Abstract
The association of estimated GFR with cardiovascular diseases risk among type 2 diabetes patients was unclear. We prospectively investigated the race-specific association of estimated GFR with the risk of coronary heart disease and stroke among 11 940 White and 16 451 African American patients. During mean follow up of 6.1–6.8 years, 6 647 coronary heart disease and 2 750 stroke incident cases were identified. Age- and sex-adjusted hazard ratios of coronary heart disease associated with baseline estimated GFR (≥90, 75–89, 60–74, 30–59, and 15–29 mL/min/1.73 m2) were 1.00, 1.04 (95% CI 0.95–1.14), 1.13 (1.02–1.26), 1.37 (1.22–1.53), and 2.07 (1.58–2.71) (Ptrend<0.001) for African Americans, and 1.00, 1.09 (0.99–1.19), 1.10 (0.99–1.21), 1.31 (1.18–1.46), and 2.18 (1.66–2.85) (Ptrend<0.001) for whites, respectively. Significantly increased stroke risk was observed among both African American and white participants with estimated GFR<60 mL/min/1.73 m2. When using the updated mean values of estimated GFR, these significant associations became stronger. Participants with mildly decreased estimated GFR (60–89 mL/min/1.73 m2) during follow-up were also at significantly higher risk of coronary heart disease and stroke. The present study demonstrated that even mildly reduced estimated GFR at baseline (<75 mL/min/1.73 m2) and during follow-up (<90 mL/min/1.73 m2) increased risk of incident coronary heart disease and stroke among both African American and white type 2 diabetes patients.
Keywords: estimated glomerular filtration rate, coronary heart disease, stroke, type 2 diabetes
Chronic kidney disease (CKD) and diabetes independently increase cardiovascular disease (CVD) risk1, 2. Approximately 40% of patients with diabetes develop CKD, manifested as albuminuria, impaired estimated glomerular filtration rate (eGFR), or both2, 3. Compared with people without diabetes, those with diabetes are already at high risk for CVD4, and the additional development of diabetic kidney disease greatly increases their risk for CVD3, 5. Studies have found a significant association between severity of CKD (assessed by eGFR) and CVD risk among the general population6–9 and among multiple high risk patient populations, with existing CVD, heart failure, diabetes, and hypertension1, 5, 10, 11. However, most studies only provided a single value of eGFR which may produce potential bias in understanding the magnitude of the association of CKD with incident CVD. Moreover, few studies have addressed the race-specific association of kidney function with the risk of coronary heart disease (CHD) and stroke among diabetic patients although there are significant racial differences in prevalence of diabetes in the general population and in the prevalence of end-stage renal disease among diabetic patients12, 13. The present study aims to assess the race-specific association of kidney function with the risk of CHD and stroke among type 2 diabetes patients within the Louisiana State University Hospital-Based Longitudinal Study.
RESULTS
General characteristics of the study population at baseline are presented by race and eGFR categories in online Table 1. Both African American type 2 diabetes patients and white type 2 diabetes patients who had eGFR ≤60 mL/min/1.73m2 were generally older, and had higher triglycerides, higher portion of cholesterol lowering medication use, and higher portion of anti-hypertension medication use, when compared with those who had eGFR >60 mL/min/1.73m2. There was no significant interaction of eGFR and sex on the risk of CHD and stroke (All P>0.05). The interaction of eGFR and race were significant on the risk of incident stroke (P<0.001), but not CHD (P=0.383).
Table 1.
Baseline characteristics of African American and white patients with type 2 diabetes*
| Glomerular filtration rate at baseline (mL/min/1.73 m2) | P value | |||||
|---|---|---|---|---|---|---|
| Characteristics | ≥90 | 75–89 | 60–74 | 30–59 | 15–29 | |
| African American | ||||||
| No. of participants | 9 293 | 3 483 | 2 147 | 1 369 | 159 | |
| Age (year) | 47.9 (9.2) | 51.1 (9.5) | 54.8 (9.4) | 57.0 (10.6) | 54.4 (11.6) | <0.001 |
