Abstract
Individuals with ASD show differences in face processing abilities from early in development. To examine whether these differences reflect an atypical versus delayed developmental trajectory, neural responses to familiar and unfamiliar faces in 24 18-to 47-month-old children with ASD were compared to responses of 32 12- to 30-month-old typically developing children. Results of two experiments revealed that neural responses to faces in children with ASD resembled those observed in younger typically developing children, suggesting delayed development. Electrophysiological responses to faces were also related to parent-report of adaptive social behaviors for both children with ASD and typical development. Slower development of the face processing system in ASD may be related to reduced self-directed ‘expected’ experience with faces in early development.
Keywords: autism, autism spectrum disorders, ASD, event-related potentials, ERP, face processing, face memory, toddlers
Autism Spectrum Disorder (ASD) is a group of neurodevelopment disorders characterized by impairments in social and communication functioning, and a restrictive and repetitive pattern of behaviors and/or interests. Studies of face processing in ASD provide information about the development of basic aspects of social brain circuitry and have demonstrated that individuals with ASD show impaired face processing ability when assessed using behavioral paradigms (e.g., Blair, Frith, Smith, Abell, & Cipolotti, 2002; Hauck, Fein, Maltby,Waterhouse, & Feinstein, 1998; for review Webb, Faja & Dawson, 2009), electrophysiology (e.g., McPartland, Dawson, Webb, Panagiotides, & Carver, 2004; O'Connor, Hamm & Kirk, 2005; Webb, Merkle et al.; 2009), or functional MRI (e.g., Kleinhans et al., 2008; Schultz et al., 2000).
One line of evidence that processing and recognizing faces may also be an area of weakness for very young children with ASD comes from work with event-related potentials (ERPs), derived from scalp-recorded electroencephalography (EEG). The EEG signal originates from summed post-synaptic potentials of synchronously-firing cortical neurons, and scalp-recorded EEG thus provides a non-invasive method of recording neural responses with extreme temporal precision. Dawson, Webb and colleagues used ERPs to examine neural responses to familiar and unfamiliar faces in 3- to 4-year-old children with ASD, and age-matched children with typical development (Dawson et al., 2002; Webb, Dawson, Bernier, & Panagiotides, 2006). While typically developing children showed amplitude differences in three key ERP components in response to familiar versus unfamiliar faces (the Nc or anterior early negative component; the P400 or mid-latency posterior positive component; and the PSW or late anterior positive slow-wave response), the ASD group did not (Dawson et al., 2002). Furthermore, an early posterior response to faces (the prN170 or N290) peaked at a longer latency in the children with ASD than the typically developing children (Webb et al., 2006). Thus, the children with ASD showed differences in their ERP responses to familiarity, as well as slower neural responses to faces in general. In both of these reports, the morphology of the ERP responses to faces was very similar between children with typical development and ASD (also see Dawson, Webb, Carver, Panagiotides, & McPartland, 2004) and ERP responses to object familiarity did not differ between groups despite significant group differences in mental ability (Dawson et al., 2002).
What can account for these apparent differences in face processing? Current accounts of the typical development of face processing stress ‘experience-expectant’ processes, that is, the importance of interaction between pre-existing neural structures and experiences that are typically available for all infants (Greenough & Black, 1992; Nelson, 2001). Many disruptions in aspects of this system in ASD have been proposed, and are reviewed in detail elsewhere (e.g., Dawson, Webb, & McPartland, 2005; Johnson et al., 2005; Grelotti, Gauthier & Schultz, 2002; Webb et al., 2009). To summarize, some propose initial atypicalities include impairments in the fusiform gyrus or low-level perception (e.g., Behrmann, Thomas & Humphreys, 2006; Dawson et al., 2005). These theories predict that fundamental atypicalities in face processing should be apparent from very early in the development of individuals with ASD. Alternatively, the progressive specialization of the face processing system may be disrupted (e.g., Johnson et al., 2005), possibly because children with ASD exhibit fewer of the typical social behaviors that are likely to provide ‘expected’ experiences (e.g., Grelotti et al., 2002; Dawson et al., 2005). If this is the case, disruptions in face processing in ASD may emerge over development, as the system fails to develop at a typical rate.
Examining the early development of face processing in toddlers with ASD is central to evaluating these proposals. The familiar/unfamiliar face paradigm used by Dawson et al. (2002) is particularly interesting in this regard, as much is understood about its typical developmental trajectory, summarized below.
Developmental Changes in ERP Responses to Familiar and Unfamiliar Faces
The Nc (Courchesne, Ganz & Norcia,1981) is a negative-going deflection that occurs around 300 to 800 ms after the onset of a stimulus, and is recorded over central and anterior scalp regions. Because it is typically greater in amplitude (more negative) to novel or unexpected stimuli and to stimuli presented during periods of heart-rate defined attention versus inattention (Richards, 2003), the Nc may be modulated by the engagement of attention to a stimulus. The Nc response to facial familiarity changes over development: in 6- and 9-month-old infants, the Nc is more negative amplitude to a familiar than an unfamiliar face (de Haan and Nelson, 1997, 1999). This difference is also marginally apparent in 18- to 24-month-old infants (Carver et al., 2003). No significant difference is found at 24- to 45-months (Carver et al., 2003); while 3- to 5-year-old children show greater negativity to unfamiliar than familiar faces (Carver et al., 2003; Dawson et al., 2002; Moulson, Westerlund, Fox, Zeanah, & Nelson, 2009). Thus, there is a general transition from greater responses to the familiar face, to greater responses to an unfamiliar face.
The Nc is followed over anterior electrodes by a prolonged positive deflection (the Positive Slow Wave, or PSW). The PSW is thought to be involved in memory updating (Nelson, 1994; 1996), as it is typically greater in amplitude to novel stimuli than familiar stimuli (e.g., Nelson & Collins, 1991; Nelson & de Regnier, 1992), and its amplitude decreases with multiple stimulus exposures (Snyder, Webb & Nelson, 2002). Like the Nc, the effect of facial familiarity on the PSW changes over development. The PSW is more positive in amplitude to unfamiliar than familiar faces in 4- (Webb, Long, & Nelson, 2005) and 6-month-old infants (de Haan & Nelson, 1997, 1999) and in 3- to 4-year-olds (Dawson et al., 2002). However, no effects of familiarity are observed in 6- to 12-month-old (e.g., Snyder et al., 2002; Webb, Long & Nelson, 2005) or 7- to 30-month-old infants (Parker et al., 2005). Thus, typically developing children may pass through a developmental phase where the PSW is not modulated by familiarity.
Over posterior regions, the N290 is a negative deflection between 230 and 350 ms after stimulus onset that is sensitive to face orientation and species and is thought to reflect structural processing of face stimuli (de Haan, Pascalis & Johnson, 2002; Halit, de Haan & Johnson, 2003; see de Haan, Johnson & Halit, 2003 for review). The latency of the N290 may decrease with age, from around 290 ms in 12-month-old infants to around 200 ms in 4-year-old children (Kuefner, de Heering, Jacques, Palmero-Soler, & Rossion, 2010), although the validity of cross-study comparison may be affected by methodological factors. Although in 6-month-old infants, the N290 does not appear to be modulated by pre-experimental facial familiarity (de Haan et al., 1997, 1999), Scott, Shannon and Nelson (2006) found that the N290 was more negative to a familiar than an unfamiliar face in 9-month-old infants, and Moulson et al. (2009) found that the N290 was more negative to an unfamiliar face than a familiar face in 42-month-old children who had been institutionalized. Thus, the N290 may be modulated by familiarity in certain circumstances, but the critical variables remain unclear.
