Abstract
Recently, Dientamoeba fragilis has emerged as a significant and common enteropathogen. The majority of patients with dientamoebiasis present with gastrointestinal complaints and chronic symptoms are common. Numerous studies have successfully demonstrated parasite clearance, coupled with complete resolution of clinical symptoms following treatment with various antiparasitic compounds. Despite this, there is very little in vitro susceptibility data available for the organism. Benzimidazoles are a class of antiparasitic drugs that are commonly used for the treatment of protozoan and helminthic infections. Susceptibility testing was undertaken on four D. fragilis clinical isolates against the following benzimidazoles: albendazole, flubendazole, mebendazole, nocodazole, triclabendazole and thiabendazole. The activities of the antiprotozoal compounds at concentrations ranging from 2 μg/mL to 500 μg/mL were determined via cell counts of D. fragilis grown in xenic culture. All tested drugs showed no efficacy. The beta-tubulin transcript was sequenced from two of the D. fragilis isolates and amino acid sequences predicted a susceptibility to benzimidazoles. This is the first study to report susceptibility profiles for benzimidazoles against D. fragilis, all of which were not active against the organism. This study also found that beta-tubulin sequences cannot be used as a reliable marker for resistance of benzimidazoles in D. fragilis.
Keywords: Dientamoeba fragilis, Antimicrobials, Benzimidazoles, Beta-tubulin
Abstract
Récemment, D. fragilis a émergé comme un entéropathogène important et commun. La majorité des patients avec dientamoebiase présente des troubles gastro-intestinaux et les symptômes chroniques sont fréquents. De nombreuses études ont démontré avec succès l’élimination des parasites, couplée à la résolution complète des symptômes cliniques, après traitement avec divers composés antiparasitaires. Malgré cela, il y a très peu de données disponibles sur la sensibilité in vitro de cet organisme. Les benzimidazoles sont une classe de médicaments antiparasitaires qui sont couramment utilisés pour le traitement des infections à protozoaires et helminthes. Les tests de sensibilité ont été réalisés sur quatre isolats cliniques de D. fragilis avec les benzimidazoles suivants : albendazole, flubendazole, mébendazole, nocodazole, triclabendazole et thiabendazole. Les activités des composés antiprotozoaires, à des concentrations allant de 2 μg/ml à 500 μg/ml ont été déterminées par comptage de cellules de D. fragilis cultivées en culture xénique. Tous les médicaments testés n’ont montré aucune efficacité. Le transcript de béta-tubuline a été séquencé à partir de deux isolats de D. fragilis, et les séquences d’acides aminés prédisaient une sensibilité aux benzimidazoles. Cette étude est la première à signaler des profils de sensibilité pour les benzimidazoles contre D. fragilis, qui tous étaient non actifs contre l’organisme. Cette étude a également révélé que les séquences de béta-tubuline ne peuvent pas être utilisées comme un marqueur fiable de la résistance de benzimidazoles chez D. fragilis.
Introduction
Dientamoeba fragilis Jepps and Dobell, 1918 [18] is a protozoan parasite that is the only recognised species in the genus Dientamoeba. It is classified as a trichomonad in the class Trichomonadida and has been shown to be closely related to the amoeboflagellate Histomonas meleagridis [14]. Dientamoeba is emerging as one of the most commonly encountered enteric protozoa of humans with prevalence reaching up to 43% in some studies when appropriate diagnostic methods are utilised [27]. Despite this, it continues to be neglected as a significant pathogen, with many laboratories not routinely performing adequate laboratory diagnostic testing for the parasite [2, 7, 12].
The clinical presentation of dientamoebiasis varies from asymptomatic carriage to symptomatic presentations, ranging from altered bowel motions, abdominal discomfort, nausea, and diarrhoea [28, 29, 33, 35]. The propensity of the organism to cause chronic symptoms, ranging from weeks to months, has been reported in the scientific literature [7, 15]. The life cycle and mode of transmission of D. fragilis are poorly defined. However, the recent discovery of a cyst stage in the life cycle of this parasite would suggest that direct transmission via the faecal-oral route is the most likely mode of transmission [24]. High rates of transmission between close contacts and household members have been described, highlighting the transmissible nature of the organism [31].
