Abstract
2′-Deoxy-2′-[18F]fluoro-5-ethyl-1-β-d-arabinofuranosyluracil ([18F]FEAU) is a promising radiolabeled nucleoside designed to monitor Herpes Simplex Virus Type 1 thymidine kinase (HSV1-tk) reporter gene expression with positron emission tomography (PET). However, the challenging radiosynthesis creates problems for being able to provide [18F]FEAU routinely. We have developed a routine method using a commercial GE TRACERlab FX-FN radiosynthesis module with customized equipment to provide [18F]FEAU. All radiochemical yields are decay corrected to end-of-bombardment and reported as means±SD. Radiofluorination (33±8%; n=4), bromination (85±8%; n=4), coupling reaction (83±6%; n=4), base hydrolysis steps, and subsequent high-performance liquid chromatography purification afforded purified [18F]FEAU β-anomer in 5±1% overall yield (n=3 runs) after ∼5.5 h and a β/α-anomer ratio of 7.4. Radiochemical/chemical purities and specific activity exceeded 99% and 1.3 Ci/μmol (48 GBq/μmol), respectively. In cell culture, [18F]FEAU showed significantly (P<0.05) higher accumulation in C6 cells expressing HSV1-tk/sr39tk as compared to wild-type C6 cells. Furthermore, [18F] FEAU showed slightly higher accumulation than 9-[4-[18F]fluoro-3-(hydroxymethyl)butylguanine ([18F]FHBG) in cells expressing HSV1-tk (P<0.05), whereas [18F]FHBG showed significantly higher (P<0.05) accumulation than [18F]FEAU in HSV1-sr39tk-expressing cells. micro-PET imaging of mice carrying tumor xenografts of C6 cells stably expressing HSV1-tkor HSV1-sr39tk are consistent with the cell uptake results. The [18F]FEAU mouse images also showed very low gastrointestinal signal with predominant renal clearance as compared to [18F]FHBG. The routine radiosynthesis of [18F]FEAU was successfully semiautomated using a commercial module along with customized equipment to provide the β-anomer in modest yields. Although further studies are needed, early results also suggest [18F]FEAU is a promising PET radiotracer for monitoring HSV1-tk reporter gene expression.
Keywords: [18F]FEAU, Semiautomated, Gene therapy, Reporter genes, Thymidine kinase, Molecular imaging
Introduction
Imaging reporter gene expression with positron emission tomography (PET) is a growing field with many types of reporter genes/reporter probes under development and validation [1, 2].
These approaches have major applications in gene/cell therapy, cell trafficking, transgenic models, and have recently entered clinical trials. One of the best validated reporter genes is the Herpes Simplex Virus Type 1 thymidine kinase (HSV1-tk), which can phosphorylate various substrates. A series of 5-substituted fluoroarabinofuranosyl nucleosides with high antiviral activity was first developed by Fox et al. in the late 1970s [3–7]. From these early studies, radiolabeled markers for HSV1-tk reporter gene expression and cell proliferation, such as 2′-deoxy-2′-fluoro-β-d-arabinofuranosyl-5-iodouracil ([14C] FIAU, [18F]FIAU) [8, 9] and 2′-deoxy-2′-fluoro-5-methyl-1-β-d-arabinofuranosyl-uracil ([18F]FMAU), respectively, evolved and are under active investigation. Acycloguanosine derivatives such as 9-(4-[18F]fluoro-3-hydroxylmethylbutyl)guanine ([18F]FHBG) are also under active investigation and have been recently translated into the clinic [10–13]. We have also studied the use of the mutant HSV1-sr39tk reporter gene that was specifically engineered to prefer utilization of acycloguanosine derivatives over endogenous thymidine [14].
Although [18F]FIAU and [18F]FHBG serve as good tracers for HSV1-tk reporter gene imaging, hepatic metabolism and clearance sometimes gives an undesired gastrointestinal background in the PET images, and therefore better substrates are still needed for some applications. We [8] and other groups [9, 15] have recently reported that radiolabeled FEAU, a 5-ethyl-substituted derivative of [18F]FMAU, demonstrates high sensitivity and selectivity to both HSV1-tk and HSV1-sr39tk in cell uptake studies. Unlike [18F]FMAU [16] that binds mainly to mammalian tk and is used as a marker for cell proliferation, [18F]FEAU is a substrate designed for primarily targeting viral tk. Because we were interested in studying the properties of [18F]FEAU and [18F]FHBG (Fig. 1) in tk-expressing tumors in cells and living mice, we needed to devise a reliable method to make the radiotracer routinely with less exposure to the radiochemist. We examined current [18F]FEAU procedures [17, 18] and created a safe and reliable method to provide [18F] FEAU using a commercial radiosynthesis module with secondary customized equipment. Additionally, synthesized [18F]FEAU was tested in cells and tumor mouse models and subsequently compared to [18F]FHBG.
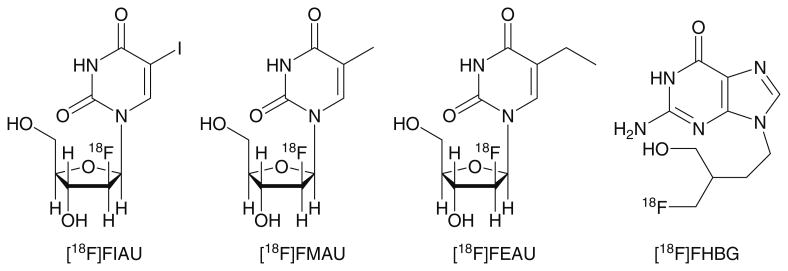
Fig. 1.
Chemical structures of 2′-deoxy-2′-[18F]fluoro-5-iodo-1-β-d-arabinofuranosyluracil ([18F]FIAU), 2′-deoxy-2′-[18F]fluoro-5-methyl-1-β-d-arabinofuranosyluracil ([18F] FMAU), 2′-deoxy-2′-[18F]fluoro-5-ethyl-1-β-d-arabinofurano-syluracil ([18F]FEAU; 8), and 9-[4-[18F]fluoro-3-(hydroxy-methyl)butylguanine ([18F]FHBG).
Materials and Methods
General
No-carrier-added [18F]fluoride was prepared by the 18O (p,n)18F nuclear reaction on a PETtrace cyclotron (GE Medical Systems; Milwaukee, WI). 18O-Enriched water (1.8 mL, >95% isotopic enrichment) was added to the silver target, bombarded with 18 MeV protons (typically 36 μA for 20–25 min), and the entire bolus of irradiated water containing up to 750–1000 mCi (28–37 GBq) was used for the subsequent [18F]FEAU radiosynthesis.