| Income ($/family) | 9 924 (6 960–14 760) | 9 864 (7 128–14 676) | 9 804 (7 236–14 400) | 9 630 (7 230–14 010) | 9 756 (7 440–13 986) | <0.001 |
| Body mass index (kg/m2) | 33.7 (8.6) | 34.4 (8.3) | 33.9 (7.9) | 33.0 (7.8) | 32.6 (8.9) | <0.001 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 145 (24) | 147 (24) | 148 (25) | 150 (27) | 154 (31) | <0.001 |
| Diastolic | 83 (13) | 82 (14) | 81 (14) | 81 (15) | 81 (17) | 0.849 |
| Total cholesterol (mg/dL) | 185 (157–214) | 184 (157–214) | 184 (156–215) | 187 (157–218) | 180 (149–216) | 0.168 |
| High-density lipoprotein cholesterol (mg/dL) | 43 (36–52) | 43 (36–52) | 44 (36–53) | 43 (35–52) | 43 (33–52) | <0.001 |
| Low-density lipoprotein cholesterol (mg/dL) | 111 (87–137) | 112 (88–138) | 110 (87–138) | 111 (87–138) | 106 (78–137) | 0.484 |
| Triglycerides (mg/dL) | 104 (74–153) | 106 (75–153) | 106 (76–156) | 118 (83–170) | 128 (93–180) | <0.001 |
| HbA1c (%) | 8.4 (2.8) | 7.9 (2.6) | 7.8 (2.5) | 7.8 (2.6) | 7.6 (2.3) | <0.001 |
| Albumin (gm/dL) | 3.75 | 3.76 | 3.73 | 3.61 | 3.30 | <0.001 |
| Obesity status (%) | <0.001 | |||||
| Normal weight (<25) | 14.1 | 10.7 | 10.6 | 13.3 | 15.7 | |
| Overweight (25–29.9) | 23.9 | 21.4 | 23.4 | 25.6 | 28.3 | |
| Obesity class I (30.0–34.9) | 23.8 | 26.4 | 26.6 | 25.4 | 26.4 | |
| Obesity class II (≥35.0) | 38.3 | 41.5 | 39.4 | 35.7 | 29.6 | |
| Current smoker (%) | 35.2 | 32.5 | 27.0 | 26.4 | 21.3 | <0.001 |
| Medication use (%) | ||||||
| Blood pressure | 92.2 | 94.5 | 96.3 | 97.2 | 96.8 | <0.001 |
| Diabetes | 87.3 | 83.8 | 84.8 | 82.7 | 80.0 | 0.004 |
| Cholesterol | 67.1 | 71.3 | 73.7 | 79.5 | 74.0 | <0.001 |
| White | ||||||
| No. of participants | 4 661 | 3 029 | 2 505 | 1 624 | 121 | |
| Age (year) | 48.7 (8.9) | 52.4 (10.1) | 54.6 (9.7) | 58.9 (9.8) | 56.8 (10.7) | <0.001 |
| Income ($/family) | 11 520 (7 476–16 380) | 11 568 (7 716–16 584) | 11 694 (7 530–16 050) | 11 436 (8 088–16 356) | 11 646 (8 333–15 636) | <0.001 |
| Body mass index (kg/m2) | 34.9 (9.1) | 35.0 (8.6) | 35.2 (8.6) | 34.7 (8.6) | 34.3 (9.3) | <0.001 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 141 (22) | 141 (21) | 142 (22) | 143 (24) | 142 (26) | 0.749 |
| Diastolic | 79 (12) | 78 (13) | 78 (12) | 75 (14) | 75 (15) | <0.001 |
| Total cholesterol (mg/dL) | 190 (161–222) | 189 (162–222) | 190 (161–221) | 186 (155–219) | 170 (137–205) | 0.025 |
| High-density lipoprotein cholesterol (mg/dL) | 40 (33–48) | 40 (33–48) | 40 (34–48) | 41 (34–48) | 38 (32–45) | <0.001 |
| Low-density lipoprotein cholesterol (mg/dL) | 110 (86–136) | 111 (86–137) | 109 (84–137) | 103 (79–133) | 90 (66–125) | <0.001 |
| Triglycerides (mg/dL) | 151 (102–216) | 153 (104–216) | 155 (107–226) | 159 (111–226) | 167 (115–245) | <0.001 |
| HbA1c (%) | 7.9 (2.3) | 7.2 (2.0) | 7.1 (2.0) | 7.1 (1.9) | 7.3 (1.9) | <0.001 |
| Albumin (gm/dL) | 3.86 | 3.90 | 3.87 | 3.79 | 3.50 | <0.001 |
| Obesity status (%) | <0.001 | |||||
| Normal weight (<25) | 12.0 | 9.1 | 8.5 | 9.9 | 13.2 | |
| Overweight (25–29.9) | 20.3 | 21.2 | 21.1 | 21.4 | 21.5 | |
| Obesity class I (30.0–34.9) | 23.3 | 25.8 | 25.3 | 27.3 | 20.7 | |
| Obesity class II (≥35.0) | 44.3 | 43.8 | 45.1 | 41.5 | 44.6 | |
| Current smoker (%) | 41.8 | 37.8 | 34.0 | 26.1 | 21.7 | <0.001 |
| Medication use (%) | ||||||
| Blood pressure | 90.0 | 91.6 | 93.9 | 96.0 | 96.6 | <0.001 |
| Diabetes | 87.6 | 83.9 | 82.3 | 83.6 | 95.4 | <0.001 |
| Cholesterol | 73.4 | 77.7 | 79.8 | 82. 8 | 85.1 | <0.001 |
Data are mean (SD) or median (interquartile range)
Body mass index was calculated as the weight in kilograms divided by the square of the height in meters.
Values are adjusted for age.
During a mean follow-up of 6.1 years, 6 647 participants developed CHD. For African Americans, The multivariate-adjusted hazard ratios of CHD associated with patients who had baseline eGFR ≥90, 75–89, 60–74, 30–59, and 15–29 mL/min/1.73 m2 were 1.00, 1.01 (95% confidence interval [CI] 0.93–1.11), 1.09 (95% CI 0.98–1.21), 1.26 (95% CI 1.12–1.41), and 1.93 (95% CI 1.47–2.53) (Table 2). A similar association was observed for white type 2 diabetes patients.
Table 2.
