Abstract
Hyperinsulinemic hypoglycemia is the most common cause of persistent hypoglycemia in children and adults. The diagnosis of hyperinsulinemic hypoglycemia relies on the evaluation of the biochemical profile at the time of hypoglycemia, however, contrary to common perception, plasma insulin is not always elevated. Thus, the diagnosis must often be based on the examination of other physiologic manifestations of excessive insulin secretion, such as suppression of glycogenolysis, lipolysis and ketogenesis, which can be inferred by the finding of a glycemic response to glucagon, and the suppression of plasma free fatty acids and beta-hydroxybutyrate concentrations during hypoglycemia.
Keywords: hypoglycemia, insulinoma, hyperinsulinism, beta cells, pancreas
Introduction
Hyperinsulinemic hypoglycemia is the most frequent cause of persistent hypoglycemia in children and adults. In adults, hyperinsulinemic hypoglycemia is most commonly an acquired problem due to an insulin-secreting tumor; while in children, with only rare exceptions, it represents a congenital disorder (1). The development of a radioimmunoassay for insulin by Yalow and Berson in 1960 (2) made it possible to demonstrate the role of endogenous insulin in hypoglycemic disorders. However, as discussed below, simply measuring plasma insulin concentrations is often not enough to establish the diagnosis of hyperinsulinemic hypoglycemia.
Methods
A literature search of PubMed (http://www.ncbi.nlm.nih.gov/pubmed) was performed for studies published up to April 2013. Keywords used included insulin, C-peptide, proinsulin, insulin assay, insulinoma, hyperinsulinemic hypoglycemia, congenital hyperinsulinism. Additional articles known to the authors or cited by others were also included.
Establishing the Diagnosis of Hyperinsulinemic Hypoglycemia
Insulin secretion by pancreatic ß-cells is tightly regulated by an array of stimulatory and inhibitory factors, of which glucose plays the leading role. The plasma glucose threshold concentration for insulin release is determined by the activity of ß-cell glucokinase and is precisely maintained at approximately 5 mmol/L in humans (3). This tight control of insulin secretion in relationship to glucose concentration results in plasma glucose concentrations in normal individuals that remain remarkably stable in the range of 3.9-7.1 mmol/L (70-128 mg/dL) during normal daily cycles of feeding and overnight fasting.
In hyperinsulinemic hypoglycemia this tight control of insulin secretion by glucose is lost. To define whether hypoglycemia is insulin-mediated, it is necessary to establish that ß-cell insulin secretion is not appropriately turned off as glucose levels decrease; however, because it is not possible to measure insulin secretion in vivo directly, reliance is ordinarily placed on measurements of plasma insulin concentrations in samples of venous blood at the time of hypoglycemia. However, it is important to note that peripheral plasma insulin concentrations can be affected by the kinetics of insulin distribution and degradation. Following secretion, insulin is distributed into both intra and extravascular spaces and thus, the volume of distribution is several times larger than plasma volume. A large fraction (~50%) of insulin secreted by the pancreas is cleared by the liver at first passage. The rate of insulin degradation is approximately 2 percent per minute (4). Therefore, the insulin concentration measured in peripheral plasma may be up to 90% lower than the initial peak plasma concentration within less than 30 minutes (5). C-peptide, secreted in a 1:1 molar ratio with insulin, is cleared primarily by the kidney and has a lower metabolic clearance rate (4.4 mL/min) than insulin and therefore a longer half-life in circulation (20-30 min vs. 3-5 min) (6, 7). Thus, peripheral C-peptide concentration reflects portal insulin secretion more accurately than the peripheral plasma insulin concentration. Normally, the molar ratio of insulin to C-peptide should be less than 1 in peripheral circulation; a higher ratio of insulin to C-peptide indicates the likelihood of exogenous insulin administration (8).