Finally, over posterior regions a positive deflection occurring between 300 and 800 ms after stimulus onset (the P400) is also modulated by facial familiarity in childhood. Specifically, responses to unfamiliar faces are more positive than to familiar faces in 45- to 54-month-old (Carver et al., 2003), and 34- to 52-month-old typically developing children (Dawson et al., 2002). However, six-month-old infants do not show differences in P400 response to familiar and unfamiliar faces (de Haan & Nelson, 1999), and differences have not been observed in 18- to 24-month-old and 24- to 45-month-old children (Carver et al., 2003). Increased allocation of early visual processing resources to unfamiliar faces may emerge in early childhood.
Neural Correlates of Face Processing in ASD
The findings of Dawson, Webb and colleagues (2002, 2006) may indicate that early-stage face processing and face familiarity processing are significantly disrupted from very early in the development of ASD. If this is the case, similar group differences (for example, no modulation of the ERP responses by facial familiarity and slowed N290 latency) should be apparent from the earliest age at which ASD is diagnosed. However, work reviewed above would also support an alternate explanation. Taken together, there is a stage between 24 and 36 months during which typically developing children do not show differential familiarity based neural responses over the Nc, P400 and PSW. Possibly, 3- to 4-year-old children with ASD are passing through this typical phase at an older age than typically developing children. This would be more consistent with delay in the rate of development rather than with an initial impairment in the face processing system.
This alternative account makes three predictions. First, toddlers with ASD should show differential ERP responses at the Nc to familiar and unfamiliar faces that resemble those seen in younger typically developing infants. Second, if typical developmental decreases in N290 latency result from progressive specialization of the face processing system, a slowed rate of specialization in ASD could result in the emergence of significant group differences in N290 latency by early childhood (c.f. Webb et al., 2006), predicting that testing earlier in development would reveal reduced or absent group differences between children with ASD and TD. Lastly, if the development of these neural correlates of face processing is indeed related to self-directed variation in ‘expected’ experiences with faces in early development, the development of ERP correlates of face processing should be related to the child's level of expression of typical social behaviors, since these are most likely to produce ‘expected’ experiences.
Present Study
Experiment 1 assessed neural responses to familiar and unfamiliar faces in 18- to 30-month-old children with ASD (ASD-18to30) and children with typical development (TD-18to30), and in 12- to 17-month-old children with typical development (TD-12to17), matched to the ASD-18to30 group on developmental (parent report) level of social ability. We predicted that if young children with ASD show delays in facial familiarity processing that are related to their level of adaptive social behaviors, responses in the ASD-18to30 group should resemble responses in the TD-12to17 group. Specifically, the Nc should be more negative to familiar than unfamiliar in both groups, but equivalent in the TD-18to30 group. Further, group differences in the N290 component relative to age-matched controls should be absent.
In Experiment 2, we examined neural responses to familiar and unfamiliar faces in the ASD-18to30 group when they were tested in a longitudinal follow-up assessment, approximately one year after their participation in Experiment 1. We predicted that the children with ASD, now 32 to 47 months (ASD-32to47 group) would show a similar pattern of effects as found in Dawson et al. (2002), who were 34 to 52 months of age. Specifically, there should be no modulation of any ERP component by facial familiarity. Finally, we used trajectory-based analyses (Thomas et al., 2009) to examine the developmental relations between social behaviors, chronological age, and key ERP responses across TD and ASD groups. We predicted that the N290 latency and the effect of familiarity on the Nc would be significantly related to children's social behaviors in both groups.
Experiment 1
Methods
Participants
Children in the ASD-18to30 (N = 59, 13 female) and typically developing (TD)-18to30 (N = 34, 10 female) groups participated in the EEG assessment as part of the NIH UW STAART Toddler Assessment Project, a larger study of the early development of autism and a randomized control trial of the Early Start Denver Model intervention, which included standardized diagnostic, cognitive, adaptive, and language assessments as well as an experimental battery including social and cognitive tasks (Dawson et al., 2010; Webb, Jones, Merkle, Namkung et al., 2010). An additional 45 TD children aged 12 to 17 months (8 female) were recruited for the EEG assessment (TD-12to17). Participants were recruited from clinics, hospitals, local advocacy groups, and the University of Washington Communications Studies Participant Pool. All children were medication free, had no history of head trauma, seizure disorder, chronic medical conditions, or known genetic syndrome associated with ASD. Children were Caucasian (N = 117), Asian (N=6 ASD-18to30; N = 1 TD-12to17), and more than one race (N=8 ASD-18to30; N = 3 TD-18to30; N=3 TD-12to18).
Diagnostic Evaluation
Children with ASD were evaluated using the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & LeCouteur, 1994), the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and clinical judgment based on DSM -IV criteria (American Psychiatric Association, 1994). The ASD group met criteria for Autism or Autism Spectrum Disorder on the ADOS-Module 1 social and communication domains, within 2 points of Autism on the ADI-R, and Autistic Disorder or Pervasive Developmental Disorder Not Otherwise Specified based on DSM-IV criteria. Of note, all children in the ASD group who participated in the longitudinal part of the study continued to meet criteria for ASD at the subsequent assessment points (1 and 2 years later), consistent with other studies (e.g., Chawarska, Klin, Paul,Macari, & Volkmar, 2009; Lord et al., 2006).
To assess the development of typical social behaviors, we used the socialization subscale of the Vineland Adaptive Behavior Scales (VABS, Sparrow, Balla, Cichetti & Doll, 1984; Sparrow, Balla & Cicchetti, 2005). This parent-report measure covers social behaviors that are approximately ordered according to their emergence in typical development, and is sensitive to slower social growth in ASD (e.g., Anderson, Oti, Lord & Welch, 2009). Examples of assessed behaviors in this age range are showing interest in other children, and seeking others out for play. Additionally, the domains of communication, motor skills, and daily living skills were assessed. Of note, age-equivalent VABS scores were used in most analyses. Although there are limitations associated with the developmental scatter present within a particular score (see Sattler, 2001), age-equivalent scores are less prone to floor-effects that may be common in young children with ASD (Akshoomoff, 2006) and facilitate interpretation of the developmental trajectories.
The Mullen Scales of Early Learning (Mullen, 1995) was used to assess cognitive function in the ASD-18to30 and TD-18to30 groups. The Mullen domains are fine motor, gross motor, visual perception, receptive language, and expressive language.
Children with TD did not meet criteria for ASD or other developmental disorders based on clinical best estimate by a licensed child psychologist (JG) and the ADOS (TD-18to30 group only). Children were included in the TD-18to30 group if they scored above 85 on the Mullen composite standard score and on at least 3 of 4 of the VABS domain scores. Children were included in the TD-12to17 group if reported as typically developing by their parents, and scored above 85 on at least 3 of 4 VABS domain scores.
Final Sample
Sixteen children in the ASD-18to30 (4 female), 17 children in the TD-18to30 (3 female) and 15 children in the TD-12to17 groups (4 female) provided adequate artifact-free trials for both conditions. Children were Caucasian (N = 46), Asian (N=1, ASD-18to30), and more than one race (N=1, ASD-18to30). Of those children who were excluded, 1 child initially in the TD-18to30 group was subsequently diagnosed with ASD, 2 children experienced experimenter error (1 ASD-18to30 and 1 TD-12to17), 3 children in the ASD-18to30 group were tested with a different electrode system, and 84 provided too few artifact-free trials (39 ASD-18to30, 16 TD-18to30, 29 TD-12to17).