Hazard ratios (95% confidence interval) for coronary heart disease and stroke by estimated glomerular filtration rate at baseline among African American and white patients with type 2 diabetes*
| Glomerular filtration rate at baseline (mL/min/1.73 m2) | P for trend | |||||
|---|---|---|---|---|---|---|
| ≥90 | 75–89 | 60–74 | 30–59 | 15–29 | ||
| Coronary heart disease | ||||||
| African Americans | ||||||
| No. of case | 1 699 | 681 | 502 | 399 | 55 | |
| Person-year | 63 047 | 22 128 | 13 535 | 8 287 | 830 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.04 (0.95–1.14) | 1.13 (1.02–1.26) | 1.37 (1.22–1.53) | 2.07 (1.58–2.71) | <0.001 |
| Multivariable adjustment HR (95% CI)† | 1 | 1.01 (0.93–1.11) | 1.09 (0.98–1.21) | 1.26 (1.12–1.41) | 1.93 (1.47–2.53) | <0.001 |
| Whites | ||||||
| No. of case | 1 101 | 835 | 704 | 615 | 56 | |
| Person-year | 27 124 | 16 596 | 13 209 | 8 487 | 498 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.09 (0.99–1.19) | 1.10 (0.99–1.21) | 1.31 (1.18–1.46) | 2.18 (1.66–2.85) | <0.001 |
| Multivariable adjustment HR (95% CI)† | 1 | 1.10 (1.00–1.21) | 1.10 (1.00–1.22) | 1.26 (1.13–1.40) | 1.97 (1.50–2.58) | <0.001 |
| Both | ||||||
| No. of case | 2 800 | 1 516 | 1 206 | 1 014 | 111 | |
| Person-year | 90 171 | 38 724 | 26 745 | 16 774 | 1 327 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.06 (1.00–1.13) | 1.11 (1.03–1.19) | 1.33 (1.23–1.44) | 2.12 (1.75–2.56) | <0.001 |
| Multivariable adjustment HR (95% CI)†‡ | 1 | 1.06 (0.99–1.13) | 1.09 (1.01–1.16) | 1.25 (1.16–1.35) | 1.95 (1.61–2.36) | <0.001 |
| Stroke | ||||||
| African Americans | ||||||
| No. of case | 744 | 306 | 232 | 202 | 25 | |
| Person-year | 68 024 | 24 183 | 15 077 | 9 415 | 1 008 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.03 (0.90–1.18) | 1.11 (0.96–1.30) | 1.44 (1.22–1.69) | 1.78 (1.20–2.66) | <0.001 |
| Multivariable adjustment HR (95% CI)† | 1 | 1.03 (0.90–1.18) | 1.10 (0.94–1.28) | 1.35 (1.15–1.59) | 1.64 (1.10–2.45) | 0.001 |
| Whites | ||||||
| No. of case | 363 | 303 | 277 | 272 | 26 | |
| Person-year | 30 480 | 18 984 | 15 195 | 10 111 | 662 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.10 (0.94–1.28) | 1.16 (0.98–1.36) | 1.41 (1.19–1.68) | 2.36 (1.58–3.53) | <0.001 |
| Multivariable adjustment HR (95% CI)† | 1 | 1.12 (0.96–1.31) | 1.18 (1.00–1.39) | 1.40 (1.18–1.67) | 2.26 (1.50–3.35) | <0.001 |
| Both | ||||||
| No. of case | 1 107 | 609 | 509 | 474 | 51 | |
| Person-year | 98 504 | 43 166 | 30 272 | 19 526 | 1 670 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.06 (0.96–1.17) | 1.13 (1.01–1.26) | 1.43 (1.27–1.61) | 2.02 (1.52–2.68) | <0.001 |
| Multivariable adjustment HR (95% CI)†‡ | 1 | 1.07 (0.96–1.18) | 1.13 (1.01–1.26) | 1.39 (1.24–1.56) | 1.91 (1.44–2.53) | <0.001 |
CI=confidence interval; HR=hazard ratio
Adjusted for age, sex, smoking, income, type of insurance, BMI, systolic blood pressure, HbA1c, LDL cholesterol, use of antihypertensive drugs, use of diabetes medications, and use of cholesterol-lowering agents
Also adjusted for race
A total of 2 750 subjects had first-time stroke diagnoses during a mean follow-up of 6.8 years. Similarly, compared with African American patients with a baseline eGFR ≥90 mL/min/1.73 m2, African American patients with a baseline eGFR <90 mL/min/1.73 m2 experienced higher risk of stroke (1.03 [95% CI 0.90–1.18] for those with eGFR 75–89 mL/min/1.73 m2, 1.10 [0.94–1.28] for those with eGFR 60–74 mL/min/1.73 m2, 1.35 [1.15–1.59] for those with eGFR 30–59 mL/min/1.73m2, and 1.64 [1.10–2.45] for those with eGFR 15–29 mL/min/1.73 m2 when adjusted for multiple factors) (Table 2). The pattern of the association between baseline eGFR and stroke risk in white patients was similar to that found in African American patients.
When white and African Americans were combined, patients who had baseline eGFR between 60 and 74 mL/min/1.73 m2 had a 9% (95% CI 1.01–1.16) increased risk for CHD and a 13% (1.01–1.26) increased risk for stroke compared with patients with a baseline eGFR ≥90 mL/min/1.73 m2. Compared with African American, White race was a risk factor for both CHD and Stroke. The multivariate-adjusted HR were 1.46 (1.39–1.54) for CHD and 1.22 (1.12–1.32) for stroke, respectively. (Table 2)
The pattern of the association between updated mean eGFR and the outcomes (i.e. CHD and stroke) was similar with that of the association between baseline eGFR and the outcomes (Table 3). Of note, higher strength was observed in the association between updated mean measurements and the outcomes compared with the association between baseline measurements and the outcomes. Also, when analyzed using updated mean eGFR, type 2 diabetes patients with even mildly decreased eGFR (60–74 mL/min/1.73 m2) and (75–89 mL/min/1.73 m2) were also at significant risk of incident CHD and stroke (Table 3).
Table 3.