Despite the discovery of the parasite nearly 100 years ago and the abundance of reports in the scientific literature regarding infections, very little research has been conducted on the use of suitable antimicrobial compounds to control infections and subsequent susceptibility testing of isolates [32]. Only three studies to date have undertaken in vitro susceptibility testing on D. fragilis isolates [3, 10, 25], and no studies to date have looked at the efficacy of the benzimidazoles. Benzimidazoles have been shown to be effective in treating both Trichomonas vaginalis [20, 21] and Giardia intestinalis [38] and ineffective against H. meleagridis [9, 17]. Benzimidazoles are a class of antiparasitic drug [5], which act on beta-tubulin by binding to a high-affinity binding site on the beta-tubulin monomer [22]. There are several different beta-tubulin residues that have been proposed as indicators of benzimidazole susceptibility. In protozoa, two residues, Glu-198 and Phe-200, have been hypothesised as an indicator for susceptibility [13, 21]. In Trichomonad parasites, agreement between beta-tubulin sequences and susceptibility to benzimidazoles in vitro has been established for T. vaginalis [20, 21]. However, a study on H. meleagridis found that while histomonal amino acid sequences predicted a susceptibility to benzimidazoles, no correlation was found with in vitro activity for these agents [16].
The aim of this study was to test the in vitro activity of albendazole, flubendazole, mebendazole, nocodazole, triclabendazole and thiabendazole against clinical isolates of D. fragilis and to determine whether beta-tubulin sequences can be used as an indicator for benzimidazole susceptibility in protozoa.
Materials and methods
Parasite culture
Four strains of D. fragilis were isolated and propagated in vitro using a biphasic xenic culture system using a Loeffler’s slope medium modified from a previously published method [6] consisting of an inspissated horse serum slope overlaid with 5 mL of PBS and supplemented with 2–5 mg of rice starch.
Genotyping of D. fragilis strains
Genotyping was performed as previously described targeting the SSU rRNA gene [30].
Antimicrobial agents and susceptibility testing
The following antimicrobial agents were used in susceptibility testing: albendazole, flubendazole, mebendazole, nocodazole, triclabendazole and thiabendazole (Sigma-Aldrich, Australia). All benzimidazoles were supplied in powdered form and dissolved in dimethylsulfoxide (DMSO) to make stock solutions of 5 mg/mL. Further doubling dilutions (PBS) were prepared from 1,000 μg/mL to 4 μg/mL. The respective dilutions were added to the PBS overlay at a 1:1 ratio to a final volume of 5 mL, giving a final dilution range of 500 μg/mL to 2 μg/mL of antimicrobial agent in the media. All susceptibility testing was performed in triplicate. A control consisting of 1 mL of 10% DMSO diluted (PBS) into a total of 5 mL and then doubling dilutions were performed (in triplicate) for all drugs to rule out inhibitory effects of DMSO on D. fragilis.
The cell concentrations were determined using Kova slides viewed under phase-contrast microscopy at a magnification of X400. Susceptibility testing with each compound was performed over 4 days. Minimum lethal concentrations (MLCs) were determined to be the concentration of the drug at which no trophozoites were observed. A control consisting of a benzimidazole sensitive strain of Trichomonas vaginalis (isolated from a local clinical sample) was used to ascertain efficacy of the antimicrobial agents tested (albendazole, flubendazole, mebendazole, nocodazole, triclabendazole and thiabendazole) as previously described [37]. A positive control was also included consisting of the D. fragilis cells and the reference drug metronidazole (Sigma Aldrich, Australia) as previously described [24].
RNA extraction for molecular analysis
Two of the four isolates of Dientamoeba used in the susceptibility testing experiments underwent further molecular testing. Ribonucleic acid was extracted from culture sediments using TRIsure reagent (Bioline, catalogue number BIO-38032) and enriched for eukaryotic mRNA using oligo (dT)-cellulose chromatography. Sequencing of the transcriptome was performed by the service provider AGRF (http://agrf.org.au/). The methods used to sequence and assemble the transcriptome of D. fragilis will be published elsewhere.