Although the 36% inclusion rate is comparable to previously published studies (Halit et al., 2003), we further examined the reasons why children did not provide sufficient data. These included: (a) intolerance of the sensor net (e.g., fussing, verbal refusal, or physical refusal) (18% TD-12to18, 42% ASD-18to30, 9% TD-18to30 group); (b) failure to attend to 30 trials (16% TD-12to18, 7% ASD-18to30, 9% TD-18to30); and (c) failure to provide 15 ‘good’ trials per condition due to excessive artifact (body movement, pulling at net, eye movements/blinks, mouth movements (31% TD-12to18 group, 17% ASD-18to30, 29% TD-18to30).
Children in the ASD-18to30 or TD-18to30, who did and did not provide data for the final sample groups, did not significantly differ on VABS socialization or communication age-equivalent scores, Mullen composite age-equivalent scores, or ADOS subdomains (ASD only)(Fs < 2, ps > 0.2). However, children in the ASD-18to30 group who did not provide data showed greater tactile sensitivity (as assessed by lower scores) on the Short Sensory Profile (Table 1; McIntosh, Miller, Shyu & Dunn, 1999; F(1,52) = 11.9, p = 0.001).
Table 1.
Mean (standard deviation) scores in children with ASD who did and did not provide sufficient artifact-free trials for analysis. Significant differences between included and excluded groups are in bold.
| ASD-18to30 | ASD-32to47 | |||
|---|---|---|---|---|
|
|
||||
| Excluded | Included | Excluded | Included | |
| ADOS Social symptoms | 11.1 (2.3) | 11.0 (2.6) | 9.1 (3.2) | 8.3 (3.3) |
| ADOS Communication symptoms | 5.3 (1.5) | 5.2 (1.5) | 4.4 (1.9) | 4.4 (1.78) |
| ADI Social Symptoms | 16.1 (3.9) | 16.9 (3.1) | Not Collected | |
| ADI Communication Symptoms | 11.5 (2.3) | 11.5 (1.4) | Not Collected | |
| Mullen Verbal AE | 10.6 (5.0) | 9.6 (2.5) | 26.6 (11.7) | 26.9 (9.1) |
| Mullen Nonverbal AE | 17.0 (3.3) | 18.1 (2.0) | 27.8 (6.8) | 28.0 (8.7) |
| VABS Socialization AE | 11.7 (3.6) | 12.1 (2.3) | 17.6 (7.3) | 18.6 (6.5) |
| VABS Communication AE | 11.3 (4.5) | 11.8 (2.2) | 20.8 (8.2) | 21.7 (6.1) |
| VABS Motor AE | 19.1 (3.4) | 19.7 (3.8) | 25.7 (6.2) | 26.5 (5.2) |
| SSP Tactile Sensitivity | 27.5 (4.1) | 31.4 (2.8) | Not Collected | |
| SSP Visual/Auditory Sensitivity | 20.2 (4.3) | 20.6 (2.8) | Not Collected | |
Key: AE – age equivalent; SSP - Short Sensory Profile
Stimuli
Two digital photographs of faces (including both internal and external features) were presented; one depicting the child's primary caregiver (familiar) and the other unfamiliar. The unfamiliar face was selected from a pool of photographs, the majority of which were the familiar face for other children in the study. The unfamiliar face was chosen to be dissimilar from the familiar person in both internal facial configuration and features but of similar gender, age, ethnicity, hair style, and head/face height. The nasion was positioned at the horizontal and vertical center of the stimulus frame. Stimulus frames were 336 pixels wide by 420 pixels high and were presented for 500 ms on an LCD monitor at a size of 18 cm by 11 cm, subtending a visual angle of 16 by 10 degrees.
ERP procedure
Children in the ASD-18to30 and TD-18to30 groups received behavioral training sessions prior to ERP recording to acclimate them to the procedure (Dawson et al., 2002). ERPs were recorded from 128-channel Geodesic sensor nets (recorded online with reference to the vertex; re-referenced offline to the average reference). Data was recorded at 250 Hz, with amplification set at 1000x, and band-pass filtering at 0.1 and 100 Hz. Children were presented with a series of 1800 ms trials (consisting of 100 ms baseline, 500 ms stimulus presentation, 1200 ms post-stimulus recording period) separated by a 500 to 1000 ms randomly jittered ITI. Testing was terminated when the child had attended to 100 of each of the stimulus types, or when the child was no longer attending. Offline, data were filtered at 20 Hz and segmented into 1800 ms epochs. Artifact detection was accomplished with both automatic artifact-detection software (NetStation 4.3) and through hand-editing (EJ, SW). Trials were rejected if the child did not attend to the picture (recorded online by a trained observer), if the signal amplitude exceeded 250 μV, if electro-ocular or muscular artifact occurred, or if there was significant drift (defined as a difference of more than 200uV between the beginning and end of the trial). Trials were corrected with respect to the 100 ms pre-stimulus baseline period.
Regions (Figure 1) and components of interest were defined with respect to the previous literature, and inspection of the grand average waveform. These regions substantially overlap those used by Dawson et al. (2002) and Webb et al. (2006). For the N290, we analyzed peak amplitude and latency; peaks were identified for each electrode using automatic peak detection software, and verified by hand (EJ). The N290 was defined as the most negative point of a negative-going deflection between 190 and 390 ms, present in at least 4/7 electrodes in a group. Peak amplitude and latency values were averaged across regions.
Figure 1.
Sensors included in each analysis region. Anterior components were analyzed over left (24,25,21,30,29,36,28,35), central (4,5,10,11,12,16,19,20) and right (3,124,119,118,117,111,112) regions. Posterior components were analyzed over left (51,52,58,59,60,65,66), central (67,68,72,73,76,77,78), and right (85,86,91,92,93,97,98) regions. 10-20 electrode names are presented for reference.
Where a child's N290 did not meet these criteria, that child's data was excluded from N290 peak analyses (e.g., Webb et al., 2009; Webb, Jones, Merkle, Murias et al., 2010). For children who did not display a N290 peak, the most negative point selected by automatic peak detection software was typically at the beginning or end of the selected time-window (i.e., 190 ms or 390 ms), which would introduce outliers into the dataset. As a second strategy, analyses were also conducted on average amplitude using 50 ms windows from 100 ms to 400 ms after stimulus including all subjects (regardless of the presence of a N290 peak). Results mirrored those observed for the N290 peak amplitude presented below and thus are not included.
For all other components (Nc, PSW, P400) where clear unitary peaks were not common in individual data, mean amplitude was computed across selected time windows for each region. Although the Nc displayed a clear peak in the grand-averaged data, many individual subject averages displayed a broad flat Nc or two peaks within the time-window. Attempting to select a peak was likely to introduce substantial noise into the data, and so analyses were confined to mean amplitude across selected time-windows. Analysis of average amplitude across 50 ms windows from 200 ms to 800 ms after stimulus onset revealed the same pattern of results as reported below, and thus are not included.
Analyses were separated into early and late time windows for the Nc, PSW and P400. Components were measured over the following time-windows (from stimulus onset): early Nc 350-550 ms; late-Nc 550-750 ms; early-PSW 800-1200 ms; late-PSW 1200-1600 ms; early-P400 350-550 ms; late-P400 550-750 ms. Components were divided into early and late time-windows (of equal duration within each component to equate signal to noise ratio) to examine whether any group differences occurred in peak activity (early time-window) or in the offset of the component (late time-window). Of note, the early and late sections of the Nc may reflect different neural sources (Reynolds & Richards, 2005) and Dawson et al. (2005) found differences specifically in the early slow-wave response.