Hazard ratios (95% confidence interval) for coronary heart disease and stroke by estimated glomerular filtration rate during follow-up among African American and white patients with type 2 diabetes*
| Glomerular filtration rate during follow-up (mL/min/1.73 m2) | P for trend | |||||
|---|---|---|---|---|---|---|
| ≥90 | 75–89 | 60–74 | 30–59 | <30 | ||
| Coronary heart disease | ||||||
| African Americans | ||||||
| No. of case | 1 371 | 726 | 534 | 565 | 140 | |
| Person-year | 59 861 | 21 935 | 13 077 | 10 944 | 2 011 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.30 (1.18–1.42) | 1.51 (1.36–1.67) | 1.85 (1.67–2.05) | 2.70 (2.27–3.22) | <0.001 |
| Multivariable adjustment HR (95% CI)† | 1 | 1.24 (1.13–1.36) | 1.39 (1.25–1.54) | 1.63 (1.46–1.80) | 2.53 (2.12–3.02) | <0.001 |
| Whites | ||||||
| No. of case | 862 | 805 | 757 | 781 | 106 | |
| Person-year | 25 031 | 16 690 | 13 520 | 9 641 | 1 032 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.25 (1.14–1.38) | 1.35 (1.22–1.50) | 1.83 (1.65–2.03) | 2.50 (2.04–3.06) | <0.001 |
| Multivariable adjustment HR (95% CI)† | 1 | 1.20 (1.08–1.32) | 1.29 (1.16–1.42) | 1.65 (1.49–1.84) | 2.15 (1.75–2.64) | <0.001 |
| Both | ||||||
| No. of case | 2 233 | 1 531 | 1 291 | 1 346 | 246 | |
| Person-year | 84 892 | 38 625 | 26 597 | 20 585 | 3 042 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.27 (1.19–1.36) | 1.42 (1.32–1.52) | 1.84 (1.72–1.98) | 2.61 (2.28–2.98) | <0.001 |
| Multivariable adjustment HR (95% CI)†‡ | 1 | 1.22 (1.14–1.30) | 1.33 (1.24–1.43) | 1.64 (1.53–1.77) | 2.35 (2.06–2.69) | <0.001 |
| Stroke | ||||||
| African Americans | ||||||
| No. of case | 597 | 325 | 325 | 278 | 67 | |
| Person-year | 63 717 | 24 309 | 14 826 | 12 469 | 2 387 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.23 (1.07–1.41) | 1.38 (1.18–1.61) | 1.82 (1.56–2.11) | 2.56 (1.99–3.31) | <0.001 |
| Multivariable adjustment HR (95% CI)† | 1 | 1.19 (1.04–1.37) | 1.30 (1.11–1.52) | 1.61 (1.39–1.88) | 2.16 (1.67–2.80) | <0.001 |
| Whites | ||||||
| No. of case | 258 | 290 | 322 | 321 | 50 | |
| Person-year | 27 414 | 19 073 | 15 810 | 11 871 | 1 263 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.34 (1.13–1.58) | 1.59 (1.34–1.89) | 1.87 (1.57–2.23) | 3.06 (2.25–4.16) | <0.001 |
| Multivariable adjustment HR (95% CI)† | 1 | 1.31 (1.10–1.55) | 1.55 (1.30–1.84) | 1.79 (1.50–2.14) | 2.73 (2.00–3.72) | <0.001 |
| Both | ||||||
| No. of case | 855 | 615 | 564 | 599 | 117 | |
| Person-year | 91 131 | 43 382 | 30 636 | 24 340 | 3 650 | |
| Age and sex adjusted HR (95% CI) | 1 | 1.26 (1.13–1.40) | 1.47 (1.31–1.64) | 1.83 (1.63–2.05) | 2.75 (2.26–3.34) | <0.001 |
| Multivariable adjustment HR (95% CI)†‡ | 1 | 1.22 (1.10–1.36) | 1.40 (1.25–1.56) | 1.68 (1.50–1.88) | 2.39 (1.97–2.91) | <0.001 |
CI=confidence interval; HR=hazard ratio
Adjusted for age, sex, smoking, income, type of insurance, BMI, systolic blood pressure, HbA1c, LDL cholesterol, use of antihypertensive drugs, use of diabetes medications, and use of cholesterol-lowering agents
Also adjusted for race
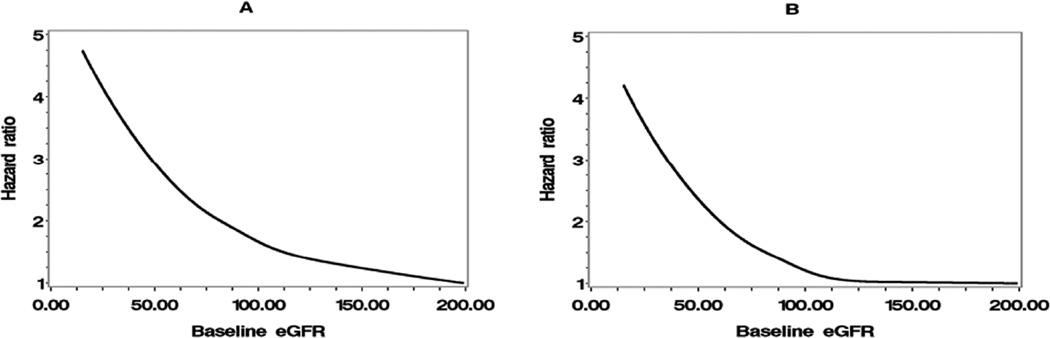
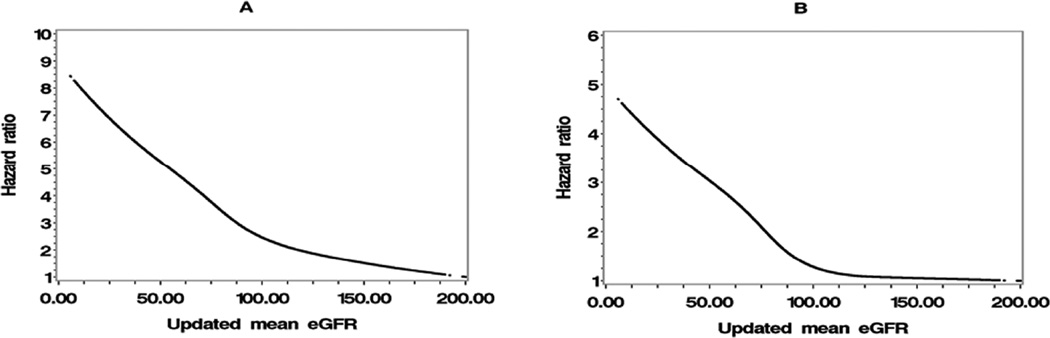
The hazard ratio curves of baseline eGFR are shown in Figure 1, and the hazard ratio curves of updated mean eGFR are shown in Figure 2. Both figures show that, when eGFR <90 mL/min/1.73 m2, incident CHD and stroke risk sharply increased as eGFR decreased.