Radioactivity measurements were carried out with a Model CRC-15 PET dose calibrator (Capintec, Ramsey, NJ). Each radiochemical yield is decay corrected and reported as a mean± SD.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified and were used without further purification. Flash chromatography was carried out using silica gel 60 with a mesh of 70–230, 6 Å (Sigma-Aldrich). Thin-layer chromatography was performed using TLC-Silica gel 60W (Merck; Germany). The crude [18F]FEAU mixture was purified with a Dionex P680 quaternary gradient pump (Sunnyvale, CA) and Knauer K-2001 UV detector (Berlin, Germany) set at 254 nm. Preparative high-performance liquid chromatography (HPLC) separation was achieved on Preparative Method A (8% MeCN:92% water, 6 mL/min, 254 nm, Phenomenex Gemini C18, 5 μm, 10×250 mm) for [18F]FEAU or Preparative Method B (50 mM NH4OAc/ethanol [94:6], 5 mL/min, 254 nm, Phenomenex Luna C18, 5 μm, 10×250 mm) for [18F]FHBG. Analytical HPLC was performed using two independent Lab Alliance (LA; State College, PA) systems with each equipped with a LA Series III HPLC pump (1 mL/min), LA Model 500 UV detector (254 nm), Carroll & Ramsey Associates Model 105S pin-diode radioactivity detector (Berkeley, CA), and an SRI Instruments Model 202 four-channel serial port chromatography data system using PeakSimple software (Torrance, CA). The identification of the [18F]FEAU intermediates and [18F]FEAU were confirmed by analytical HPLC using Analytical Method A (80% MeCN:20% water; 1 mL/min, 254 nm, Phenomenex Luna C18, 5 μm, 4.6×250 mm) and Method B (15% MeCN:85% water; 1 mL/min, 254 nm Phenomenex Gemini C18, 5 μm, 4.6×250 mm) columns (Phenomenex; Torrance, CA, respectively. Analytical Method C (15% ethanol:85% water; 1 mL/min, 254 nm, Phenomenex Gemini C18, 5 μm, 4.6×250 mm) was used for identifying [18F]FHBG.
Proton and 19F nuclear magnetic resonance (NMR) were obtained from a Varian Mercury 400-MHz spectrometer (Varian; Palo Alto, CA) using trimethylsilyl ether as an internal standard for the 1H-NMR. Samples were also analyzed (Vincent Coates Foundation Mass Spectrometry Laboratory, Stanford University, CA) using the electrospray technique in positive and negative modes with a Waters Micromass® ZQ™ single-quadrupole mass spectrometer (Waters; Milford, MA).
GE TRACERlab FX-FN and Custom-made Synthesis Modules
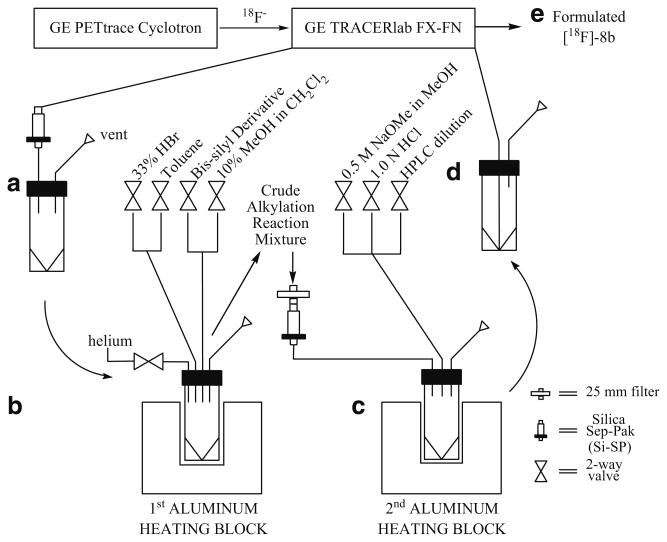
[18F]Fluoride processing, 18F radiolabeling, and preparative HPLC purification of [18F]FEAU were completed in the modified GE TRACERlab FX-FN synthesis module (GE Medical Systems; Milwaukee, WI). The remaining intermediate reaction steps and solid-phase extractions were carried out remotely incorporating a homemade synthesis module (Scheme 1) that was constructed in the laboratory using electronic components for toggle switch control (Newark InOne, Chicago, IL), 24-V Burkert solenoid valves (Consolidated International, National City, CA), and customized glassware (O.Z. Glass, Pinole, CA).
Scheme 1.
Diagram shows connection between the cyclotron, primary and secondary synthesis modules. a 5-mL collection vial receives [18F]-3b after solid phase extraction with Si-SP. b The vial with [18F]-3b is placed into aluminum heating block for the subsequent bromination and alkylation reactions. c [18F]-7a and [18F]-7b are eluted through a Si-SP to a second 5-mL vial for the remaining deprotection step in another heating block. d The vial containing the crude deprotected product is reattached to the 1° module for HPLC purification and product formulation. e Formulated [18F]-8b is sterile filtered into a sterile multidose vial.
Preparation of 4 wt% Tetrabutylammonium Bicarbonate
Carbon dioxide from dry ice was bubbled into a 100-mL graduated cylinder containing tetrabutylammonium hydroxide (10 mL, 4% by weight in water) for 4 h at room temperature. The resulting tetrabutylammonium bicarbonate solution was stored at 0–5°C for use in the radiofluorination step.
Synthesis of Precursors and Reference Standards
2-O-(Trifluoromethylsulfonyl)-1,3,5,-tri-O-benzoyl-α-d-ribofuranose 2
Triflic anhydride (0.65 mL, 3.8 mmol) was added to a solution of 1,3,5-tri-O-benzoylribofuranose 1 (1.76 g, 3.8 mmol) dissolved in anhydrous pyridine (8 mL) and stirred for 20 h at room temperature. The reaction mixture was poured into ice water (10 mL), and the product was extracted with dichloromethane (3×20 mL). The organic layer was washed with 10% HCl (2×10 mL) and saturated NaHCO3 (2×10 mL). After drying the organic fraction over with MgSO4, the solvent was evaporated under vacuum. Crude triflate (1.97 g, 88%) was purified by column chromatography using 3:1 hexane and ethyl acetate as the eluent to afford 1.81 g (81%) of 2 as a colorless viscous oil. The reference standards for the 1H NMR (400 MHz, CDCl3) δ were (ppm): 8.12 (m, 4H), 8.06 (m, 2H), 7.64 (m, 3H), 7.48 (m, 4H), 7.42 (t, 2 H), 6.86 (d, 1H), 5.78 (q, 1H), 5.54 (q, 1H), 4.86 (q, 1H), 4.76 (dd, 1H), 4.62 (dd, 1H). MS, m/z, 594 (100%, [M+Na]+) [19].