The Nc, PSW, P400 and N290 components were initially analyzed in a series of repeated measures ANOVAs, with time (early, late), condition (familiar, unfamiliar) and region (left, central, right) as within-subject variables, and group as a between-subject variable. Greenhouse-Geisser corrections were used. Where significant interactions were found, follow-up univariate ANOVAs or paired t-tests were used to clarify the pattern of findings. When testing such interactions failed to reveal statistically significant results, these are not discussed in the paper.
Results
Table 1 shows demographic and developmental data for each group. There were no effects of condition or group on the number of analyzed trials (Fs < 1.5, ps > 0.2), and no significant group differences in gender (X2(2, N = 48) = 0.43, p = 0.7). A series of univariate ANOVAs with post-hoc Tukey tests revealed that: (i) As expected, the three groups differed in chronological age (F(2,48) = 40.7, p < 0.001), due to significant differences between TD-12to17 and the other two groups, which did not differ. (ii) The three groups differed in socialization age-equivalent score (F(2,48) = 29.3, p < 0.001), due to significant differences between TD-18to30 and the other two groups (ps < 0.001), TD-12to17 and ASD-18to30 did not differ (p = 0.29). (iii) The three groups differed in communication age-equivalent scores (F(2,48) = 48.8, p < 0.001) due to significant differences between all three groups (TD-18to30 > TD-12-17 > ASD18to30). Of note, VABS data was not collected for the four oldest children in the TD-12to17 group. To avoid a misleading group comparison, for analyses (ii) and (iii) only, the socialization and communication age-equivalent scores for these children were replaced with the child's chronological age, since linear regression analyses showed the relation between chronological age and socialization/communication score in the TD group as a whole was strong (for socialization: rs > 0.7, ps < 0.001). (iv) TD-18to30 and ASD-18to30 differed in Mullen verbal and non-verbal age-equivalent scores (Fs > 20, ps < 0.001). Thus, ASD-18to30 was matched to TD-18to30 on chronological age, and to TD-12to17 on adaptive social behavior.
N290
Of note, 1 child in the ASD-18to30 group, 3 children in the TD-12to17 group, and 3 children in the TD-18to30 group did not have a visible N290 peak in all regions and conditions and were not included in this analysis. Posterior lead ERP graphs are presented in Figure 2A for the TD groups and Figure 2B for the ASD groups.
Figure 2.
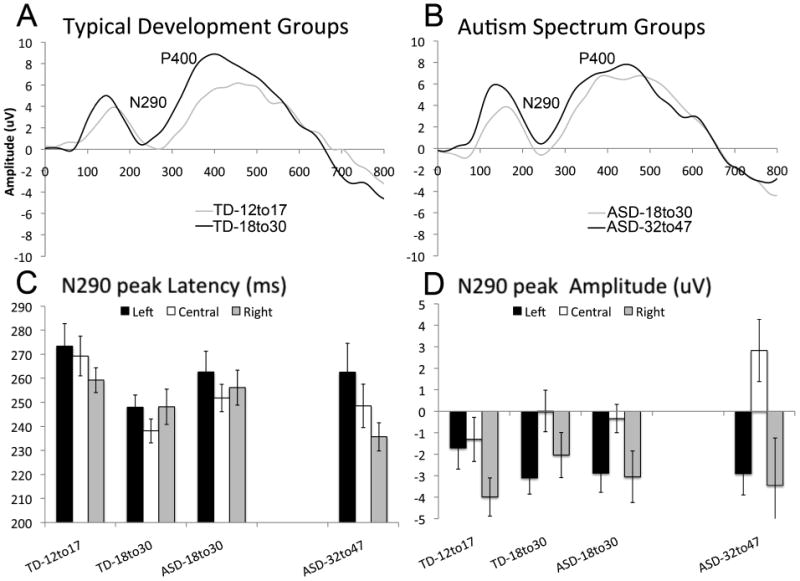
Developmental change in N290 latency, and N290 amplitude: (A) Grand average waveform for the TD-18to30 and TD-12to17 groups, averaged across posterior regions and conditions; (B) Grand average waveform for the ASD-18to30 (Experiment 1) and ASD-32to47 (Experiment 2) groups, averaged across posterior regions and conditions; (D) N290 peak latency by region and group; (D) N290 peak amplitude by region and group. Error bars indicate +/- 1 standard error.
For N290 amplitude there was a main effect of region (F(2,78) = 10.5, p = 0.001; see Figure 2D). Amplitude was more negative over the lateral regions than the central region (Fs > 10, ps < 0.001); right and left posterior regions did not differ (F(1,39) = 1.4, p = 0.25). For N290 latency, there was a main effect of group (F(2,38) = 4.0, p = 0.03). TD-12to17 had significantly longer N290 latencies than TD-18to30 (p = 0.02). The ASD-18to30 did not differ from either typically developing group (ps > 0.3; Figure 2C).
P400
For P400 amplitude, there were main effects of time (F(1,45) = 123.3, p = 0.001) and region (F(2,90) = 8.7, p = 0.001), qualified by a significant interaction between time, condition, region and group (F(4,90) = 2.9, p = 0.03). ASD-18to30 showed a significantly more positive response to the familiar versus the unfamiliar face over the left hemisphere in the later time-window (F(1,15) = 9.6, p < 0.01). TD-18to30 and TD-12to17 showed equivalent amplitude to familiar and unfamiliar at both left and right posterior regions over the later time-window. There were no other significant effects of condition.
Nc
For Nc amplitude, there was a main effect of time (F(1,45) = 117.3, p = 0.001), qualified by an interaction between time and group (F(2,45) = 6.2, p = 0.02). TD-12to17 showed a smaller (i.e., less negative) Nc over the early time-window than ASD-18to30 (p = 0.04) (Figure 3A vs 3B). TD-18to30 showed an intermediate response that did not significantly differ from the other groups (ps > 0.1; Figure 3C). There were no group differences in the later time window.
Figure 3.
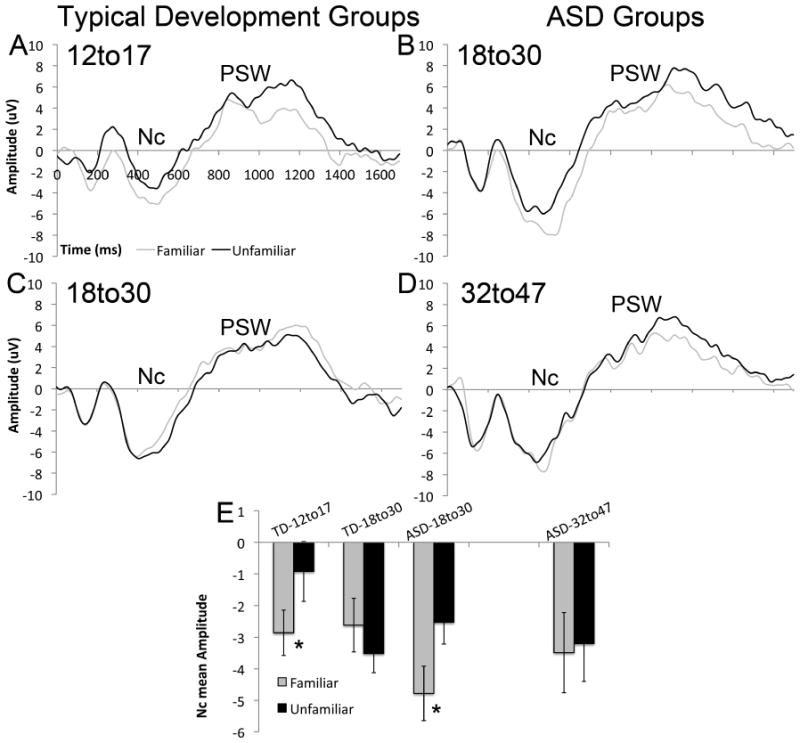
Grand average waveforms of response to familiar and unfamiliar averaged across anterior regions in (A) TD-12to17; (B) TD-18to30; (C) ASD-18to30 and (D) ASD-32to47 groups; (E) Mean amplitude of the Nc response (350 to 750ms) to familiar and unfamiliar faces in the four groups, averaged across region. Error bars indicate +/- 1 standard error.