Figure 1.
Hazard ratios for incident (A) coronary heart disease and (B) stroke by baseline eGFR.
eGFR: estimated glomerular filtration rate
Figure 2.
Hazard ratios for incident (A) coronary heart disease and (B) stroke by updated mean eGFR
eGFR: estimated glomerular filtration rate
DISCUSSION
The present study demonstrated that reduced eGFR (eGFR<60 mL/min/1.73 m2) at baseline and during follow-up were associated with increased risk of incident CHD and stroke among type 2 diabetes patients. In addition, type 2 diabetes patients with even mildly decreased eGFR (60–75 mL/min/1.73 m2) at baseline and (60–89 mL/min/1.73 m2) during follow-up were also associated with an increased risk of for incident CHD and stroke.
Although several studies have investigated the association between eGFR and CVD risk, most of these studies have been limited to apparently healthy individuals or individuals with preexisting CVD or at high risk for CVD6–10. The overall epidemiological evidence suggests that individuals with preexisting CVD or at high risk for CVD who had eGFR<60 ml/min/1.73 m2 were at increased risk of CVD outcomes10. However, in the general population, the associations of eGFR and with fatal and non-fatal CVD are inconsistent. Although there are significant associations between eGFR and CVD mortality6, 9, 14, some8, 12 but not all studies6, 9 have found a significant inverse association between eGFR and the risk of incident CVD8, 12. In addition, limited data exist regarding the association of kidney function with CVD risk among patients with type 2 diabetes1, 5, 11. This risk association may be particularly of interest because, in patients with type 2 diabetes, the additional development of diabetic kidney disease would markedly amplify their risk for CVD3, 5. In the current study, we found that incident CHD and stroke risk increased at eGFR <60 mL/min/1.73 m2, when compared with eGFR ≥90 mL/min/1.73 m2 at baseline, which are consistent with the results from two other cohorts5, 11. In addition, we found an increased risk of CHD and stroke even in subjects with mildly decreased baseline eGFR (60–74 ml/min/1.73 m2) and updated mean eGFR (60–89 ml/min/1.73 m2) during follow-up. This result may better reflect the magnitude of the association between kidney function and the risk of incident CVD because kidney function may change over time potentially diluting their association with subsequent events5.
The current study, in which the participants have the same health care access and similar socioeconomic status, provides a unique opportunity to investigate racial differences in the association between eGFR and incident CVD. In our study, there was a higher percentage of white type 2 diabetes patients who had eGFR <60 mL/min/1.73 m2 than African American type 2 diabetes patients. This result is different from the result from the Young Adults study that 20-year CKD incidence was higher among African Americans than whites15. However, our result is in line with the results from two other studies12, 16. Our study is the only one that investigated the racial differences in the association between eGFR and incidence CHD and stroke in patients with type 2 diabetes. We found significant race interaction in the associations of eGFR with the risk of stroke, but not in the association of eGFR with CHD risk. The incident stroke risk associated with reduced eGFR was higher in whites than in African Americans. In the pooled analysis performed by Weiner et al,12 the risk of the composite study outcome (a composite of myocardial infarction, fatal CHD, stroke, and death) associated with CKD, defined as a eGFR between 15 and 60 ml/min per 1.72 m2, was higher in African American than in white healthy community-based population. Unlike participants in the current study, participants from different races in Weiner et al’ s study12 were likely to experience socioeconomic disparities in access and utilization of health care. Except for these two reasons, the authors noted that differences in the four studies involved in their study may also contribute to the racial differences in the association12.