Mining the transcriptome for tubulin sequences
Contigs from the D. fragilis transcriptome were used to construct a blast database using the makeblastdb program available from the NCBI website. Histomonas meleagridis beta-tubulin 1, (GenBank accession no.: AEN84279) was used as a query sequence in a tblastn search (default parameters, version 2.2.28+) against this database to identify homologues within the D. fragilis transcriptome. Putative D. fragilis beta-tubulin sequences detected in this blast search were then subjected to blastn and blastx searches against the NCBI nucleotide and protein databases, respectively, to confirm their identity. Putative D. fragilis beta-tubulins were translated into their protein sequences using the “Translate” component of the “Sequence manipulation suite” (Stothard 2000) (website: http://www.bioinformatics.org/sms2/translate.html). Alignments of the resulting amino acid sequences were performed using clustalW (default parameters).
Results
Genotyping
All four D. fragilis strains used in the experiments were identified as genotype 1.
MLCs
All benzimidazoles tested (albendazole, flubendazole, mebendazole, nocodazole, triclabendazole and thiabendazole) had no effect on the in vitro D. fragilis cultures with MLCs of >500 μg/mL. Metronidazole, however, was effective with an MLC of 31 μg/mL. The T. vaginalis control strain was susceptible to all benzimidazoles with MLCs ranging from 4 to 16 μg/mL. Thus, the observed lack of activity against D. fragilis is not due to benzimidazole degradation at any point during the experiment.
Identification of beta-tubulin transcripts in the D. fragilis transcriptome
Three D. fragilis contigs from D. fragilis isolate 1 were identified as close homologues of H. meleagridis beta-tubulin (GenBank accession no.: AEN84279) by tblastn search. However, only two of these could be translated into a full length tubulin amino acid sequence. The two full length tubulin contigs achieved significant blastn and blastx hits to beta-tubulin sequences from other trichomonads when blasted against the NCBI web server, confirming that at least two beta-tubulin isoforms are present in D. fragilis. These two D. fragilis beta-tubulin sequences can be found in GenBank under accession nos. KM186141 and KM186142.
Examination for amino acids predictive of albendazole susceptibility
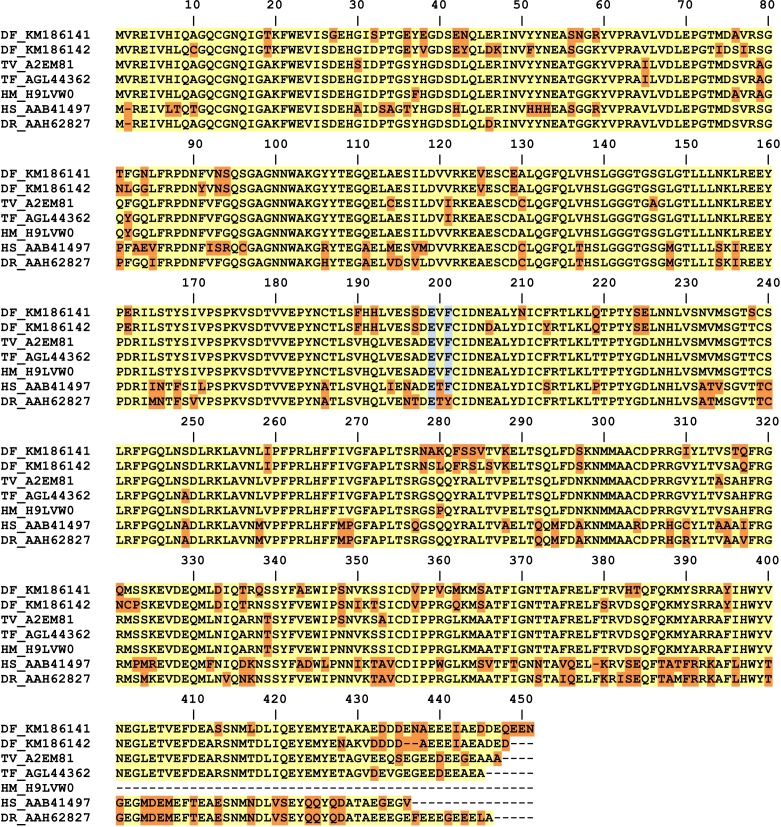
Alignment of D. fragilis amino acid sequences of beta-tubulin 1 and 2 to beta-tubulins from other Trichomonads (Fig. 1) confirmed that D. fragilis possesses the amino acids which are predictive of albendazole susceptibility. Based on these alignments, it became apparent that Trichomonad beta-tubulins possess an additional valine residue which follows the first methionine amino acid. This valine residue was not present in other beta-tubulin sequences examined (such as Candida sp., Aspergillus sp. and Ascaris sp – data not shown) and the implications of this are that the amino acids predictive for albendazole susceptibility are moved forward by one additional position (see Fig. 1), compared to previous reports describing beta-tubulin sequences [16, 21].