2-Deoxy-2-fluoro-1,3,5-tri-O-benzoyl-α-d-arabinofuranose 3a
2-O-(Trifluoromethylsulfonyl)-1,3,5,-tri-O-benzoylribo-furanose 2 (1.155 g, 2.5 mmol) was dissolved in dichloromethane (20 mL). Diethyl amino sulfur trifluoride (1 mL) was added under nitrogen to the solution. The reaction was heated to 40°C overnight (∼16 h). After cooling, the reaction was quenched with saturated sodium bicarbonate (10 mL). The organic layer was washed with brine (2×10 mL) and dried over MgSO4. Column chromatography with chloroform afforded pure fluoro product (0.65 g, 56%). The reference standards for the 1H NMR (400 MHz, CDCl3) δ were (ppm): 8.07–7.42 (m, 15H), 6.76 (d, 1H), 5.63 (dd, 1H), 5.40 (d, 1H), 4.74 (m, 3H). 19F NMR (400 MHz, CDCl3) δ ppm -191.27 (dq, 1F). MS, m/z, 464 (100%, [M+Na]+) [20].
2-Deoxy-2-fluoro-3,5-di-O-benzoyl-α-d-arabinofuranosyl bromide 4a
1,3,5-Tri-O-benzoyl-2-deoxy-2-fluoro-α-d-arabinofuranose 3a (140 mg, 3.1 μmol) was dissolved in anhydrous dichloromethane (3 mL). Hydrobromic acid in acetic acid (0.6 mL, 33 wt%) was added to the solution and stirred at room temperature overnight (∼16 h). The solution was washed with saturated NaHCO3 (3×3 mL) and dried over MgSO4. The solvent was removed in vacuo, and the residue (120 mg, 94.3%) was used in the next step without further purification. The reference standards for the 1H NMR (400 MHz, CDCl3) δ were (ppm): 8.09 (m, 4H), 7.53 (m, 6H), 5.60 (d, 1H), 5.55 (dq, 1H), 4.79 (m, 3H). 19F NMR (400 MHz, CDCl3) δ ppm -170.22 (dq, 1F). MS, m/z, 422 (100%, [M+Na]+) [20].
2,4-Bis-O-(trimethylsilyl)-5-ethyluracil 6
Method A
Hexamethyldisilazane (0.24 mL, 1.2 mmol) was added to a mixture of 5-ethyluracil 5 (140 mg, 1 mmol) and ammonium sulfate (13 mg, 0.1 mmol) in acetonitrile (5 mL). The reaction mixture was refluxed for 3 h, the solvent was removed in vacuo, and the product was used immediately in the next step [19, 21].
Method B (for Radiochemistry)
Hexamethyldisilazane (0.2 ml, 0.96 μmol) was added to a 5-mL round-bottom flask containing 5-ethyluracil 5 (70 mg, 0.5 μmol), ammonium sulfate (6.6 mg, 0.05 μmol), and anhydrous acetonitrile (0.3 mL). The resulting mixture was heated under reflux for 2.5 h under argon. The reaction mixture was cooled and concentrated under reduced pressure to give a cloudy syrup. The residue was diluted with anhydrous acetonitrile (0.1 mL) and used for the subsequent step [19, 21].
1-(3′,5′-Di-O-benzoyl-2′-deoxy-2′-fluoro-d-arabinofuranose)-5-ethyluracil 7a and 7a′
Crude 2,4-bis-O-(trimethylsilyl)-5-ethyluracil 6 from Method A was dissolved in chloroform (2 mL). 2-Deoxy-2-fluoro-3,5-di-O-benzoyl-α-d-arabinofura-nosyl bromide (120 mg, 0.28 mmol) in chloroform (3 mL) was added to the solution, and the reaction mixture was refluxed for 24 h. After cooling to room temperature, the organic layer was washed with water (2×3 mL) and subsequently dried with Na2SO4. After the solvent was evaporated under vacuum, the white residue was dissolved in hot ethanol (∼2 mL) and kept undisturbed at room temperature for 2 days to yield 106 mg (85.7%) of desired anomeric mixture as a white solid. The reference standards for the 1H NMR (400 MHz, d6-DMSO) δ were (ppm): 8.02–7.54 (m, 12H), 7.30 (s, 1H), 6.31 (dd, 1H), 5.71 (dd, 1H), 5.54 (dd, 1H), 4.80 (m, 1H), 4.64 (m, 2H), 2.03 (m, 2H), 0.830 (t, 3H). 19F NMR (400 MHz, d6-DMSO) δ ppm -199.93 (dq, 1F). MS, m/z, 482 (100%, [M+H]+) [19, 21].
1-(2′-Deoxy-2′-fluoro-d-arabinofuranosyl)-5-ethyl uracil 8a and 8a′
The mixture of 7a and 7a′ (100 mg, 0.21 mmol) was dissolved in 2 M ammonia in methanol (2 ml), and the solution was stirred at room temperature overnight. The solvent was removed in vacuo, and FEAU was recrystallized with acetonitrile to give 8a/8a′ (49.8 mg, 89.6%). The reference standards for the 1H NMR (400 MHz, CD3OD) δ were (ppm): missing ∼11 (s, 1H), 7.63 (d, 1H), 6.18 (dd, 1H), 5.00 (dq, 2H), 4.57 (dq, 1H), 3.73 (m, 3H), 3.30 (m, 1H), 2.30 (m, 2H), 1.11 (m, 3H). F NMR (400 MHz, CD3OD) δ ppm -201.11 (dq, 1F). MS, m/z, 274 (100%, [M-H]+) [21, 22].
Radiochemistry
Synthesis of 2-deoxy-2-[18F]fluoro-1,3,5-tri-O-benzoyl-α-d-arabinofuranose[18 F]-3b
Cyclotron-produced [18F]fluoride ion in 18O-water was delivered directly to the glass reactor of a TRACERlab FX-FN module followed by an addition of freshly-prepared 4 wt% tetrabutylammonium bicarbonate (5 μL, 0.8 μmol) in anhydrous MeCN (1.5 mL). The mixture was taken to dryness at 85°C under reduced pressure with helium flow. A further cycle of acetonitrile (2 mL) addition and evaporation was performed to ensure the complete removal of water. Triflate 2 (5 mg, 8.4 μmol) in MeCN (0.5 mL) was added to the anhydrous [18F]fluoride residue and heated to 85°C for 30 min. The reaction mixture is cooled and loaded onto a Silica Sep-Pak plus cartridge. [18F]-3b (33±8%; n=4) was eluted off the cartridge with ethyl acetate (3 mL) into a 5-mL conical vial and used directly in the next step. Product identity (tR=9.6 min) was confirmed by using analytical HPLC Method A [16, 18].