Although the mechanism behind the association between impaired kidney function and the risk of incident CVD is not completely understood, several mechanisms have been proposed. Traditional CVD risk factors, such as older age, smoking, hypertension, and dyslipidemia, often coexist with CKD1, 2, 17 and the positive association of these factors with incident CVD were well documented18–20. Elevated asymmetric dimethyl arginine, reduced nitric oxide bioavailability, and endothelial dysfunction in renal disease, which are associated with atherosclerosis, are also recognized as factors linking impaired kidney function and the risk of incident CVD21, 22. Furthermore, inflammatory markers such as C-reactive protein, fibrinogen, interleukin-6, tumor necrosis factor α, factor VIIc, factor VIIIc, plasmin-antiplasmin complex, D-dimer, and the adhesion molecules E-selection, VCAM-1 and ICAM-1 are often elevated, and the activation of renin-angiotensin system, which may in part depend on the adaptation to loss of renal mass that results in changes in renal hemodynamics, frequently occurs in CKD. These factors may alter the progression of atherosclerosis through their contribution to the production of reactive oxygen species21, 23. Besides, increased promoters of calcification and reduced inhibitors of calcification may partly responsible for the linkage between kidney dysfunction and CVD risk21, 24. As to eGFR, factors including activation of the renin-angiotensin system, anemia, elevated asymmetric dimethyl arginine and hyperhomocysteinemia could represent a link between reduced eGFR and increased CVD risk22, 23, 25, 26.
There are several strengths in our study, including the large sample size, high proportion of African Americans, long follow-up time, and the use of administrative databases to avoid the problem of differential recall bias. In addition, participants in this study use the same public health care system and have the same socioeconomic status, which minimizes the influence from the accessibility of health care, particularly when comparing African Americans and whites. Moreover, updated mean values of eGFR during follow-up were used to predict the outcomes in this analysis, which can avoid potential bias from a single baseline measurement. One limitation of our study is that our analysis was not performed on a representative sample of the state of Louisiana’s population which limits the generalizability of this study; however, Louisiana State University Health Care Services Division hospitals are public hospitals and cover over 1.6 million patients most of which were low income persons in Louisiana. Second, even though our analyses were adjusted for an extensive set of confounding factors, residual confounding due to the measurement error in the assessment of confounding factors, unmeasured factors such as physical activity, education, dietary factors, and family history of diabetes, CVD and other chronic diseases cannot be excluded. Third, ascertainments of CHD and stroke status were based on hospital discharge register. Although we haven’t finished a validation study of CHD and stroke, the method of using the hospital discharge register to diagnose the major non-fatal diseases has been widely used in American cohort studies, such as the Kaiser Permanente Medical Care Program13, 27, the Framingham Offspring Study28, etc. The validity of the diagnoses of myocardial infarction and stroke by using hospital discharge register in these cohort studies is high13.
In conclusion, we found that reduced eGFR (<75 mL/min/1.73 m2 at baseline, <90 mL/min/1.73 m2 during follow-up) were associated with increased risk of incident CHD and stroke among both African American and white patients with type 2 diabetes. Our results indicate that the updated mean eGFR which can identify CVD risk at an earlier stage may be a more important predictor of incident CVD although a single baseline eGFR was predictive of CVD outcomes.
METHODS
Study Population
Louisiana State University Health Care Services Division operates seven public hospitals and affiliated clinics in Louisiana, which provide quality medical care to the residents of Louisiana regardless of their income or insurance coverage29–35. Overall, Louisiana State University Health Care Services Division facilities have served about 1.6 million patients (35% of the Louisiana population) since 1997. Administrative (name, address, date of birth, gender, race/ethnicity, types of insurance, family income, and smoking status), anthropometric (date of examination, measurements of body weight, height, and blood pressure for each visit), laboratory (test code, test collection date, test result values, and abnormal flag), clinical diagnosis, and medication data collected at these facilities are available in electronic form for both inpatients and outpatients from 1997. Using these data, we have established the Louisiana State University Hospital-Based Longitudinal Study. A cohort of diabetic patients was set up by using the ICD-9 (250) through the Louisiana State University Hospital-Based Longitudinal Study database between January 1, 1999, and December 31, 2009. Both inpatients and outpatients were included and all patients were under primary care. Louisiana State University Health Care Services Division’s internal diabetes disease management guidelines call for physician confirmation of diabetes diagnoses by applying the American Diabetes Association criteria: a fasting plasma glucose level ≥126 mg/ dl; 2-hour glucose level ≥200 mg/dl after a 75-g 2-hour oral glucose tolerance test; one or more classic symptoms plus a random plasma glucose level ≥200 mg/dl36. We have validated the diabetes diagnosis in Louisiana State University Health Care Services Division hospitals. The agreement of diabetes diagnosis was 97%: 20,919 of a sample of 21,566 hospital discharge diagnoses based on ICD codes also had physician-confirmed diabetes by using the American Diabetes Association diabetes diagnosis criteria36. The first record of diabetes diagnosis was used to establish the baseline for each patient in the present analyses due to the design of the cohort study. These newly diagnosed diabetes participants had used Louisiana State University Health Care Services Division hospitals for median 2.8 (interquartile ranges 1.8–6.1) years prior to the baseline33.
After excluding subjects with a history of CHD and stroke at baseline, patients with incomplete data on any required variables, and those with eGFR<15 mL/min/1.73 m2 at baseline, the present analyses included 4,452 non-Hispanic white men, 7,488 non-Hispanic white women, 5,629 African American men and 10,822 African American women. In these type 2 diabetes patients, about 79.0% of patients qualify for free care (by virtue of being low income and uninsured – any individual or family unit whose income is at or below 200% of Federal Poverty Level), about 5.0% of patients are self-pay (uninsured, but incomes not low enough to qualify for free care), about 5.0% of patients are covered by Medicaid, about 8.8% of patients have Medicare, and about 2.2% of patients are covered by commercial insurance. The study and analysis plan were approved by both the Pennington Biomedical Research Center and Louisiana State University Health Sciences Center Institutional Review Boards, LSU System. We did not obtain informed consent from participants involved in our study because we used anonymized data compiled from electronic medical records.