Measurement of plasma insulin concentration: pitfalls
When first developed, more than 40 years ago (2), the insulin assay was mainly used in studies aimed at understanding the physiology and pathophysiology of insulin regulation. These studies involved small numbers of subjects and, therefore, reproducibility and standardization were not priorities. Later, in an attempt to limit the large variation in reported values from different laboratories, the World Health Organization provided purified preparations of porcine insulin that could be used as external standards (9). The development of recombinant DNA technology in the 1980s (10) made available large quantities of purified human insulin and led to further improvements in the performance of the assay. Subsequent improvements have included the development of monoclonal antibodies for use in immunoassays (11) and of guidelines standards for assay performance (12), which have allowed for better reproducibility, sensitivity and specificity. Nevertheless, a complete reference system in conformance with International Organization for Standardization requirements is yet to be established for insulin (13).
The main clinical application of plasma insulin assay is for the diagnosis of hyperinsulinemic hypoglycemia. However, despite the improved performance of the assay, interference by the presence of anti-insulin antibodies (14) and of hemolysis decreases the utility of the assay for this purpose. Hemolysis of the sample, a particular problem in children in whom blood drawing is difficult, can result in a dose- and time-dependent decrease in immunoreactive insulin (15) due to the release of insulin degrading enzyme from red blood cells. To avoid the effects of hemolysis it has been recommended to analyze the samples promptly after sampling and to discard any samples with a concentration of free hemoglobin equal or above 125 mg/dL (16). In this instance, C-peptide measurements, which are not affected by hemolysis (17), may be useful to rule out analytical interference with the assay.
Insulin concentration in hyperinsulinemic hypoglycemia
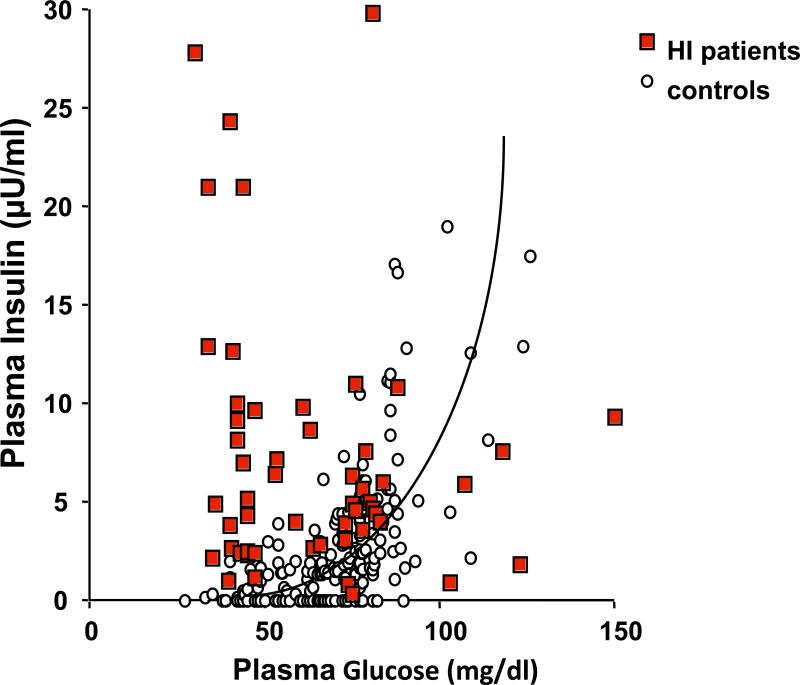
It has been long recognized that absolute hyperinsulinemia can not always be documented in cases of hyperinsulinemic hypoglycemia (5, 18); i.e., that hyperinsulinemia is not always manifested in hyperinsulinism. Normally, plasma insulin concentrations should be suppressed at a time of hypoglycemia to below the limits of assay detection. Thus, any detectable level of insulin at the time of hypoglycemia can be considered abnormal and should be regarded as evidence of inappropriate insulin secretion (19, 20). In a series of children with congenital hyperinsulinism, plasma insulin concentrations at the time of hypoglycemia overlapped with those of children with hypoglycemia not due to hyperinsulinism. In many of the hyperinsulinism cases, plasma insulin concentrations were within the “normal range” for the assay (Figure 1). In individuals with hyperinsulinemic hypoglycemia due to an insulinoma, a plasma insulin concentration of ≥ 3μU/mL (20.8 pmol/L) at the time of hypoglycemia (above the limit of assay detection) had a sensitivity and specificity of 93% and 95%, respectively (21). Thus, it is clear that an undetectable plasma insulin concentration does not exclude the diagnosis of hyperinsulinemic hypoglycemia. The sensitivity is higher, at 100%, for C-peptide concentrations ≥ 0.6 ng/mL (0.2 nmol/L), but the specificity is only 60% (21). With the introduction of more specific insulin assays which do not detected proinsulin, it is recommended to also measure proinsulin concentrations in individuals with suspected insulinomas as tumor cells secrete more proinsulin than normal ß-cells (19). Different thresholds for proinsulin concentrations at the time of hypoglycemia have been recommended for the diagnosis of insulinomas (22-24). The sensitivity of a plasma proinsulin concentrations ≥ 5 pmol/L is low at 60%, but the specificity is 100 % (21). Very different results were reported in another study using different cut-offs (25) and including a smaller sample size. Overall, there is some agreement that when blood glucose is < 2 mmol/L, a proinsulin concentration > 5 pmol/L is highly suggestive of an insulinoma. There are no data regarding proinsulin levels in children with congenital hyperinsulinemic hypoglycemia.
Figure 1.
Plasma insulin concentration in relationship to plasma glucose concentration in children with congenital hyperinsulinism and control children.
Variables derived from simultaneous measurements of plasma glucose, insulin, C-peptide and proinsulin have also been used in the diagnosis of hyperinsulinism hypoglycemia. The utility of an amended insulin to glucose ratio in the diagnosis of insulinoma was evaluated in a retrospective study of 114 individuals with suspected hypoglycemia (26). The amended insulin (in pmol/L) to glucose (in mmol/L) ratio is calculated after subtracting 1.7 mmol/L from the glucose concentration. In this study, the sensitivity and specificity for the amended insulin to glucose ratio [> 53.6 (pmol/L)/(mmol/L)] at the end of a prolonged fast were both 98% (26). The difficulty in utilizing this ratio for the diagnosis is that, frequently, insulin may be undetectable and therefore the ratio can not be calculated. One of the reasons why these investigators were able to use this measure was that the insulin assay used in the study was not specific and detected not only insulin but also proinsulin. In the same study, the amended C-peptide to glucose ratio [>0.61 (nmol/L)(mmol/L)] had a sensitivity of 95% and a specificity of 94% to diagnose insulinoma. A proinsulin (in pmol/L) to insulin (in mIU/L) ratio of > 7.8 at the end of a diagnostic fast (plasma glucose 2.5-3.3 mmol/L) was reported to have a low sensitivity of 47% but a specificity of 95% (25).