Figure 1.
Full alignment of beta-tubulin amino acid sequences from D. fragilis with tubulin sequences derived from Trichomonads and other eukaryotes. The residues highlighted blue are those thought to be predictors of albendazole susceptibility in protozoa as described in previous studies. Amino acids shaded yellow represent the most common amino acid at that position (predicted consensus based on this alignment). Amino acids shaded orange are those which differ from the predicted consensus. Note however that at positions 8, 430, 434 and 446, a consensus cannot be resolved. TV: Trichomonas vaginalis, DF: Dientamoeba fragilis, HM: Histomonas meleagridis, TF: Tritrichomonas foetus, HS: Homo sapiens, DR: Danio rerio. For Histomonas meleagridis and Trichomonas vaginalis the species acronym is followed by the corresponding UniprotKB identifier. For all other organisms, the species acronym is followed by the corresponding Genbank accession number.
Based on the results of the current study, amino acid positions 198 (199 for Trichomonads) and 200 (201 for Trichomonads) cannot be used as predictors of albendazole resistance (or susceptibility). We suggest, therefore, that there may be other amino acids in the beta-tubulin protein which may be predictive of albendazole susceptibility in protozoa. Alternatively, it may be that the beta-tubulin sequence alone cannot be used as a reliable predictor for albendazole resistance (or susceptibility) in protozoa.
Discussion
Dientamoeba is a frequently encountered enteric protozoan, yet despite the relatively high prevalence of this organism [2, 27], very little research has been undertaken on susceptibility testing to drugs. There is no gold standard treatment for D. fragilis, and the majority of treatment data is based on a small number of case reports [26]. Many cases of treatment failure have been reported [4, 28, 36] leading some researchers to postulate that current treatment options may be suboptimal for the eradication of Dientamoeba [26]. This highlights the need for further study on antiprotozoal agents that have potential activity against D. fragilis. While Dientamoeba can be readily cultured from fresh un-refrigerated clinical samples, long-term cultures have been shown to be notoriously difficult to maintain [23]. This has hampered many in vitro studies of this organism in particular susceptibility testing. However, recent advances in culturing techniques have allowed for long-term subculture of isolates [6, 23].
Current data is lacking on susceptibility profiles for D. fragilis isolates with only three previous studies conducted to date [3, 10, 25]. Only two of these used clinical samples, with one using the no longer available D. fragilis ATCC strain 30948 which was of the rarely encountered genotype 2 type, which is not the predominant genotype found in clinical samples [30]. The current study used four clinical isolates of D. fragilis, all of which were genotype 1.
Benzimidazoles have been widely used since the 1960s as anthelmintic agents in veterinary and human medicine and as antifungal agents in agriculture. Initially, benzimidazole activity seemed to be limited to helminths and fungi however in 1985 T. vaginalis was reported to be inhibited by the benzimidazole derivatives mebendazole and flubendazole [19]. Subsequently, susceptibility of benzimidazoles was shown for G. intestinalis and microsporidia [21]. More recently, the activity of benzimidazoles was tested against H. meleagridis and they were shown to be an ineffective agent for treatment in vitro [16]. Resistance to the benzimidazoles has been observed in parasitic nematodes of livestock animals since the early 1960s [11]. The beta-tubulin protein confers benzimidazole sensitivity in the helminth Caenorhabditis elegans and clear evidence exists that three different single amino acid substitutions (Thr-167, Glu-198 and Phe-200) in the beta-tubulin protein of different nematode species can be responsible, each leading separately to resistance [8]. However in protists, it seems that only two may play a role, namely Glu-198 and Phe-200 [21].