Synthesis of 2-deoxy-2-[18F]fluoro-3,5-di-O-benzoyl-α-d-arabinofuranosyl bromide [18F]-4b
Ethyl acetate was completely evaporated from the [18F]-3b solution with heating at 68°C and helium flow (pressure=250 kPa) in approximately 10 min. After solvent evaporation was complete, 33 wt% hydrobromic acid (100 μL) in dichloroethane (0.4 mL) was added to the [18F]-3b residue. The acidic mixture was sealed and heated to 80°C for 10 min. An aliquot (5 μL) was removed for HPLC analysis by Method A. The remaining solvent was evaporated at 68°C and helium flow. An additional evaporation cycle with toluene (1 mL) was also completed. After product identity (tR= 8.0 min) was confirmed, the [18F]-4 residue (85±8%; n=4) was used directly in the coupling step [16].
Synthesis of 1-(3′,5′-di-O-benzoyl-2′-deoxy-2′-[18F]fluoro-β-d-arabinofuranose)-5-ethyluracil 7b and 7b′
Dichloroethane (0.5 mL) was added to the crude 2,4-bis-O-(trimethylsilyl)-5-ethyluracil 6 from the Method B, and the entire mixture was transferred to the conical vial containing the dried [18F]-4b residue. The reaction mixture was sealed and heated to 100°C for 1 h. After the reaction was cooled with an ice bath, an aliquot (5 μL) was removed for HPLC analysis by Method A. The remaining mixture was loaded onto a silica gel Sep-pak plus cartridge, and the cartridge was eluted with 10% methanol in dichloromethane (3 mL). After coupled products were confirmed (α-anomer: tR=4.4 min; β-anomer: tR = 4.8 min) by standard coinjection, the anomeric [18F]-7 mixture (83 ±6%; n=4) was not separated and used directly in the final deprotection step [16].
Synthesis of 1-(2′-deoxy-2′-[18F]fluoro-β-d-arabinofurano-syl)-5-ethyl uracil [18F]-8b
[18F]-8b was made following a modified literature procedure for [18F]FMAU [16]. The organic solvents were evaporated from the [18F]-7 mixture via heating at 45°C and helium flow. Once dryness was achieved, 0.5 M sodium methoxide in methanol (0.5 mL) was added to the dried [18F]-7 residue. The reaction mixture was sealed and heated to 100°C for 5 min. After the reaction was cooled with an ice bath, 1.0 N hydrochloric acid (0.25 mL) was added to quench the reaction, and the mixture was diluted with preparative HPLC mobile phase (2 mL). The crude mixture was reintroduced to the TRACERlab FX-FN module for HPLC separation using Preparative Method A to easily isolate pure β-anomer, [18F]-8b, away from the α-form. The pure HPLC fraction was collected (tR= 16.5 min), subsequently processed by solid phase extraction using a C-18 Sep-pak plus cartridge, formulated with ethanol (1 mL) and 0.9% saline (9 mL), and sterile filtered with a Millex-MP filter (25 mm). HPLC analysis was performed on the formulated product using Method B for confirmed product identification (tR=7.0 min). Base hydrolysis steps and subsequent HPLC purification separated the anomeric mixture to afford purified [18F]FEAU β-anomer in 5±1% overall yield (n=3 runs) after ∼5.5 h and a β/α-anomer ratio of 7.4.
Synthesis of 9-[4-[18F]fluoro-3-(hydroxymethyl)butylgua-nine
In a TRACERlab FX-FN module, a Chromafix® 18F-separation cartridge (45 mg PS-HCO3) was used to trap the cyclotron-produced [18F]fluoride. The radioactivity was transferred into the reactor by elution with Kryptofix 2.2.2 (15 mg)/potassium carbonate (3.5 mg) in 9:1 acetonitrile/water (1 mL). A dried [18F]fluoride/Kryptofix 2.2.2/potassium carbonate complex resulted when the mixture was taken to dryness at 85°C under reduced pressure with helium flow. Tosyl-FHBG (0.5 mg, 0.53 μmol; ABX Advanced Biochemical Compounds, Germany) in MeCN (0.5 mL) was added to the anhydrous [18F]fluoride residue and heated to 160°C for 20 min. The reaction mixture was cooled, diluted with water (6 mL), and passed through a preconditioned C-18 Sep-pak plus cartridge. Another aliquot of water (6 mL) was used to rinse the reactor and wash the loaded C-18 cartridge. The trapped 18F-radiolabeled intermediate was eluted off the cartridge with methanol (2 mL) back into the TRACERlab FX-FN reactor. Hydrochloric acid (1.0 N, 0.4 mL) was added to the reactor, and the mixture was heated to 105°C for 7 min. After cooling the reactor to 40°C, sodium hydroxide (1.0 N, 0.2 mL) and 50 mM ammonium acetate (NH4OAc) in ethanol (94:6 v/v, 2 mL) were added to neutralize and dilute the mixture for preparative HPLC purification. Purified [18F]FHBG was isolated (tR=11.5 min) using Preparative Method B and passed through four preconditioned C-18 Sep-Pak plus cartridges connected in series. The loaded cartridges were eluted with 10% ethanol in 0.9% saline (5 mL), and the eluent was sterile filtered (Millex-GS; 33 mm; 0.2 μm) into a sterile multidose vial (30 mL) to give the final formulated product in 6±2% (n=69 runs). Product identity (tR=5.5 min) was confirmed by using analytical HPLC Method C [13].
Cell Culture and Uptake Studies
All the uptake studies were performed using rat glioma C6 cells (ATCC, Manassas, VA) cultured in high-glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution. Two stable clones of C6 cells expressing either HSV1-sr39 thymidine kinase (C6-sr39tk+) or a triple-fusion vector carrying a wild-type HSV1-thymidine kinase (C6-tk+) were utilized. C6, C6-tk+, and C6-sr39tk+ cells (5×105) were plated in 12 well dishes [23]. After 24 h, [18F]FEAU or [18F]FHBG (1 μCi) was added in each well and incubated at 37°C for 1 h. Cells were then washed three times with 1× phosphate-buffered saline and lysed in thymidine kinase lysis buffer, and the radioactivity of each well was counted in a Cobra II gamma counter (Perkin-Elmer). Protein concentration of the cell lysates were measured after the decay of radioactivity. All results were expressed as mean ± standard error (n=4) of counts per minute normalized to total protein (CPM/μg P). Selective indices were calculated as accumulation ratios between transfected cell and control cell to compare the selectivity to HSV1-tk or HSV1-sr39tk between probes.