Other markers of increased insulin action in hyperinsulinemic hypoglycemia
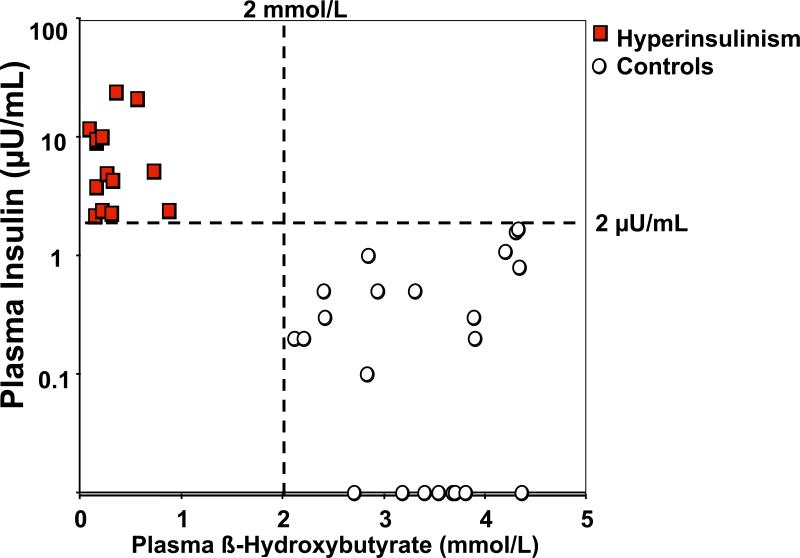
Because of the difficulties in diagnosing hyperinsulinemic hypoglycemia by measuring insulin concentration at the time of hypoglycemia, the diagnosis must often be based on the examination of other physiologic manifestations of excessive insulin secretion, such as suppression of glycogenolysis, lipolysis and ketogenesis, which can be inferred by the finding of a glycemic response to glucagon, and the suppression of plasma free fatty acids and beta-hydroxybutyrate concentrations during hypoglycemia (Table 1) (27, 28). In the series of children discussed above, those with congenital hyperinsulinism and those with hypoglycemia not due to hyperinsulinism could best be differentiated by using both plasma insulin and plasma beta-hydroxybutyrate concentrations (Figure 2). The sensitivity and specificity of a plasma beta-hydroxybutyrate concentration of ≤ 2.7 mmol/L in the diagnosis of insulinoma is 100% (21). The availability of point-of-care devices to measure beta-hydroxybutyrate at the bedside facilitates the diagnostic process, especially in children, by providing a rapid and reliable indicator of insulin action during diagnostic fasting tests. The plasma concentrations of free fatty acids are also suppressed at the time of hypoglycemia in hyperinsulinemic hypoglycemia; however, in most institutions, these results are not promptly available to make the diagnosis.
Table 1.
Diagnostic Criteria for Hyperinsulinemic Hypoglycemia
| Parameter | Value detected when plasma glucose < 50 mg/dL |
|---|---|
| Plasma insulin | > 2 μU/mL (13.9 pmol/L) |
| Plasma C-peptide | > 0.2 mmol/L (0.07 nmol/L) |
| Plasma free fatty acids | < 0.5 mmol/L |
| Plasma beta-hydroxybutyrate | < 0.6 mmol/L |
| Glycemic response to glucagon | ≥ 30 mg/dL (1.7 mmol/L) |
Figure 2.
Plasma insulin concentration in relationship to plasma beta-hydroxybutyrate in children with congenital hyperinsulinism and control children.
Evaluation of the glycemic response to injection of glucagon at the end of the fast or during a spontaneous hypoglycemic event provides a rapid, reliable measure of increased insulin action and is very helpful in establishing the diagnosis of hyperinsulinemic hypoglycemia. An increase in plasma glucose concentration of greater than 30 mg/dL (1.7 mmol/L) in response to administration of glucagon (1 mg intravenously or intramuscularly) indicates inappropriate conservation of liver glycogen during hypoglycemia and indicates suppression of liver glycogenolysis by excessive insulin action (18). The utility of the glucagon stimulation test at the end of a fast in establishing the diagnosis of congenital hyperinsulinism was demonstrated in a study of 14 children with congenital hyperinsulinism, 11 children with ketotic hypoglycemia, and 14 children with normal endocrine and metabolic response to fasting that served as controls (18). The mean plasma glucose concentration at the end of the fast was 30.5 ± 2.5 mg/dL (1.7 ± 0.1 mmol/L) in the children with hyperinsulinism, 35.7 ± 1.6 mg/dL (2.0 ± 0.1 mmol/L) in the children with ketotic hypoglycemia, and 65.5 ± 3.7 mg/dL (3.6 ± 0.2 mmol/L) in the normal control children. In response to administration of glucagon (0.5-1 mg) the mean change in plasma glucose for the three groups was: 71.5 ± 8.5 mg/dL (4.0 ± 0.5 mmol/L) in the children with hyperinsulinism, 8.1 ± 2.4 mg/dL (0.4 ± 0.1 mmol/L) in the children with ketotic hypoglycemia, and 32 ± 6.2 mg/dL (1.8 ± 0.3 mmol/L) in the controls. The plasma glucose change was less than 30 mg/dL (1.7 mmol/L) in 13 out of the 14 ketotic hypoglycemia and control children in whom plasma glucose was less than 40 mg/dL (2.2 mmol/L) at the end of the fast, while 10 out of the 11 children with hyperinsulinism had a glycemic response to glucagon greater than 30 mg/dL (1.7 mmol/L). Thus, in this small series, a response of greater than 30 mg/dL (1.7 mmol/L) was diagnostic of hyperinsulinism with a sensitivity of 91% and a specificity of 93% (18). In adult individuals, a plasma glucose response to glucagon greater than 25 mg/dL (1.4 mmol/L) at the time of hypoglycemia has a sensitivity of 91% and a specificity of 95% in the diagnosis of insulinoma (21).