There was a significant condition by group interaction (F(2,45) = 4.1, p = 0.02; Figure 3E) with responses more negative to familiar than unfamiliar for ASD-18to30 (F(1,15) = 5.9, p = 0.03) and TD-12to17 (F(1,14) = 5.9, p = 0.03), but not for TD-18to30 (F(1,16) = 1.1, p = 0.3). There was no significant effect of group on responses to either the familiar or the unfamiliar face (Fs < 2.5, ps > 0.1), indicating that it was the differential response that varied between the groups.
There was also a main effect of region (F(2,90) = 6.4, p < 0.01), qualified by a region by group interaction (F(4,90) = 6.1, p = 0.001). Responses were more negative over the right than left hemisphere for TD-12to17 (F(1,14) = 9.1, p < 0.01) and ASD-18to30 (F(1,15) = 4.8, p = 0.04), but not for TD-18to30 (F(1,16) = 0.9, p = 0.4). There was also a significant condition by region interaction (F(2,90) = 4.4, p = 0.02); responses to the unfamiliar were more negative over the right than left hemisphere (F(1,45) = 11.0, p < 0.01), but responses to the familiar face were not (F(1,45) = 1.7, p = 0.2).
Anterior positive slow-wave
There were main effects of time (F(1,45) = 46.9, p < 0.001) and region (F(2,90) = 3.9, p = 0.04), qualified by a significant time, region by group interaction (F(4,90) = 3.4, p =0.02). In the second time-window, right region amplitude was more positive for ASD-18to30 than TD-12to17 and TD-18to30 (ps = 0.03, 0.04), who did not differ (p = 0.99).
Summary
Findings suggest that facial familiarity responses in early ASD are delayed but not necessarily atypical. The ASD-18to30 and TD-12to17 groups both showed more negative Nc responses to a familiar versus an unfamiliar adult, whilst the TD-18to30 group showed no effect of familiarity over any component. The Nc was right-lateralized in both the TD-12to17 group and the ASD-18to30 group, but bilateral in the TD-18to30 group. Also suggestive of delay, the N290 peaked at a significantly faster latency in the TD-18to30 compared to the TD-12to17 group, with the ASD-18to30 group responses averaging somewhere in the middle. However, the ASD group also showed a pattern of responses that differed from both typical groups. Specifically, the late anterior positive slow wave over the right hemisphere was more positive in the ASD-18to30 group than either TD group and the late P400 over the left hemisphere had more positive amplitude to the familiar versus the unfamiliar face in the ASD-18to30 group only.
Discussion
Why might the Nc response to facial familiarity in young children with ASD resemble that in social age-matched typically developing children? The Nc component is thought to reflect the engagement of attention to a stimulus (e.g., Richards, 2003; Ackles & Cook, 2007, 2009; Reynolds & Richards, 2005), and as such must be interpreted in the context of the eliciting paradigm (in this case, the primary caregiver's face versus an unfamiliar face). Under experience-expectant models, self-directed social experiences could influence the development of many aspects of face processing, including face recognition. Group differences in Nc response to the primary caregiver's face versus an unfamiliar face could thus reflect the effect of disrupted social experiences on the development of this system. Indeed, young children with ASD show early disruptions in face memory (Chawarska & Volkmar, 2007; Chawarska & Shic, 2009), and habituate more slowly to an unfamiliar face (Webb, Jones, Merkle, Namkung, et al., 2010).
Recognition memory is likely not the only critical factor in modulating Nc responses to the primary caregiver's face: indeed, familiarizing infants with a face at the start of an experiment can decrease the negativity of the Nc at ages that typically show enhanced Nc to their caregiver's face (e.g., Reynolds & Richards, 2005; Courchesne et al., 1981). Rather, the affective significance of the primary caregiver's face likely also influences the modulation of the Nc by personal familiarity: Nc responses in the primary caregiver/unfamiliar face paradigm are related to variables relating to the infant's attachment to their caregiver (Swingler, Sweet & Carver, 2007; 2010), and Carver et al. (2003) suggest that changes in the Nc familiarity effect over typical development may relate to the child's developing independence from their caregiver.
Experiment 2
Since the ASD-18to30 group resembled the TD-12to17 group in the modulation of the Nc component by facial familiarity, it is possible that the 3- to 4-year-old children with ASD tested by Dawson et al. (2002) showed no modulation of the Nc component by facial familiarity because they were also responding like younger typically developing children, such as the TD-18to30 group in Experiment 1. The ASD-18to30 group also showed an N290 latency that lay between the N290 latency of the TD-12to17 and TD-18to30 groups and that was not significantly different from either, suggesting that the delay in this component observed in older cohorts (Webb et al., 2006) may only be emerging in toddlerhood. Finally, the P400 component showed a pattern of familiarity differentiation in the ASD-18to30 group that was not observed in either typically developing group, nor observed in previous work with older children (Dawson et al., 2002). Possibly, the unusual P400 familiarity response observed in toddlers with ASD resolves with age. There is of course an alternative to all these explanations: subtle differences in analysis method or participant population may account for the difference in results between Experiment 1 and previous work.
To address this possibility, the ASD-18to30 group was retested with the familiar face/unfamiliar face paradigm approximately 1 year after their participation in Experiment 1. This testing was conducted as part of the “Time 2” assessment of the wider longitudinal study in which they were participating (NIH UW STAART; see Dawson et al., 2010). This provided the opportunity to examine whether the cohort of children with ASD tested in Experiment 1 would show a similar pattern to that reported by Dawson et al. (2002) and Webb et al. (2006) when tested at an older age and with the Experiment 1 analysis strategy. However, to note an important caveat, the NIH UW STAART was also a randomized clinical trial of the Early Start Denver Model (ESDM) intensive intervention. After data were collected at time 1 (included in Experiment 1), for those families who chose to continue participation in the clinical trial, 50% of children with ASD were randomly assigned to the ESDM group and over the course of the next 24 to 30 months, received an average of 20.4 hours per week in intervention; the Assessment and Monitoring group received an average of 18.4 hours per week of community based therapies (Dawson et al., 2010). Since the numbers of participants with good data were too low to allow us to compare responses in the two groups (see below), it is important to recognize that we cannot separate effects of age from effects of early diagnosis and subsequent treatment.
Methods
Participants
Of the 59 children with ASD tested in Experiment 1, 47 children with ASD aged between 32 and 47 months (11 female) participated in Experiment 2; the remaining 12 children were no longer participating in the wider study. Children were Caucasian (N=35), Asian (N=5) and more than one race (N=7). Children were evaluated as in Experiment 1 (Table 1).