Micro-PET imaging of [18F]FEAU and [18F] FHBG Uptake in Tumor-bearing Mice
Animal care and euthanasia were performed with the approval of the Administrative Panels on Laboratory Animal Care of Stanford University. One million of C6-tk+ and C6-sr39tk+ cells were implanted on two shoulders of 4–6-week-old nude mice (n=5). When the tumors were 3–5 mm in diameter, ∼200 μCi per mouse of [18F]FEAU was injected via tail vein, and micro-PET scans (Siemens, R4) were performed after 1 h for 10 min. Next day, the same mice were again scanned for [18F]FHBG (∼200 μCi/mouse) uptake. The micro-PET images were reconstructed with the ordered-subsets expectation maximization algorithm [24] and were analyzed by using a Medical Imaging Data Examiner (AMIDE) software [25]. The percentage-injected dose per gram (% ID/g) was calculated by dividing the region of interest counts by the injected dose (decay corrected) and reported as mean±standard error.
Statistical Analysis
Data are reported as mean±standard error. A paired Student's t test was used to compare results across groups. A P value of less than 0.05 was considered statistically significant.
Results
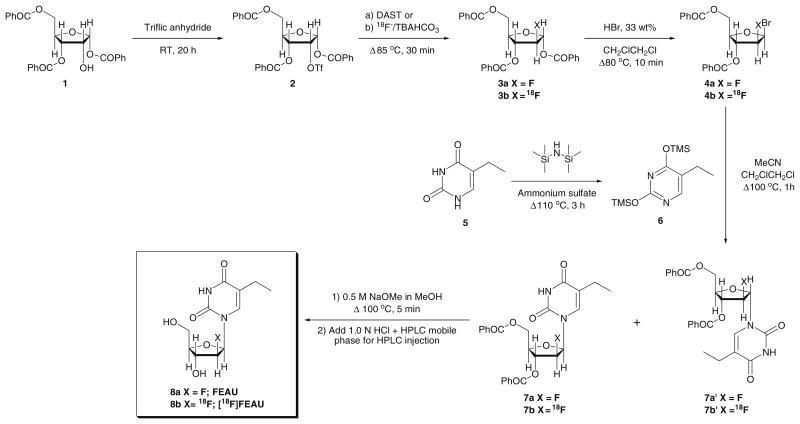
The radiofluorination of 1 in the GE TRACERlab FX-FN synthesis module produced the radiolabeled intermediate 2 in moderate but reliable yields (33±8%; n=4) after purification using a silica gel Sep-Pak cartridge (Scheme 2). Purified intermediate 2 was transferred to a second custom module for the remaining reaction steps. Bromination of the purified material with 33% hydrobromic acid in acetic acid provided the bromo-derivative 3 in 85±8% (n=4) and was used directly in the next step after the solvent was removed by evaporation with toluene. Thymine bis-silyl derivative 5 was prepared in parallel to the initial two steps and immediately before the subsequent alkylation reaction which gave the coupled product in 83±6% yield (n=4). Deprotection of 5 using sodium methoxide afforded [18F]FEAU as a mixture of α and β anomers. Special care and handling (i.e., maintaining inert environment and proper storage of sodium methoxide) of 5 and sodium methoxide were important in the success of making [18F]FEAU. HPLC purification of the anomeric mixture provided the β-anomer 8 (5±1% overall yield; n=3) after ∼5.5 h and revealed a β/α-anomer ratio of 7.4 after the completion of the multistep synthesis. Radiochemical/chemical purities (Fig. 2) and specific activity were greater than 99% and greater than 1.3 Ci/μmol, respectively.
Scheme 2.
The radiosynthetic pathway for 2′-deoxy-2′-fluoro-5-ethyl-1-β-d-arabinofuranosyluracil 8a and 2′-deoxy-2′-[18F] fluoro-5-ethyl-1-β-d-arabinofuranosyluracil 8b.
Fig. 2.
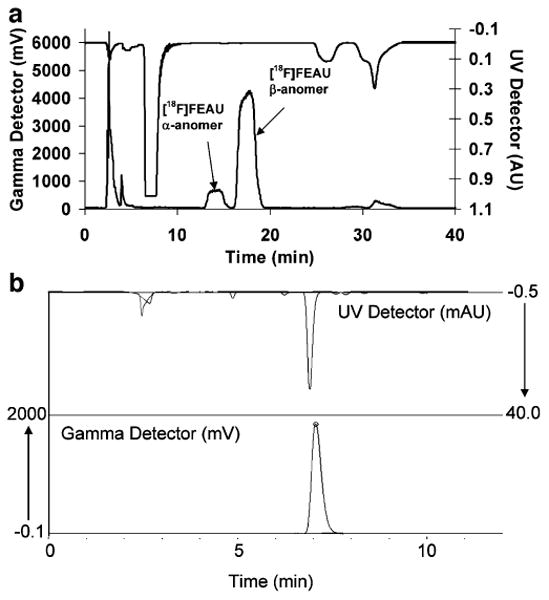
UV absorbance (λ=254 nm) and radioactivity HPLC chromatograms for [18F]FEAU and carrier: a preparative purification and b QC analysis.
In cell culture, both [18F]FEAU and [18F]FHBG show low accumulation at 1 h in control C6 cells with a slightly higher and statistically significant (P<0.05) accumulation of [18F]FHBG (Fig. 3). C6 cells stably expressing HSV1-tk show slightly higher accumulation of [18F]FEAU than [18F]FHBG (P<0.05), and C6 cells stably expressing HSV1-sr39tk show statistically greater (P<0.05) accumulation of [18F]FHBG over [18F]FEAU (Fig. 3). The selectivity index of both tracers is shown in Fig. 4. As seen, the selectivity indices of [18F]FEAU is significantly higher than [18F]FHBG for C6-tk+ cells (P<0.05), whereas the selectivity indices for [18F]FHBG is greater than [18F]FEAU (P<0.05) in C6-sr39tk+ cells. Overall, the C6-sr39tk+ cells show a 100-fold higher accumulation of both [18F]FEAU and [18F] FHBG than the C6-tk+ cells (Fig. 3).
Fig. 3.
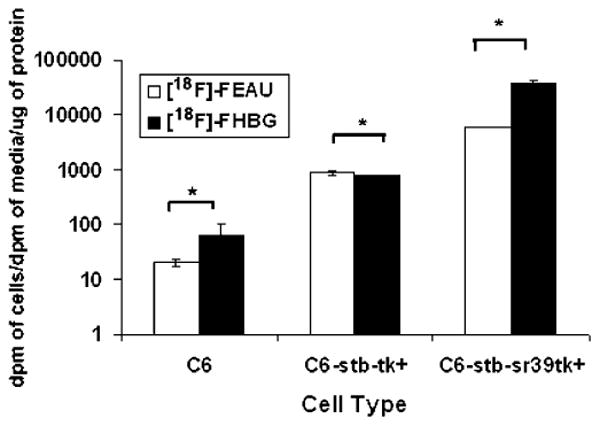
Accumulation of [18F]FEAU and [18F]FHBG in control cells and cells stably transfected with HSV1-tk or HSV1-sr39tk. C6 rat glioma cells (C6, C6-stb-tk+, and C6-stb-sr39tk+). Values are accumulation of each tracer (dpm cells/dpm medium), normalized by μg of total protein. Y-axis is log scale (asterisk denotes P<0.05).