Disorders that may mimic hyperinsulinemic hypoglycemia
The most difficult form of hypoglycemia to distinguish from endogenous hyperinsulinemic hypoglycemia is hypoglycemia induced by exogenous administration of insulin or of drugs that stimulate insulin secretion, such as sulfonylureas. It is particularly important to recognize cases with surreptitious administration of medications, either by the patient (Munchausen syndrome) or, especially in children, by another individual (Munchausen by proxy). Occasionally, these cases can be recognized by the finding of unusually elevated plasma concentrations of insulin at a time of hypoglycemia (>100-200 μU/mL). Suppressed concentrations of C-peptide at a time of hypoglycemia can provide a clue to the possibility of exogenous insulin administration. In contrast, administration of sulfonylureas will stimulate release of both insulin and C-peptide. Specific tests may be required to detect various sulfonylurea and other insulin secretogogues in plasma or urine.
It is also important to recognize that conditions in which activation of insulin signaling is independent of insulin should be considered in cases of hypoglycemia with undetectable plasma concentrations of insulin but evidence of increased insulin actions. These include individuals with activating mutations in AKT2 (29) and those with activating insulin receptor antibodies (30). Hypoglycemia has also been reported in patients with auto-antibodies that bind to and alter the pharmacokinetics of insulin (31, 32); in these cases, depending on the insulin assay employed, measurement of plasma insulin concentrations may be altered to produce either falsely lowered or falsely elevated values.
Summary
In summary, assays of plasma insulin concentrations are the mainstay for the diagnosis of hyperinsulinemic hypoglycemia. However, the diagnosis does not rely solely on the demonstration of elevated plasma insulin or C-peptide levels at the time of hypoglycemia. Rather, it relies heavily on the evidence of inappropriate increased insulin action provided by measurements of free fatty acids, beta-hydroxybutyrate, and glycemic response to glucagon. Used in combination with assays of plasma insulin concentrations, these measurements greatly enhance the accuracy of diagnosing hyperinsulinemic hypoglycemia.
