:2095–2100. doi: 10.2337/dc11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sone H, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S, Ishibashi S, Katayama S, Ohashi Y, Akanuma Y, Yamada N. Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS) The Journal of clinical endocrinology and metabolism. 2011;96:3448–3456. doi: 10.1210/jc.2011-0622. [DOI] [PubMed] [Google Scholar]
- 38.Sone H, Tanaka S, Tanaka S, Iimuro S, Ishibashi S, Oikawa S, Shimano H, Katayama S, Ohashi Y, Akanuma Y, Yamada N G. Japan Diabet Complications Study. Comparison of Various Lipid Variables as Predictors of Coronary Heart Disease in Japanese Men and Women With Type 2 Diabetes Subanalysis of the Japan Diabetes Complications Study. Diabetes Care. 2012;35:1150–1157. doi: 10.2337/dc11-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badimon L, Vilahur G. LDL-cholesterol versus HDL-cholesterol in the atherosclerotic plaque: inflammatory resolution versus thrombotic chaos. Annals of the New York Academy of Sciences. 2012;1254:18–32. doi: 10.1111/j.1749-6632.2012.06480.x. [DOI] [PubMed] [Google Scholar]
- 40.Verges B. Lipid modification in type 2 diabetes: the role of LDL and HDL. Fundamental & clinical pharmacology. 2009;23:681–685. doi: 10.1111/j.1472-8206.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 41.Vidal F, Colome C, Martinez-Gonzalez J, Badimon L. Atherogenic concentrations of native low-density lipoproteins down-regulate nitric-oxide-synthase mRNA and protein levels in endothelial cells. European Journal of Biochemistry. 1998;252:378–384. doi: 10.1046/j.1432-1327.1998.2520378.x. [DOI] [PubMed] [Google Scholar]
- 42.Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thrombosis and haemostasis. 2011;105(Suppl 1):S34–42. doi: 10.1160/THS10-11-0717. [DOI] [PubMed] [Google Scholar]
- 43.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padro T, Pena E, Garcia-Arguinzonis M, Llorente-Cortes V, Badimon L. Low-density lipoproteins impair migration of human coronary vascular smooth muscle cells and induce changes in the proteomic profile of myosin light chain. Cardiovascular research. 2008;77:211–220. doi: 10.1093/cvr/cvm045. [DOI] [PubMed] [Google Scholar]
- 45.Duvillard L, Florentin E, Lizard G, Petit JM, Galland F, Monier S, Gambert P, Verges B. Cell surface expression of LDL receptor is decreased in type 2 diabetic patients and is normalized by insulin therapy. Diabetes Care. 2003;26:1540–1544. doi: 10.2337/diacare.26.5.1540. [DOI] [PubMed] [Google Scholar]
- 46.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- 47.Garg A, Grundy SM. Management of dyslipidemia in NIDDM. Diabetes Care. 1990;13:153–169. doi: 10.2337/diacare.13.2.153. [DOI] [PubMed] [Google Scholar]
- 48.Sniderman AD. How, when, and why to use apolipoprotein B in clinical practice. Am J Cardiol. 2002;90:48i–54i. doi: 10.1016/s0002-9149(02)02633-4. [DOI] [PubMed] [Google Scholar]