Although the complete crystallographic structure of the beta-tubulin monomer and the mechanism of action of benzimidazoles are still unknown, a recent study used homology modelling techniques along with molecular docking studies to advance this area of research [1]. The study was undertaken on Trichinella spiralis and the researchers were able to suggest a binding site for benzimidazoles that contains several amino acids associated with resistance (Phe-167, Glu-198 and Phe-200). This further supports the role of these amino acid positions in albendazole resistance or susceptibility in helminths.
The current study used several benzimidazole derivatives: albendazole, flubendazole, mebendazole, nocodazole, triclabendazole and thiabendazole. All were shown to be ineffective anti-Dientamoeba agents. Concentrations ranging from 2 μg/mL to 500 μg/mL resulted in D. fragilis trophozoite cell counts similar to that of the control. Although both Giardia and Trichomonas have been shown to be susceptible to benzimidazoles, the closely related H. meleagridis was shown to be resistant to benzimidazoles [9, 16, 17]. The exact mechanism for resistance is however unknown [16].
Based on this study, positions 198 and 200 of the beta-tubulin protein are not predictive of albendazole resistance, indicating that we need to look elsewhere to understand the phenomenon of resistance to benzimidazoles in Trichomonads. It should also be noted that this phenomena has not only been reported in Trichomonads. Giardia strains can reportedly become resistant to albendazole without having mutations in Glu-198 or Phe-200 [34]. Taken with the results of the current study, this detracts from the importance of Glu-198 and Phe-200 in albendazole susceptibility as seen in protozoa. Clearly, other mechanisms of albendazole resistance must be explored in protozoa.
Conclusion
The results of this study show that benzimidazoles have no effect on D. fragilis in culture. As such, no therapeutic response could be expected from the treatment of dientamoebiasis with benzimidazoles. The preliminary data presented would also suggest that beta-tubulin sequences cannot be used as a reliable marker for resistance of benzimidazoles in D. fragilis and as a result, other markers of benzimidazole resistance need to be explored.
Acknowledgments
We would like to thank SydPath (St. Vincent’s pathology) research committee for providing funding for this project.
Cite this article as: Stark D, Barratt JLN, Roberts T, Marriott D, Harkness JT & Ellis J: Activity of benzimidazoles against Dientamoeba fragilis (Trichomonadida, Monocercomonadidae) in vitro and correlation of beta-tubulin sequences as an indicator of resistance. Parasite, 2014, 21, 41.
References
- 1.Aguayo-Ortiz R, Mendez-Lucio O, Medina-Franco JL, Castillo R, Yepez-Mulia L, Hernandez-Luis F, Hernandez-Campos A. 2013. Towards the identification of the binding site of benzimidazoles to beta-tubulin of Trichinella spiralis: insights from computational and experimental data. Journal of Molecular Graphics Modelling, 41, 12–19 [DOI] [PubMed] [Google Scholar]
- 2.Al-Hindi AI, Shammala BM. 2013. Dientamoeba fragilis in Gaza Strip: a neglected protozoan parasite Iranian. Journal of Parasitology, 8(2), 249–255 [PMC free article] [PubMed] [Google Scholar]
- 3.Balamuth W. 1953. Comparative action of selected amebicidal agents and antibiotics against several species of human intestinal amebae. American Journal of Tropical Medicine and Hygiene, 2(2), 191–205 [DOI] [PubMed] [Google Scholar]
- 4.Banik GR, Barratt JL, Marriott D, Harkness J, Ellis JT, Stark D. 2011. A case-controlled study of Dientamoeba fragilis infections in children. Parasitology, 138(7), 819–823 [DOI] [PubMed] [Google Scholar]
- 5.Bansal Y, Silakari O. 2012. The therapeutic journey of benzimidazoles: a review. Bioorganic & Medicinal Chemistry, 20(21), 6208–6236 [DOI] [PubMed] [Google Scholar]