Final sample
Twelve children (one female) provided adequate artifact-free trials for both familiar and unfamiliar conditions. Children were Caucasian (N = 7), Asian (N=2), and more than one race (N=3). This 26% inclusion rate is comparable with the 29% inclusion rate for the ASD-18to30 group in Experiment 1.
Of those children who were excluded, 1 child experienced experimenter error and 34 provided too few artifact-free trials. Reasons children did not provide sufficient artifact-free trials for analysis were: (a) 28% intolerance of the sensor net (e.g., fussing, verbal refusal, or physical refusal); (b) 6% failure to attend to 30 trials; and (c) 38% failure to provide 15 ‘good’ trials per condition due to excessive artifact (body movement, pulling at net, eye movements/blinks, mouth movements.
Children contributing data to Experiment 2 were not always the same children who contributed data to Experiment 1. Four of the twelve children included in Experiment 2 were also included in the final sample for Experiment 1. Of the remaining eight, one child was tested with a different electrode system and 7 children did not provide sufficient artifact-free trials in Experiment 1. Of the twelve children who were included in the final sample in Experiment 1 but not Experiment 2, two were no longer participating in the wider longitudinal study, one child experienced experimenter error and nine did not provide sufficient artifact-free trials in Experiment 2. Although the low number of children who provided valid data in both experiments (n = 4) prevented statistical comparison of developmental and diagnostic variables between children who provided data for one versus both experiments, we addressed the representativeness of the final ASD-32to47 group in two ways. First, using inclusion in the final ASD-32to47 group as the between-subjects variable, we conducted a series of univariate ANOVAs on developmental (Mullen, VABS) and diagnostic (ADOS, ADI-R) data collected concurrent with Experiment 2. There were no significant developmental or diagnostic differences between children who did (n = 12) and did not (n = 47) provide valid ERP data in the ASD-32to47 group at “Time 2” (see Table 1; all Fs < 2, ps > 0.2). This suggests that children included in the final ASD-32to47 group were representative of their cohort (Dawson et al., 2010). Second, again using inclusion in the final ASD-32to47 group as the between-subjects variable, we conducted a series of univariate ANOVAs on developmental (Mullen, VABS, Short Sensory Profile) and diagnostic (ADOS, ADI-R) data collected concurrent with Experiment 1. This revealed that there were no significant “Time 1” developmental or diagnostic differences between children who did (n = 12) and did not (n = 47) provide valid ERP data in the ASD-32to47 group (all Fs < 2, ps > 0.2). Thus, the group was representative of their cohort at “Time 1” (Dawson et al., 2010).
ERP Procedure
The stimuli, ERP procedures, and analysis strategy were identical to those described in Experiment 1. A new picture was taken of the familiar person and a novel unfamiliar face was used.
Results
N290
Of note, data from 2 children were excluded from the N290 analysis due to the absence of a visible peak. For the number of electrodes with a visible N290 peak, there was a main effect of region (F(2,22) = 13.1, p < 0.001); there were significantly fewer electrodes with visible peaks over the left region (mean = 5.5) than the right or central regions (means = 6.7, 6.5; Fs(1,11) > 10, ps < 0.01).
For N290 peak amplitude, there was a main effect of region (F(2,18) = 5.4, p = 0.02; see Figure 2D); amplitude was less negative centrally than in the right or left regions (Fs(1,11) > 8, ps < 0.02), which did not differ (F(1,9) = 0.05, p = 0.8).
For N290 latency there was a marginally significant effect of region (F(2,18) = 3.8, p = 0.07); responses were slower over the left versus the right region (F(1,9) = 6.1, p = 0.04; Figure 2C). There were no effects of familiarity.
P400
There was a significant main effect of time (F(1,11) = 32.8, p < 0.001), qualified by a significant region by time interaction (F(2,16) = 9.0, p < 0.01). The right region was marginally more positive than the left region for the early time window (F(1,11) = 3.4, p = 0.09). There were no effects of familiarity.
Nc
There was a significant main effect of time (F(1,11) = 29.8, p < 0.001), such that amplitude was more negative in the earlier time window. There were no other significant effects, including condition (F(1,11) = 0.05, p = 0.8; Figure 3D) or region (F(1,11) = 0.5, p = 0.5).
PSW
There was a main effect of time (F(1,11) = 9.9, p < 0.01) such that amplitude was more positive over anterior regions in the early versus the later time window.
Summary
When tested at 32 to 47 months, children with ASD from the cohort tested in Experiment 1 showed no evidence of differentiation between a familiar and an unfamiliar adult over any component measured, replicating the findings of Dawson et al. (2002). These results are consistent with the hypothesis that the response to familiar and unfamiliar faces changes over development, and that this pattern is delayed in children with ASD.
Trajectory analysis
To complement our research strategy of comparing young children with ASD to matched control groups, we utilized the trajectory analysis method as described by Thomas and colleagues (2009) to examine: (a) whether developmental change in neural responses to faces within the typically developing group was related to their chronological age, and/or their adaptive social behaviors; and (b) whether the developmental trajectory observed in the ASD group was significantly different in either slope or intercept from that observed in the typically developing group.
Methods
Participants
Children in Experiments 1 and 2 were combined into two diagnostic groups, TD-all and ASD-all. Analyses for the ASD group were conducted without data from the first time-point for the four children that were included in both Experiment 1 and Experiment 2, such that analyses for both diagnostic groups would be completely cross-sectional and with a relatively even age distribution.
Analysis
Based on results from Experiment 1, the Nc and N290 were chosen because they were differentiated diagnostic group and age. Dependent variables were mean Nc amplitude to familiar minus unfamiliar over central regions (‘Nc familiarity effect’) and the peak latency of the N290 response averaged across regions and conditions (‘N290 latency’). Of note, the central region was chosen for the Nc analysis to avoid contamination by group differences in Nc lateralization patterns.
Predictor variables were chronological age and VABS socialization age-equivalent scores. Predictor variables were transformed such that the lowest score that was present in both diagnostic groups was subtracted from each child's individual score (socialization - 10 months, chronological age -18 months); this enables comparison of the intercept of the two diagnostic groups at a predictor level that was measured for both groups (Thomas et al., 2009). We used a three-step analysis strategy: (i) A linear regression to examine the predictor/dependent relation in the TD-all group only, to first establish whether the variable was sensitive to developmental change. (ii) If a significant trajectory was present in typical development, ANCOVAs were used to determine whether there was a significant difference between groups in the intercept or slope of the relation between the predictor and dependent variable. (iii) If this relation differed between groups, linear regression was used to characterize the nature of the relation in the ASD-all group.
Results
Nc familiarity effect
For the TD-all group, there was no significant linear relation with chronological age (F(1,31) = 2.5, p = .1), Figure 4A. In contrast, there was a significant linear relation with socialization age-equivalent scores (int. = -2.65, slope = 0.3, F(1,27) = 4.9, p = .04) such that the familiarity effect was greater in children with younger socialization age-equivalent scores (Figure 4B).
Figure 4.