Baseline and follow-up measurements
The patient’s characteristics, including age of diabetes diagnosis, gender, race/ethnicity, family income, smoking status, types of health insurance, body weight, height, body mass index (BMI), blood pressure, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, HbA1c, creatinine concentration, and medication (antihypertensive drug, cholesterol lowing drug and antidiabetic drug) within half year after the diabetes diagnosis (baseline) and during follow-up after the diabetes diagnosis (follow-up) were extracted from the computerized hospitalization records. The updated mean values of HbA1c, LDL cholesterol, BMI, blood pressure and eGFR over time were measured firstly at baseline and secondly as an updated mean of annual measurement, calculated for each participant from baseline to each year of follow up. For example, at one year the updated mean is the average of the baseline and one year values and at three years it is the average of baseline, one year, two year, and three year values. In case of an event during follow-up, the period for estimating updated mean value was from baseline to the year before this event occurred.37, 38 The average number of clinic laboratory measurements for serum creatinine concentration during the follow-up period was 16 times. Plasma total, LDL cholesterol, HDL cholesterol and triglycerides were measured by enzymatic colorimetric methods. Serum glucose was measured by the glucose-oxidase method. HbA1c was measured by immunoassay. Serum creatinine, which was measured using the modified kinetic Jaffe method, was standardized to isotope dilution mass spectrometry. Creatinine concentrations were reduced by 5%, the established calibration factor.39 In Louisiana State University Health Care Services Division hospitals, eGFR is estimated using Modification of Diet in Renal Disease equation: eGFR= (in ml/mom/1.73 m2) = 186 × [serum creatinine (in mg/dl)−1.154 × Age−0.203 × 0.742 (if female) × 1.210 (if black)]40.
Prospective follow-up
Follow-up information was obtained from the Louisiana State University Hospital-Based Longitudinal Study database by using the unique number assigned to every patient who visits the Louisiana State University Health Care Services Division hospitals. Two diabetic complications were used as outcomes in this study, including CHD (ICD-9 Codes 410–414) and stroke (ICD-9 Codes 430–436). Since 1997, diagnoses of CHD and stroke were made by the treating physicians, based on a clinical assessment and examinations as considered relevant by the clinician in charge of treatment. Follow-up of each cohort member continued until the date of the diagnosis of the outcome disease, death from causes other than the outcomes, the date of the last visit if the subject stopped use of Louisiana State University Health Care Services Division hospitals, or May 31, 201233.
Statistical analyses
Patients were categorized according to eGFR stages (≥ 90 [reference group], 75–89, 60–74, 30–59, and 15–29 mL/min/1.73 m2) in an expanded version of the GFR stages used for chronic kidney disease classification recommended by National Kidney Foundation.1 The associations between eGFR and the risk of incident CHD or stroke were analyzed by using Cox proportional hazards models in the following 2 ways: (1) as 5-category variable, and (2) as a continuous variable. eGFR categories were included in the models as dummy and categorical variables, and the significance of the trend over different categories of eGFR was tested in the same models by giving an ordinal numeric value for each dummy variable. All proportionality assumptions were appropriate. Confounders, comorbidity indicators, and treatment, which had been shown to be associated with the risk of CHD and stroke in our studies and other studies, were included as covariates5, 11, 34, 35. All the above analyses were first carried out adjusting for age and sex (age- and sex-adjusted model) and further for smoking, income, type of insurance, BMI, systolic blood pressure, glycated hemoglobin, LDL cholesterol, use of antihypertensive drugs, use of diabetes medications, and use of cholesterol-lowering agents (multivariate-adjusted model). Race was also adjusted when African Americans and whites were combined. LDL cholesterol was logarithmically transformed due to skewed distributions. When we analyzed association between updated mean of eGFR and incident CHD or stroke risk, we adjusted for updated means of BMI, LDL cholesterol, systolic blood pressure and HbA1c instead of baseline values of these variables. Restricted cubic splines with 5 knots were used in Cox models to test whether there is a dose-response or non-linear association of eGFR as a continuous variable with incident CHD or stroke risk. Statistical significance was considered to be P < 0.05. All statistical analyses were performed by using SAS for Windows, version 9.3 (SAS Institute, Cary, NC).