Fig. 4.
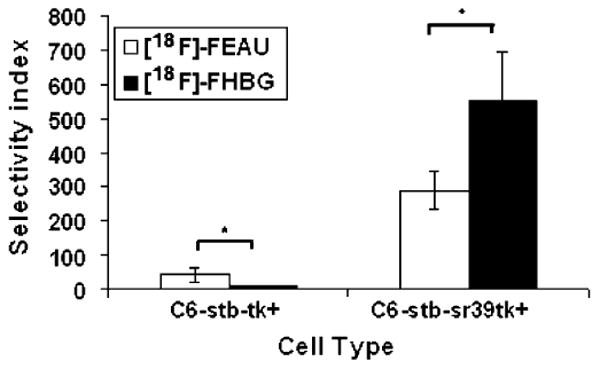
Selectivity index (average ratio of tracer accumulation between C6-sr39tk+ and C6-tk+ cells) of [18F]FHBG and [18F]FEAU (asterisk denotes P<0.05).
Micro-PET imaging of mice carrying tumor xenografts of C6-tk+ and C6-sr39tk+ imaged with both [18F]FEAU and [18F]FHBG are shown in Fig. 5. In accordance with the cell culture results, the C6-tk+ tumors show higher accumulation of [18F]FEAU as compared to [18F]FHBG (0.484±0.012 vs 0.155±0.028% ID/g; P<0.05), while the C6-sr39tk+ tumors show threefold greater accumulation of [18F]FHBG than [18F]FEAU (8.84±1.26 vs 2.75±0.45% ID/g; P<0.05) in Fig. 6. The [18F]FEAU images show very low gastrointestinal signal with predominant renal clearance as compared to [18F]FHBG.
Fig. 5.
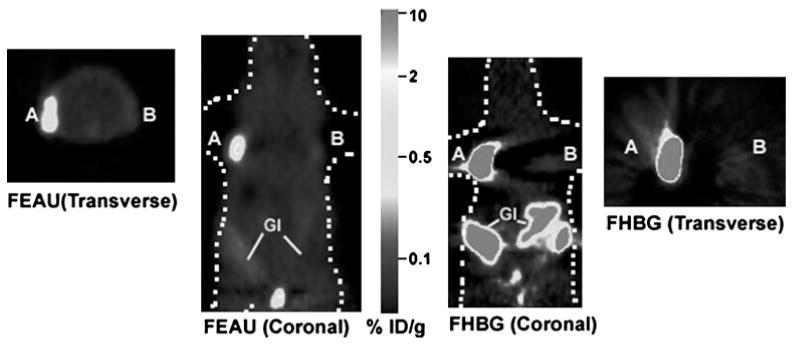
Micro-PET images of [18F]FEAU and [18F]FHBG uptake in a mouse xenograft model. One million of C6-sr39tk+ (A) and C6-tk+ (B) cells were implanted on the left and right shoulder of a nude mouse, and tumors were allowed to grow till they reach 3–5 mm of diameter. The mouse was first injected with ∼200 μCi of [18F]FEAU via tail vein and scanned in a micro-PET for 10 min after 1 h. Specific uptake of [18F] FEAU is seen in both tumors (A and B). After 24 h, the same mouse was injected with ∼200 μCi of [18F]FHBG via tail vein, and specific accumulation is again observed in both the tumors. Both transverse and coronal images of [18F]FHBG and [18F]FEAU scans are shown. [18F]FHBG showed relatively high signal in the gastro-intestinal tract (GI) because of clearance of the probe whereas [18F]FEAU exhibited very low signal in the gastro intestinal tract due to predominantly renal clearance. The color scale is in %ID/g tissue.
Fig. 6.
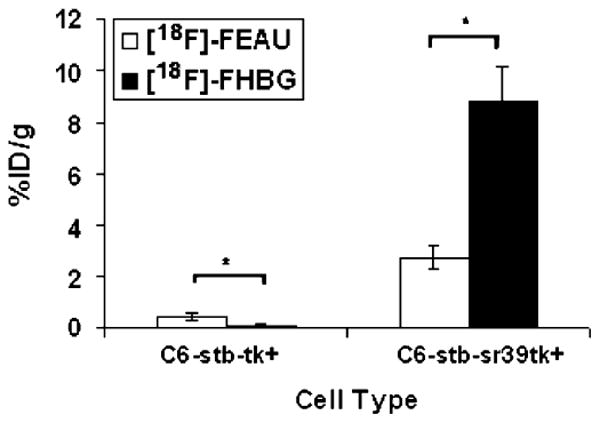
Comparison of [18F]FEAU and [18F]FHBG accumulation in five nude mice bearing both C6-sr39tk+ and C6-tk+ tumors. Percent injected dose per gram (%ID/g) of [18F] FHBG and [18F]FEAU uptakes were calculated for the respective ROIs drawn over the tumors of the experiment mentioned in Fig. 5 (A and B). C6-sr39tk+ tumors showed significantly higher uptake of [18F]FHBG than [18F]FEAU, while C6-tk+ tumors showed higher uptake of [18F]FEAU than [18F]FHBG (asterisk denotes P<0.05).
Discussion
Many studies have been performed to develop optimized radiolabeled reporter probes for imaging HSV1-tk and HSV1-sr39tk reporter gene expression. Most of these studies have used stably transfected cell lines in cell culture and/or in xenograft models to compare the efficiency between pyrimidine nucleoside and acycloguanosine derivatives, while some have used adenoviral-mediated gene delivery [26]. Pyrimidine nucleoside derivatives such as FIAU not only show higher cell uptake than acycloguanosine analogues such as fluoropencyclovir (PCV) or FHBG in HSV1-tk stably transfected cells but also show higher uptake in nontransfected tumor cells as compared to acycloguanosines [27].
Because of a similar structure to natural thymidine, FIAU has been used for imaging HSV1-tk reporter gene expression [28–32]. However, FIAU is not suitable for antiviral therapeutic applications because FIAU is severely toxic to mitochondria in normal cells and fatally toxic to neurons and liver in clinical studies. Another 5-substituted nucleoside, FEAU, was also developed as an anti-Herpes Simplex agent [33, 34] FEAU demonstrated lower cellular toxicity but is still as effective as FIAU to treat cells infected with Herpes Simplex Virus. We [8] and others [35] have demonstrated the potential utility of [3H]FEAU as a reporter probe for HSV1-tk/sr39tk reporter gene expression. A few groups have made [18F]FEAU following two slightly different synthetic routes for making various 18F-labeled arabinofuranosyl nucleosides [16, 17]. In the current study, we examined these routes and developed methods for synthesizing [18F] FEAU with both commercial and custom-made radiochemistry modules in our laboratory. Furthermore, we studied [18F]FEAU and compared it to [18F]FHBG for quantifying HSV1-tk/sr39tk reporter gene expression in cell culture and imaging in mouse tumor xenograft models.