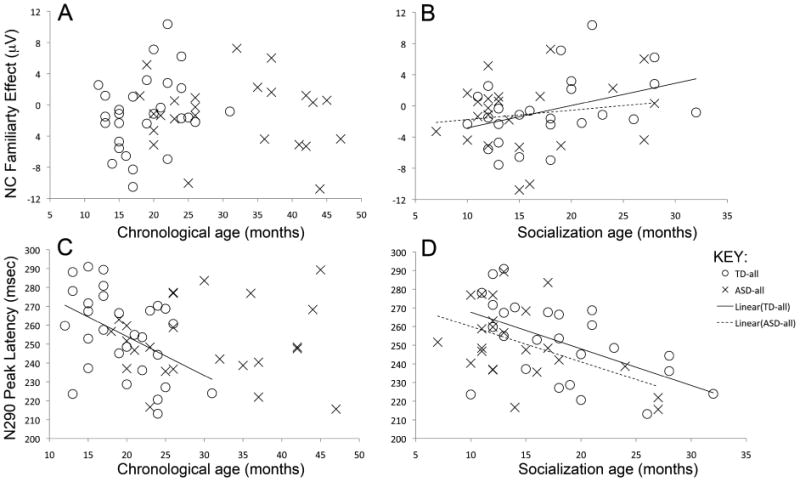
Relations between the Nc familiarity effect (mean amplitude of response to familiar minus mean amplitude of response to unfamiliar face across central regions) and (A) chronological age and (B) socialization age-equivalent scores. Relation between the N290 peak latency and (C) chronological age and (D) socialization age.
An ANCOVA including both TD-all and ASD-all groups revealed a significant main effect of socialization age-equivalent scores (F(1,51) = 4.0, p =.05), and no main effects or interactions with group (Fs < 1, p =.2). The effect of socialization score remained if chronological age was included as a covariate (F(1,51) = 6.1, p = .02). Thus, the significant linear relation between the modulation of the Nc by facial familiarity and social behavior did not significantly differ in the two groups (see Figure 4A, 4B).
N290 latency
For the TD-all group, there was a significant linear relation with chronological age (intercept = 258.2, slope = -2.1, F(1,27) = 6.9, p = .01), and with socialization age-equivalent scores (intercept = 267.7, slope = -2.0, F(1,24) = 9.4, p < .01), such that N290 latencies were longer in the younger children (Figure 4C) and the children with lower socialization age-equivalent scores (Figure 4D).
An ANCOVA for both TD-all and ASD-all groups, with chronological age, showed a significant interaction between diagnostic group and chronological age (F(1,49) = 5.0, p = .02), and a significant main effect of chronological age (F(1,49) = 5.1, p = .03). This indicates that the developmental trajectories were significantly different in the two groups. An individual regression analysis for the ASD-all group revealed no evidence of a significant linear relation with age (intercept = 251.7, slope = -0.01, F(1,23) = 0.0, p = 0.98) (Figure 4C). In the ANCOVA with socialization age-equivalent scores, there was a main effect of socialization score (F(1,45) = 15.6, p <0.001)(Figure 4D; dotted line) and no effects involving group (Fs < 1, p > 0.2). The effect of socialization score remained if chronological age was also included as a covariate (F(1,45) = 16.5, p <0.001). Thus, the groups did not significantly differ in the linear relation between the latency of the N290 and their socialization age-equivalent score (Figure 4D).
Discussion
For both TD-all and ASD-all groups, higher socialization age-equivalent scores, were related to less differentiation between a familiar and an unfamiliar face over the central Nc and were linearly related to shorter N290 peak latencies. These relations remained when chronological age was included as a covariate. For the TD-all group, linear relations between N290 latency and chronological age were also apparent; these were not seen in the ASD-all group.
General Discussion
ERP responses to faces of 18- to 30-month-old toddlers with ASD differed from those of chronological age-matched typically developing children. However, responses in these toddlers with ASD broadly resembled patterns seen in younger, 12- to 17-month-old typically developing children who had similar developmental levels of socialization behaviors. Further, both typically-developing children and children with ASD show similar a relationship between the development of socialization skills and their ERP responses to familiar versus unfamiliar faces, suggesting a similar developmental trajectory for both groups. Of note, this is the first study to demonstrate relations between ERP responses to faces and social behaviors in 1- to 3-year-old children with either typical development or ASD, though findings are consistent with previous reports with other age groups (Key, Stone, & Williams, 2009; Dawson et al., 2005). These patterns suggest that ‘impairments’ in face processing observed in previous work with young children with ASD (Dawson et al., 2002; Webb et al., 2006) may represent delays in the development of the face processing system that may be associated with slowed development of adaptive social behaviors. These findings will be discussed in turn, and their implications for models of face processing in ASD considered.
Evidence for delay in the development of the neural correlates of face processing in ASD
Modulation of the Nc response by facial familiarity in the two typically developing groups was consistent with patterns observed in previous work (e.g., Carver et al., 2003; Moulson et al., 2009; Webb et al., 2005). Specifically, in the typically developing 18to30-month group, facial familiarity did not modulate the Nc, while in the typically developing 12to17-month group, the Nc component was larger (more negative) to the familiar than unfamiliar face. The 18to30-month group with ASD showed significantly different Nc responses compared to their age-matched control group, but similar responses to those of the younger typically developing 12to17-month group. When children with ASD from the same cohort were tested 1 year later (ASD-32to47), Nc responses were no longer modulated by familiarity, similar to the typically developing18to30-month group. This suggests that facial familiarity processing progresses through relatively typical developmental stages in ASD, but at a slower rate.
Intriguingly, using an average reference, the Nc was right-lateralized in the ASD 18to30-month group and the TD 12to17-month group, and bilateral in the TD 18to30-month group. Previous studies provide mixed evidence about Nc lateralization patterns, making this pattern difficult to interpret. For example, using a linked mastoid reference Dawson et al. (2002) found right-lateralization in 3- to 4-year-old children with ASD and typical development, and Webb et al. (2005) found right-lateralization in 4- to 12-month-old typically developing infants. However, using an average reference Carver et al. (2003) found left-lateralization in typically developing children aged 24- to 45- months. Reference scheme influences observed lateralization patterns (e.g., Nunez & Srinivasan, 2006). Alternatively, there may be alterations or shifts in the lateralization pattern with development. Of note, developmental changes in lateralization have also been observed in ERP responses to language (for review, Mills & Sheehan, 2007) and alternations in lateralization have been found in ASD (e.g., Chiron et al. 1995; Kleinhans et al., 2008; McCleery, Akshoomoff, Dobkins, & Carver, 2009; Strogonova et al., 2007).
Developmental change was also observed over the posterior N290 component. Latency decreased significantly with chronological age in the typically developing children, but not in the children with ASD. Very few other studies have examined the N290 in this age range. However, cross-study comparisons provide supporting evidence for a latency decrease in typical development between 1 and 4 years (Halit et al., 2003; Kuefner et al., 2010). Consistent with predictions, the 18to30-month group with ASD did not show prolonged latencies relative to the typically developing 18to30-month group, although inspection of Figure 2 indicates that a trend in that direction. Two recent reports found no significant differences in N290 latencies in 10-month-old infants with an older sibling with ASD relative to infants with a typically developing older sibling (Elsabbagh et al., 2009; McCleery et al., 2009). Although it is presently unclear how many of these siblings will develop ASD, these reports are consistent with the possibility that N290 differences are not apparent in the very early development of ASD. The prolonged N290 latency to faces observed in 3- to 4-year-olds with ASD relative to children with typical development (Webb et al., 2006) is consistent with a plateau in the development of face processing development in early childhood in children with ASD.