- 6.Barratt JL, Banik GR, Harkness J, Marriott D, Ellis JT, Stark D. 2010. Newly defined conditions for the in vitro cultivation and cryopreservation of Dientamoeba fragilis: new techniques set to fast track molecular studies on this organism. Parasitology, 137(13), 1867–1878 [DOI] [PubMed] [Google Scholar]
- 7.Barratt JL, Harkness J, Marriott D, Ellis JT, Stark D. 2011. A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes, 2(1), 3–12 [DOI] [PubMed] [Google Scholar]
- 8.Beech RN, Skuce P, Bartley DJ, Martin RJ, Prichard RK, Gilleard JS. 2011. Anthelmintic resistance: markers for resistance, or susceptibility? Parasitology, 138(2), 160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callait MP, Granier C, Chauve C, Zenner L. 2002. In vitro activity of therapeutic drugs against Histomonas meleagridis (Smith, 1895). Poultry Science, 81(8), 1122–1127 [DOI] [PubMed] [Google Scholar]
- 10.Chan FT, Guan MX, Mackenzie AM, Diaz-Mitoma F. 1994. Susceptibility testing of Dientamoeba fragilis ATCC 30948 with iodoquinol, paromomycin, tetracycline, and metronidazole. Antimicrobial Agents and Chemotherapy, 38(5), 1157–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drudge JH, Szanto J, Wyant ZN, Elam G. 1964. Field studies onparasite control in Sheep: Comparison of Thiabensazole, Ruelene, and Phenothiazine. American Journal of Veterinary Research, 25, 1512–1518 [PubMed] [Google Scholar]
- 12.Fletcher SM, Stark D, Harkness J, Ellis J. 2012. Enteric protozoa in the developed world: a public health perspective. Clinical Microbiology Reviews, 25(3), 420–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzen C, Salzberger B. 2008. Analysis of the beta-tubulin gene from Vittaforma corneae suggests benzimidazole resistance. Antimicrobial Agents and Chemotherapy, 52(2), 790–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerbod D, Edgcomb VP, Noel C, Zenner L, Wintjens R, Delgado-Viscogliosi P, Holder ME, Sogin ML, Viscogliosi E. 2001. Phylogenetic position of the trichomonad parasite of turkeys, Histomonas meleagridis (Smith) Tyzzer, inferred from small subunit rRNA sequence. Journal of Eukaryotic Microbiology, 48(4), 498–504 [DOI] [PubMed] [Google Scholar]
- 15.Gray TJ, Kwan YL, Phan T, Robertson G, Cheong EY, Gottlieb T. 2013. Dientamoeba fragilis: a family cluster of disease associated with marked peripheral eosinophilia. Clinical Infectious Disease, 57(6), 845–848 [DOI] [PubMed] [Google Scholar]
- 16.Hauck R, Hafez HM. 2009. Partial sequence of the beta-tubulin of Histomonas meleagridis and the activity of benzimidazoles against H. meleagridis in vitro. Parasitology Research, 104(5), 1183–1189 [DOI] [PubMed] [Google Scholar]
- 17.Hu J, McDougald LR. 2004. The efficacy of some drugs with known antiprotozoal activity against Histomonas meleagridis in chickens. Veterinary Parasitology, 121(3–4), 233–238 [DOI] [PubMed] [Google Scholar]
- 18.Jepps MW, Dobell C. 1918. Dientamoeba fragilis, a new intestinal amoeba from man. Parasitology, 10, 352–367 [Google Scholar]
- 19.Juliano C, Martinotti MG, Cappuccinelli P. 1985. “In vitro” effect of microtubule inhibitors on Trichomonas vaginalis. Microbiologica, 8(1), 31–42 [PubMed] [Google Scholar]
- 20.Katiyar SK, Edlind TD. 1994. Beta-tubulin genes of Trichomonas vaginalis. Molecular and Biochemistry Parasitology, 64(1), 33–42 [DOI] [PubMed] [Google Scholar]
- 21.Katiyar SK, Gordon VR, McLaughlin GL, Edlind TD. 1994. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrobial Agents and Chemotherapy, 38(9), 2086–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubega GW, Geary TG, Klein RD, Prichard RK. 1993. Expression of cloned beta-tubulin genes of Haemonchus contortus in Escherichia coli: interaction of recombinant beta-tubulin with native tubulin and mebendazole. Molecular and Biochemistry Parasitology, 62(2), 281–292 [DOI] [PubMed] [Google Scholar]