Mutant HSV1-tk's were initially developed to more effectively utilize acyclovir and ganciclovir (GCV) and to less effectively utilize thymidine to allow for more effective suicide gene therapy [36]. We have attempted to improve the sensitivity of imaging reporter gene expression by using an alternate substrate such as GCV, PCV, and FHBG and the mutant reporter gene HSV1-sr39tk [14]. It is interesting to note that this mutant worked well for PCV and its derivatives although not initially selected utilizing those drugs [14]. Accumulation of [18F]PCV and [18F]FHBG were significantly greater than that of FIAU in both stably transfected and adenoviral-infected cells expressing a mutant HSV1-sr39tk [14].
In the current study, we observed a relatively higher accumulation of [18F]FEAU as compared to [18F]FHBG in cells expressing the HSV1-tk reporter gene as we reported earlier with [3H]FEAU [8]. However for cells expressing HSV1-sr39tk, [18F]FHBG accumulation was higher than [18F]FEAU. We are not able to make absolute comparisons between [18F]FEAU/HSV1-tk vs [18F]FHBG/HSV1-sr39tk as this depends on the exact levels of the reporter enzyme in each cell line studied. Furthermore, we did not study effects of viral delivery of reporter as compared to stable expression of the reporter, and this may also affect results as we have previously reported [27].
To be a good candidate for in vivo imaging, the reporter probe needs to have not only high sensitivity and selectivity for HSV1-tk/sr39tk but also favorable in vivo pharmacokinetic profiles. The lipophilic FHBG reveals both hepatobiliary and renal excretion pathways, sometimes making it harder to interpret reporter gene expression in the abdominal/pelvic areas. The use of [124I]FIAU can be limited because of deiodination in vivo, constraints in 124I availability, and a relatively long half-life (t1/2=4.2 days) limiting rapid serial imaging We demonstrated in the current work the favorable in vivo imaging characteristics of [18F]FEAU. There is relatively low background signal with good renal clearance. Future studies will need to look at accumulation in additional cell lines, effects of extracellular thymidine on uptake, and studies in living subjects with both tumor models and adenoviral/lentiviral-mediated gene delivery.
Conclusion
The routine radiosynthesis of [18F]FEAU was successfully semiautomated using a commercial module along with customized equipment to provide the β-anomer in modest yields. Although further studies are needed, early results in cells and living mice suggest [18F]FEAU is a promising PET radiotracer for monitoring HSV1-tk reporter gene expression in living subjects.
Acknowledgments
This research was supported in part by ICMIC P50 (SSG) and NIH R05 CA 082214 (SSG).
References
- 1.Penuelas I, Gambhir SS. Imaging studies for evaluating gene therapy in translational research. Drug Discov Today Technol. 2005;2:335–343. doi: 10.1016/j.ddtec.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Min JJ, Gambhir SS. Gene therapy progress and prospects. Noninvasive imaging of gene therapy in living subjects. Gene Therapy. 2004;11:115–125. doi: 10.1038/sj.gt.3302191. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe KA, Reichman U, Hirota K, Lopez C, Fox JJ. Nucleosides. 110, synthesis and antiherpes virus activity of some 2′-fluoro-2′deoxyarabinofuranosylpyrimidine nucleosides. J Med Chem. 1979;22:21–24. doi: 10.1021/jm00187a005. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe KA, Su TL, Klein RS, et al. Nucleosides. 123. Synthesis of antiviral nucleosides: 5-substituted of 1-(2-deoxy-2-halogeno-β-D-arabinofuranosyl)cytosines and-uracils. some structure-activity relationships. J Med Chem. 1983;26:152–156. doi: 10.1021/jm00356a007. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe KA, Su TL, Reichman U, Greenberg N, Lopez C, Fox JJ. Nucleosides. 129. Synthesis of antiviral nucleosides: 5-alkenyl-1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)uracils. J Med Chem. 1984;27:91–94. doi: 10.1021/jm00367a020. [DOI] [PubMed] [Google Scholar]
- 6.Perlman ME, Watanabe KA, Schinazi RF, Fox JJ. Nucleosides. 133. Synthesis of 5-alkenyl-1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl) cytosines and related pyrimidine nucleosides as potential antiviral agents. J Med Chem. 1985;28:741–748. doi: 10.1021/jm00383a009. [DOI] [PubMed] [Google Scholar]
- 7.Su TL, Watanabe KA, Shinazi RF, Fox JJ. Nucleosides. 136. Synthesis and antiviral effects of several 1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-5-alkyluracils. Some structure–activity relationships. J Med Chem. 1986;29:151–154. doi: 10.1021/jm00151a025. [DOI] [PubMed] [Google Scholar]
- 8.Kang KW, Min JJ, Chen X, Gambhir SS. Comparison of [14C] FMAU, [3H]FEAU, [14C]FIAU, and [3H]PCV for monitoring reporter gene expression of wild type and mutant herpes simplex virus type 1 thymidine kinase in cell culture. Mol Imaging Biol. 2005;7:296–303. doi: 10.1007/s11307-005-0010-7. [DOI] [PubMed] [Google Scholar]
- 9.Buursma AR, Rutgers V, Hospers GAP, Mulder NH, Vaalburg W, de Vries EFJ. [18F]FEAU as a radiotracer for herpes simplex virus thymidine kinase gene expression: in-vitro comparison with other PET tracers. Nucl Med Commun. 2006;27:25–30. doi: 10.1097/01.mnm.0000186609.12895.20. [DOI] [PubMed] [Google Scholar]
- 10.Yaghoubi S, Barrio JR, Dahlbom M, et al. Human pharmacokinetic and dosimetry studies of [18F]FHBG: a reporter probe for imaging herpes simplex virus type-1 thymidine kinase reporter gene expression. J Nucl Med. 2001;42:1225–1234. [PubMed] [Google Scholar]