Acknowledgments
Funding: This work was supported by Louisiana State University’s Improving Clinical Outcomes Network (LSU ICON)
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCE
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boer IH, Rue TC, Hall YN, et al. Temporal Trends in the Prevalence of Diabetic Kidney Disease in the United States. Jama-Journal of the American Medical Association. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu G, Jousilahti P, Tuomilehto J. Joint effects of history of hypertension at baseline and type 2 diabetes at baseline and during follow-up on the risk of coronary heart disease. Eur Heart J. 2007;28:3059–3066. doi: 10.1093/eurheartj/ehm501. [DOI] [PubMed] [Google Scholar]
- 5.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and Kidney Function Independently Predict Cardiovascular and Renal Outcomes in Diabetes. Journal of the American Society of Nephrology. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurth T, de Jong PE, Cook NR, et al. Kidney function and risk of cardiovascular disease and mortality in women: a prospective cohort study (vol 338, pg b2392, 2009) British Medical Journal. 2009;339 doi: 10.1136/bmj.b2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan DJ, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New England Journal of Medicine. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 8.Cirillo M, Lanti MP, Menotti A, et al. Definition of kidney dysfunction as a cardiovascular risk factor. Arch Intern Med. 2008;168:617–624. doi: 10.1001/archinte.168.6.617. [DOI] [PubMed] [Google Scholar]
- 9.Foster MC, Hwang S-J, Larson MG, et al. Cross-classification of microalbuminuria and reduced glomerular filtration rate - Associations between cardiovascular disease risk factors and clinical outcomes. Arch Intern Med. 2007;167:1386–1392. doi: 10.1001/archinte.167.13.1386. [DOI] [PubMed] [Google Scholar]
- 10.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney International. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 11.So WY, Kong APS, Ma RCW, et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care. 2006;29:2046–2052. doi: 10.2337/dc06-0248. [DOI] [PubMed] [Google Scholar]
- 12.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. Journal of the American Society of Nephrology. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 13.Karter AJ, Ferrara A, Liu JY, et al. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 14.Astor BC, Hallan SI, Miller ER, III, et al. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. American Journal of Epidemiology. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 15.Muntner P Fau, Newsome B, Newsome B Fau, Kramer H, Kramer H Fau, Peralta CA, et al. Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:101–107. doi: 10.2215/CJN.06450611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. American Journal of Kidney Diseases. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 17.Hannedouche T, Albouze G, Chauveau P, et al. Effects of blood pressure and antihypertensive treatment on progression of advanced chronic renal failure. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1993;21:131–137. doi: 10.1016/0272-6386(93)70104-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Tuomilehto J, Jousilahti P, et al. Lifestyle factors in relation to heart failure among finnish men and women. Circ Heart Fail. 2011;4:607–612. doi: 10.1161/CIRCHEARTFAILURE.111.962589. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Tuomilehto J, Jousilahti P, et al. Lifestyle factors and antihypertensive treatment on the risks of ischemic and hemorrhagic stroke. Hypertension. 2012;60:906–912. doi: 10.1161/HYPERTENSIONAHA.112.193961. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Tuomilehto J, Jousilahti P, et al. Total and High-Density Lipoprotein Cholesterol and Stroke Risk. Stroke. 2012;43:1768–1774. doi: 10.1161/STROKEAHA.111.646778. [DOI] [PubMed] [Google Scholar]
- 21.Schiffrin EL, Lipman ML, Mann JFE. Chronic kidney disease - Effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 22.Kielstein JT, Boger RH, Bode-Boger SM, et al. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. Journal of the American Society of Nephrology. 2002;13:170–176. doi: 10.1681/ASN.V131170. [DOI] [PubMed] [Google Scholar]
- 23.Levey A, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 24.Kestenbaum Br Fau, Adeney KL, Adeney Kl Fau, de Boer IH, de Boer Ih Fau, Ix JH, et al. Incidence and progression of coronary calcification in chronic kidney disease: the Multi-Ethnic Study of Atherosclerosis. Kidney Int. 2009;76:991–998. doi: 10.1038/ki.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarnak Mj Fau, Levey AS, Levey As Fau, Schoolwerth AC, Schoolwerth Ac Fau, Coresh J, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 26.European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Kanaya AM, Adler N, Moffet HH, et al. Heterogeneity of Diabetes Outcomes Among Asians and Pacific Islanders in the US The Diabetes Study of Northern California (DISTANCE) Diabetes Care. 2011;34:930–937. doi: 10.2337/dc10-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson PW, D'Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Wang Y, Chen L, et al. Increasing prevalence of diabetes in middle or low income residents in Louisiana from 2000 to 2009. Diabetes Research and Clinical Practice. 2011;94:262–268. doi: 10.1016/j.diabres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Chen L, Xiao K, et al. Increasing incidence of gestational diabetes mellitus in louisiana, 1997–2009. Journal of women's health. 2012;21:319–325. doi: 10.1089/jwh.2011.2838. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Chen L, Horswell R, et al. Racial Differences in the Association Between Gestational Diabetes Mellitus and Risk of Type 2 Diabetes. Journal of Womens Health. 2012;21:628–633. doi: 10.1089/jwh.2011.3318. [DOI] [PubMed] [Google Scholar]
- 32.Hu G, Horswell R, Wang Y, et al. Body mass index and the risk of dementia among Louisiana low income diabetic patients. PLoS One. 2012;7:e44537. doi: 10.1371/journal.pone.0044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Katzmarzyk PT, Horswell R, et al. Racial disparities in diabetic complications in an underinsured population. The Journal of clinical endocrinology and metabolism. 2012;97:4446–4453. doi: 10.1210/jc.2012-2378. [DOI] [PubMed] [Google Scholar]
- 34.Zhao W, Katzmarzyk PT, Horswell R, et al. Aggressive Blood Pressure Control Increases Coronary Heart Disease Risk Among Diabetic Patients. Diabetes Care. 2013 doi: 10.2337/dc13-0189. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao W, Katzmarzyk PT, Horswell R, et al. Blood Pressure and Stroke Risk among Diabetic Patients. The Journal of clinical endocrinology and metabolism. 2013 doi: 10.1210/jc.2013-1757. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 37.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cederholm J, Gudbjornsdottir S, Eliasson B, et al. Systolic blood pressure and risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish national diabetes register. J Hypertens. 2010;28:2026–2035. doi: 10.1097/HJH.0b013e32833c8b75. [DOI] [PubMed] [Google Scholar]
- 39.Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clinical Chemistry. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 40.National Kidney Foundation. clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002;39:S1–S266. [PubMed] [Google Scholar]