References
- 1.De Leon DD, Stanley CA. Mechanisms of Disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nature Clinical Practice Endocrinology Metabolism. 2007;3:57–68. doi: 10.1038/ncpendmet0368. [DOI] [PubMed] [Google Scholar]
- 2.Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. The Journal of Clinical Investigation. 1960;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matschinsky FM. Regulation of pancreatic beta-cell glucokinase: from basics to therapeutics. Diabetes. 2002;51(Suppl 3):S394–404. doi: 10.2337/diabetes.51.2007.s394. [DOI] [PubMed] [Google Scholar]
- 4.Berson SA, Yalow RS, Bauman A, Rothschild MA, Newerly K. Insulin-I131 metabolism in human subjects: demonstration of insulin binding globulin in the circulation of insulin treated subjects. The Journal of Clinical Investigation. 1956;35:170–190. doi: 10.1172/JCI103262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yalow RS, Berson SA. Dynamics of Insulin Secretion in Hypoglycemia. Diabetes. 1965;14:341–349. doi: 10.2337/diab.14.6.341. [DOI] [PubMed] [Google Scholar]
- 6.Faber OK, Kehlet H, Madsbad S, Binder C. Kinetics of human C-peptide in man. Diabetes. 1978;27(Suppl 1):207–209. doi: 10.2337/diab.27.1.s207. [DOI] [PubMed] [Google Scholar]
- 7.Polonsky KS, Licinio-Paixao J, Given BD, Pugh W, Rue P, Galloway J, Karrison T, Frank B. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. The Journal of Clinical Investigation. 1986;77:98–105. doi: 10.1172/JCI112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Service FJ, Rubenstein A, Horwitz DL. C-peptide analysis in diagnosis of factitial hypoglycemia in an insulin-dependent diabetic. Mayo Clinic Proceedings Mayo Clinic. 1975;50:697–701. [PubMed] [Google Scholar]
- 9.Bristow AF, Das RE, Bangham DR. World Health Organization International Standards for highly purified human, porcine and bovine insulins. Journal of Biological Standardization. 1988;16:165–178. doi: 10.1016/0092-1157(88)90004-2. [DOI] [PubMed] [Google Scholar]
- 10.Johnson IS. Human insulin from recombinant DNA technology. Science. 1983;219:632–637. doi: 10.1126/science.6337396. [DOI] [PubMed] [Google Scholar]
- 11.Deberg M, Houssa P, Frank BH, Sodoyez-Goffaux F, Sodoyez JC. Highly specific radioimmunoassay for human insulin based on immune exclusion of all insulin precursors. Clinical Chemistry. 1998;44:1504–1513. [PubMed] [Google Scholar]
- 12.Robbins DC, Andersen L, Bowsher R, Chance R, Dinesen B, Frank B, Gingerich R, Goldstein D, Widemeyer HM, Haffner S, Hales CN, Jarett L, Polonsky K, Porte D, Skyler J, Webb G, Gallagher K. Report of the American Diabetes Association's Task Force on standardization of the insulin assay. Diabetes. 1996;45:242–256. doi: 10.2337/diab.45.2.242. [DOI] [PubMed] [Google Scholar]
- 13.Miller WG, Thienpont LM, Van Uytfanghe K, Clark PM, Lindstedt P, Nilsson G, Steffes MW. Toward standardization of insulin immunoassays. Clinical Chemistry. 2009;55:1011–1018. doi: 10.1373/clinchem.2008.118380. [DOI] [PubMed] [Google Scholar]
- 14.Sapin R. The interference of insulin antibodies in insulin immunometric assays. Clinical Chemistry and Laboratory Medicine : CCLM / FESCC. 2002;40:705–708. doi: 10.1515/CCLM.2002.121. [DOI] [PubMed] [Google Scholar]
- 15.Chevenne D, Letailleur A, Trivin F, Porquet D. Effect of hemolysis on the concentration of insulin in serum determined by RIA and IRMA. Clinical Chemistry. 1998;44:354–356. [PubMed] [Google Scholar]
- 16.Bellomo G, Sulas MG, Mairate E, Bardone MB, Rolla R. Hemolysis is a major cause of variability in insulin measurement during oral glucose tolerance test in children. Clinical Laboratory. 2012;58:67–74. [PubMed] [Google Scholar]
- 17.O'Rahilly S, Burnett MA, Smith RF, Darley JH, Turner RC. Haemolysis affects insulin but not C-peptide immunoassay. Diabetologia. 1987;30:394–396. doi: 10.1007/BF00292540. [DOI] [PubMed] [Google Scholar]