- 23.Munasinghe VS, Stark D, Ellis JT. 2012. New advances in the in-vitro culture of Dientamoeba fragilis. Parasitology, 139(7), 864–869 [DOI] [PubMed] [Google Scholar]
- 24.Munasinghe VS, Vella NG, Ellis JT, Windsor PA, Stark D. 2013. Cyst formation and faecal-oral transmission of Dientamoeba fragilis-the missing link in the life cycle of an emerging pathogen. International Journal of Parasitology, 43(11), 879–883 [DOI] [PubMed] [Google Scholar]
- 25.Nagata N, Marriott D, Harkness J, Ellis JT, Stark D. 2012. In vitro susceptibility testing of Dientamoeba fragilis. Antimicrobial Agents and Chemotherapy, 56(1), 487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata NH, Marriott D, Ellis JT, Stark D. 2012. Current treatment options for Dientamoeba fragilis infections. International Journal of Parasitology: Drugs and Drug Resistance, 2, 204–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roser D, Simonsen J, Nielsen HV, Stensvold CR, Molbak K. 2013. Dientamoeba fragilis in Denmark: epidemiological experience derived from four years of routine real-time PCR. European Journal of Clinical Microbiology and Infectious Disease, 32(10), 1303–1310 [DOI] [PubMed] [Google Scholar]
- 28.Schure JM, de Vries M, Weel JF, van Roon EN, Faber TE. 2013. Symptoms and treatment of Dientamoeba fragilis infection in children, a retrospective study. Paediatric Infectious Disease Journal, 32(4), e148–e150 [DOI] [PubMed] [Google Scholar]
- 29.Stark D, Barratt J, Roberts T, Marriott D, Harkness J, Ellis J. 2010. A review of the clinical presentation of dientamoebiasis. American Journal of Tropical Medicine and Hygiene, 82(4), 614–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2005. Prospective study of the prevalence, genotyping, and clinical relevance of Dientamoeba fragilis infections in an Australian population. Journal of Clinical Microbiology, 43(6), 2718–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stark D, Roberts T, Marriott D, Harkness J, Ellis JT. 2012. Detection and transmission of Dientamoeba fragilis from environmental and household samples. American Journal of Tropical Medicine and Hygiene, 86(2), 233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stark DJ, Beebe N, Marriott D, Ellis JT, Harkness J. 2006. Dientamoebiasis: clinical importance and recent advances. Trends in Parasitology, 22(2), 92–96 [DOI] [PubMed] [Google Scholar]
- 33.Ter Schure JM, de Vries M, Weel JF, van Roon EN, Faber TE. 2013. Symptoms and treatment of Dientamoeba fragilis infection in children, a retrospective study. Pediatric Infectious Disease Journal, 32(4), e148–e150 [DOI] [PubMed] [Google Scholar]
- 34.Upcroft J, Mitchell R, Chen N, Upcroft P. 1996. Albendazole resistance in Giardia is correlated with cytoskeletal changes but not with a mutation at amino acid 200 in beta-tubulin. Microbiology Drug Resistance, 2(3), 303–308 [DOI] [PubMed] [Google Scholar]
- 35.Vandenberg O, Peek R, Souayah H, Dediste A, Buset M, Scheen R, Retore P, Zissis G, van Gool T. 2006. Clinical and microbiological features of dientamoebiasis in patients suspected of suffering from a parasitic gastrointestinal illness: a comparison of Dientamoeba fragilis and Giardia lamblia infections. International Journal of Infectious Disease, 10(3), 255–261 [DOI] [PubMed] [Google Scholar]
- 36.Vandenberg O, Souayah H, Mouchet F, Dediste A, van Gool T. 2007. Treatment of Dientamoeba fragilis infection with paromomycin. Pediatric Infectectious Disease Journal, 26(1), 88–90 [DOI] [PubMed] [Google Scholar]
- 37.Wright JM, Dunn LA, Kazimierczuk Z, Burgess AG, Krauer KG, Upcroft P, Upcroft JA. 2010. Susceptibility in vitro of clinically metronidazole-resistant Trichomonas vaginalis to nitazoxanide, toyocamycin, and 2-fluoro-2′-deoxyadenosine. Parasitology Research, 107(4), 847–853 [DOI] [PubMed] [Google Scholar]
- 38.Wright JM, Dunn LA, Upcroft P, Upcroft JA. 2003. Efficacy of antigiardial drugs. Expert Opinion Drug Safety, 2(6), 529–541 [DOI] [PubMed] [Google Scholar]