- 11.Panuelas I, Mazzolini G, Boan JF, et al. Positron emission tomography imaging of adenoviral-mediated transgene expression in living cancer patients. Gastroenterology. 2005;128:1787–1795. doi: 10.1053/j.gastro.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Panuelas I, Haberkorn U, Yaghoubi S, Gambhir SS. Gene therapy imaging in patients for oncological applications. Eur J Nucl Med Mol Imaging. 2005;32:S384–403. doi: 10.1007/s00259-005-1928-3. [DOI] [PubMed] [Google Scholar]
- 13.Yaghoubi S, Couto MA, Chen C, et al. Preclinical safety evaluation of 18F-FHBG: a PET reporter probe for imaging herpes simplex virus type 1 Thymidine Kinase (HSV1-tk) or mutant HSV1-sr39tk's expression. J Nucl Med. 2006;47:706–715. [PubMed] [Google Scholar]
- 14.Gambhir SS, Bauer E, Black ME, et al. A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc Natl Acad Sci USA. 2000;97:2785–2790. doi: 10.1073/pnas.97.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajitou A, Trepel M, Lilley CE, et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell. 2006;125:385–398. doi: 10.1016/j.cell.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 16.Alauddin MM, Conti PS, Fissekis JD. Synthesis of [18F]-labeled 2′deoxy-2′fluoro-5-methyl-1-β-D-arabinofuranosyluracil ([18F]-FMAU) J Label Compd Radiopharm. 2002;45:583–590. [Google Scholar]
- 17.Mangner TJ, Klecker RW, Anderson L, Shields AF. Synthesis of 2′-deoxy-2′-fluoro-5-methyl-1-β-D-arabinofuranosyl nucleosides, [18F] FAU, [18F]FMAU, [18F]FBAU, and [18F]FIAU, as potential PET agents for imaging cellular proliferation. Nucl Med Biol. 2003;30:215–224. doi: 10.1016/s0969-8051(02)00445-6. [DOI] [PubMed] [Google Scholar]
- 18.Alauddin MM, Conti PS, Fissekis JD. A general synthesis of 2′-deoxy-2′-fluoro-5-methyl-1-β-D-arabinofuranosyluracil and its 5-substituted nucleosides. J Label Compd Radiopharm. 2003;46:285–289. [Google Scholar]
- 19.Tann CH, Brodfuehrer PR, Brundidge SP, Sapino C, Howell HG. Fluorocarbohydrates in synthesis. An efficient synthesis of 1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-5-iodouracil (β-FIAU) and 1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)thymine (β-FMAU) J Org Chem. 1985;50:3644–3647. [Google Scholar]
- 20.Wilds CJ, Damha MJ. 2′-Deoxy-2′-fluoro-β-D-arabinonucleosides and oligonucleotides (2′F-ANA): synthesis and physicochemical studies. Nucleic Acids Res. 2000;28:3625–3635. doi: 10.1093/nar/28.18.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansuri MM, Ghazzouli I, Chen MS, et al. 1-(2-Deoxy-2-fluoro-.beta.-D-arabinofuranosyl)-5-ethyluracil. A highly selective anti-herpes simplex agent. J Med Chem. 1987;30:867–871. doi: 10.1021/jm00388a021. [DOI] [PubMed] [Google Scholar]
- 22.Howell HG, Brodfuehrer PR, Brundidge SP, Benigni DA, Sapino C. Antiviral nucleosides. A stereospecific, total synthesis of 2′-fluoro-2′-deoxy-beta-D-arabinofuranosyl nucleosides. J Org Chem. 1988;53:85–88. [Google Scholar]
- 23.Ray P, Tsien R, Gambhir SS. Construction and validation of improved triple fusion reporter gene vectors for molecular imaging of living subjects. Cancer Res. 2007;67:3085–3093. doi: 10.1158/0008-5472.CAN-06-2402. [DOI] [PubMed] [Google Scholar]
- 24.Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13:601–619. doi: 10.1109/42.363108. [DOI] [PubMed] [Google Scholar]
- 25.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2:131–137. doi: 10.1162/15353500200303133. [DOI] [PubMed] [Google Scholar]
- 26.Min JJ, Iyer M, Gambhir SS. Comparison of [18F]FHBG and [14C]FIAU for imaging of HSV1-tk reporter gene expression: adenoviral infection vs. stable transfection. Eur J Nucl Med Mol Imaging. 2003;30:1547–1560. doi: 10.1007/s00259-003-1238-6. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs A, Tjuvajev JG, Dubrovin M, et al. Positron emission tomography-based Imaging of transgene expression mediated by replication-conditional, oncolytic Herpes Simplex Virus Type 1 mutant vectors in vivo. Cancer Res. 2001;61:2983–2995. [PubMed] [Google Scholar]
- 28.Tjuvajev JG, Doubrovin M, Akhurst T, et al. Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J Nucl Med. 2002;43:1072–1083. [PubMed] [Google Scholar]
- 29.Tjuvajev JG, Stockhammer G, Desai R, et al. Imaging the expression of transfected genes in vivo. Cancer Res. 1995;55:6126–6132. [PubMed] [Google Scholar]
- 30.Tjuvajev JG, Finn R, Watanabe K, et al. Noninvasive imaging of herpes virus thymidine kinase gene transfer and expression: a potential method for monitoring clinical gene therapy. Cancer Res. 1996;56:4087–4095. [PubMed] [Google Scholar]
- 31.Tjuvajev JG, Avril N, Oku T, et al. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58:4333–4341. [PubMed] [Google Scholar]
- 32.Tjuvajev JG, Chen SH, Joshi A, et al. Imaging adenoviral-mediated herpes virus thymidine kinase gene transfer and expression in vivo. Cancer Res. 1999;59:5186–5193. [PubMed] [Google Scholar]
- 33.Watanabe KA, Su TL, Reichman U, Greenberg N, Lopez C, Fox JJ. Nucleosides. 129. Synthesis of antiviral nucleosides: 5-alkenyl-1-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)uracils. J Med Chem. 1984;1:91–94. doi: 10.1021/jm00367a020. [DOI] [PubMed] [Google Scholar]
- 34.Perlman ME, Watanabe KA, Schinazi RF, Fox JJ. Nucleosides. 133. Synthesis of 5-alkenyl-1-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl) cytosines and related pyrimidine nucleosides as potential antiviral agents. J Med Chem. 1985;28:741–748. doi: 10.1021/jm00383a009. [DOI] [PubMed] [Google Scholar]
- 35.Kong XB, Vidal P, Tong WP, Chiang J, Gloff CA, Chou TC. Preclinical pharmacology and pharmacokinetics of the anti-hepatitis virus agent 2′-fluoro-5-ethyl-1-beta-D-arabinofuranosyluracil in mice and rats. Antimicrob Agents Chemother. 1992;36:1472–1477. doi: 10.1128/aac.36.7.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black ME, Newcomb TG, Wilson HM, Loeb LA. Creation of drug-specific herpes simplex virus type 1 thymidine kinase mutants for gene therapy. Proc Natl Acad Sci USA. 1996;93:3525–3529. doi: 10.1073/pnas.93.8.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]