- 18.Finegold DN, Stanley CA, Baker L. Glycemic response to glucagon during fasting hypoglycemia: an aid in the diagnosis of hyperinsulinism. Journal of Pediatrics. 1980;96:257–259. doi: 10.1016/s0022-3476(80)80817-1. [DOI] [PubMed] [Google Scholar]
- 19.Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology and Metabolism. 2009;94:709–728. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 20.Palladino AA, Bennett MJ, Stanley CA. Hyperinsulinism in infancy and childhood: when an insulin level is not always enough. Clinical Chemistry. 2008;54:256–263. doi: 10.1373/clinchem.2007.098988. [DOI] [PubMed] [Google Scholar]
- 21.Placzkowski KA, Vella A, Thompson GB, Grant CS, Reading CC, Charboneau JW, Andrews JC, Lloyd RV, Service FJ. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. The Journal of Clinical Endocrinology and Metabolism. 2009;94:1069–1073. doi: 10.1210/jc.2008-2031. [DOI] [PubMed] [Google Scholar]
- 22.Hirshberg B, Livi A, Bartlett DL, Libutti SK, Alexander HR, Doppman JL, Skarulis MC, Gorden P. Forty-eight-hour fast: the diagnostic test for insulinoma. The Journal of clinical endocrinology and metabolism. 2000;85:3222–3226. doi: 10.1210/jcem.85.9.6807. [DOI] [PubMed] [Google Scholar]
- 23.Marks V. Recognition and differential diagnosis of spontaneous hypoglycaemia. Clinical Endocrinology. 1992;37:309–316. doi: 10.1111/j.1365-2265.1992.tb02329.x. [DOI] [PubMed] [Google Scholar]
- 24.Kao PC, Taylor RL, Service FJ. Proinsulin by immunochemiluminometric assay for the diagnosis of insulinoma. The Journal of Clinical Endocrinology and Metabolism. 1994;78:1048–1051. doi: 10.1210/jcem.78.5.8175958. [DOI] [PubMed] [Google Scholar]
- 25.Vezzosi D, Bennet A, Fauvel J, Caron P. Insulin, C-peptide and proinsulin for the biochemical diagnosis of hypoglycaemia related to endogenous hyperinsulinism. European Journal of Endocrinology / European Federation of Endocrine Societies. 2007;157:75–83. doi: 10.1530/EJE-07-0109. [DOI] [PubMed] [Google Scholar]
- 26.Nauck MA, Meier JJ. Diagnostic accuracy of an “amended” insulin-glucose ratio for the biochemical diagnosis of insulinomas. Annals of Internal Medicine. 2012;157:767–775. doi: 10.7326/0003-4819-157-11-201212040-00004. [DOI] [PubMed] [Google Scholar]
- 27.Stanley CA, Baker L. Hyperinsulinism in infancy: diagnosis by demonstration of abnormal response to fasting hypoglycemia. Pediatrics. 1976;57:702–711. [PubMed] [Google Scholar]
- 28.Lord K, De Leon DD. Monogenic hyperinsulinemic hypoglycemia: current insights into the pathogenesis and management. International Journal of Pediatric Endocrinology. 2013;2013:3. doi: 10.1186/1687-9856-2013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussain K, Challis B, Rocha N, Payne F, Minic M, Thompson A, Daly A, Scott C, Harris J, Smillie BJ, Savage DB, Ramaswami U, De Lonlay P, O'Rahilly S, Barroso I, Semple RK. An activating mutation of AKT2 and human hypoglycemia. Science. 2011;334:474. doi: 10.1126/science.1210878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor SI, Barbetti F, Accili D, Roth J, Gorden P. Syndromes of autoimmunity and hypoglycemia. Autoantibodies directed against insulin and its receptor. Endocrinology and Metabolism Clinics of North America. 1989;18:123–143. [PubMed] [Google Scholar]
- 31.Hirata Y, Uchigata Y. Insulin autoimmune syndrome in Japan. Diabetes Research and Clinical Practice. 1994;24(Suppl):S153–157. doi: 10.1016/0168-8227(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 32.Basu A, Service FJ, Yu L, Heser D, Ferries LM, Eisenbarth G. Insulin autoimmunity and hypoglycemia in seven white patients. Endocrine Practice : Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2005;11:97–103. doi: 10.4158/EP.11.2.97. [DOI] [PubMed] [Google Scholar]