There is also evidence to suggest that some aspects of face processing ‘catch up’ in later development for children with ASD, consistent with the contention that some early difficulties do not represent fundamental atypicalities in perception or processing of a static face. For example, Wilson, Pascalis and Blades (2007) found that 7- to 10-year-old children with ASD were as accurate in recognizing familiar faces (teachers from the child's school) as children with developmental delay. Further, relative to age-matched controls, the presentation of personally familiar faces may normalize the early neural responses in both adults and children with ASD (e.g., Pierce & Redcay, 2008; Webb, Jones, Merkle, Murias et al., 2010). Concerning early responses to faces, Grice et al. (2005) found no group differences in N290 latency in 4- to 6-year-old children with ASD. However, other aspects of face processing may remain delayed: for example, Grice et al. (2005) also found that the N290 was modulated by gaze direction in 4- to 6-year-old children with ASD, a pattern seen in 4-month-old typically developing infants (Farroni, Csibra, Simion, & Johnson, 2002). Longitudinal studies employing comparable paradigms would be required to fully evaluate the possibility that some aspects of face processing are atypical in early childhood but resolve in later development, and to identify which aspects may remain problematic.
Relation to models of face processing
Within typical development, external experiences with faces are thought to play a critical role in the developing face processing system. For example, between 6 and 9 months, infants lose the ability to discriminate faces from ethnicities or species with whom they do not have experience (e.g., Pascalis, de Haan, & Nelson, 2002; Kelly, Quinn et al., 2007), but exposure to individuated (named) faces from these groups enables infants to maintain discrimination (Pascalis et al., 2005; Scott & Monesson, 2009). Infants who have been deprived of social experiences through institutionalization also show attenuated early neural responses to faces relative to infants who were randomly assigned to foster care or never-institutionalized children (Parker et al., 2005; Moulson et al., 2009). Thus, individual differences in the social experiences available in a child's environment are likely to impact the early development of the face processing system.
The availability of faces is likely to be relatively typical in young children with ASD; however, the self-directed social experiences are likely to be atypical or decreased. While little is known about the role of the infant's social behaviors in the development of their face processing system, it is known that active experience plays a critical role in learning in a number of domains. For example, providing infants with active grasping experience helps them to understand the grasping actions of others (Sommerville, Woodward & Needham, 2005), and infants maintain the ability to discriminate vowels from a foreign language only if they experience the language in the context of a social interaction (Kuhl, Tsao & Lui, 2005). General theories of child development such as neuroconstructivism emphasize the critical role of the child's actions (‘proactivity’) in shaping the developing brain (Mareschal et al., 2007). Thus, it is at least plausible that the child's social behaviors impact the development of the face processing system.
This study is one of the first to explicitly address whether social behaviors are related to the early development of face processing in either typical development or ASD and the first to compare relations in both groups. The significant relations between modulation of the Nc by familiarity, N290 latency, and socialization behaviors in the current research are consistent with the possibility that a child's adaptive social behaviors influence the development of their face processing system in early childhood. Indeed, the social skills examined in the VABS Socialization scale for this age group include behaviors such as showing interest in other children, playing co-operatively with others, and seeking others out for play or companionship. These behaviors may influence the degree and quality of exposure that the child has to other faces, which under an experience-expectant model supports the development of face processing skills. Critically, this perspective would predict that the Nc response to familiarity, and the N290 latency, would be typical in young infants who are later diagnosed with ASD, because recent work has suggested that individuals with ASD exhibit relatively typical social behaviors in the first months of life (for review, Rogers, 2009). Current prospective studies with younger siblings of children with ASD will provide opportunities to test this prediction.
However, the existence of a cross-sectional relation between parent-reports of social behaviors, and face processing skills, does not imply causality. The relation between neural structures involved in face processing and social behavior is likely complex and bidirectional. For example, Dawson et al. (2005) argue that slower face processing may prevent the correct temporal binding of facial expressions with contextual information, disrupting the child's ability to appropriately respond to their social environment and thus affecting their social behavior. Alternatively, relations between ERP responses to faces and measures of social behavior may correlate due to their common reliance on the wider social brain network, including structures such as the fusiform gyrus, the amygdala, the superior temporal sulcus (STS), the prefrontal cortex, and the orbitofrontal cortex. Source analysis of the Nc and N290 components in typically developing infants has localized them to generators in this network, specifically the prefrontal and anterior cingulate cortices (Reynolds & Richards, 2005) and the STS and fusiform gyrus (Johnson et al., 2005), respectively. Johnson et al. (2005) propose that a failure of specialization within the social brain network may contribute to social symptoms of ASD. Combining ERP measures with brain imaging and behavior in a longitudinal study commencing in infancy may provide insight into such developmental interrelations.
More challenging paradigms or alternative analytical techniques may reveal fundamental atypicalities in face processing in young children with ASD. For example, Milne, Scope, Pascalis, Buckley, & Makeig (2009) recently used independent components analysis (ICA) to show that 8- to 18-year-old children with ASD had atypical early visual responses to Gabor patches of different spatial frequencies. Using a similar technique may show that apparently similar ERP responses in young children with ASD and TD differ in their underlying components.
Notably, the P400 and slow-wave components in the 18to30-month group with ASD did not match those seen in either the typically developing 12to17 or 18to30-month groups. Since these findings were not predicted, drawing strong conclusions from these patterns requires replication. However, these findings do not resemble patterns seen in typical development and may be interpreted as atypicalities. For example, the late P400 greater response to the familiar than unfamiliar face, seen in the 18to30-month group with ASD, has not been previously observed in typically developing infants or toddlers (e.g., de Haan & Nelson, 1997, 1999; Carver et al., 2003). In typical development, modulation by familiarity appears to emerge in the form of greater responses to the unfamiliar face around 3 to 4 years (Dawson et al., 2002). Possibly, the presentation of a highly familiar face “normalizes” the response of face-sensitive processes or regions that are otherwise less consistently activated in ASD. Indeed, recent work with fMRI indicates that presentation of a personally familiar face activates face-processing regions that are otherwise under-activated in both children and adults with ASD (e.g., Pierce, Haist, Sedaghat, & Courchesne, 2004; Pierce & Redcay, 2008).
Limitations and future directions
One limitation to the present findings is that data from the typically developing children were cross-sectional rather than of a longitudinal nature. Longitudinal studies are particularly important with clinical groups, in part because their heterogeneity makes comparisons between groups more difficult. Longitudinal studies covering the period from infancy to early childhood would provide further insight into the similarities and differences between the developmental trajectories of familiar face processing in ASD and typical development. Of note, the relatively high attrition rate associated with ERPs is also a significant limitation both within this study and to conducting longitudinal studies. The ASD group in Experiment 1 and Experiment 2 were from the same cohort, with relatively poor overlap in the contribution of data to both time points. Attrition was due to inattention and movement artifact in addition to non-compliance with wearing the net, suggesting that training of attention may also be warranted. Although the group of children who did provide valid ERP data were representative of the wider group in terms of their scores on the cognitive, social, and diagnostic measures, children with ASD who provided valid data in Experiment 1 did have lower levels of tactile sensitivity that children who did not. The lack of a clear conceptual relation between tactile sensitivity and face processing suggests that it is unlikely that this observation presents a significant confound to the results, although it highlights the difficulty in using psychophysiological methods requiring skin contact in some children with ASD. The difficulty with obtaining good signal from children with ASD at two time points is not trivial, and complementing ERP studies of face processing with behavioral tasks with lower attrition rates (e.g., Webb, Jones, Merkle, Namkung et al., 2010) is an important goal for future work. Further, employing multiple measures of social development will be critical. Although the relation between ERP measures and socialization age-equivalent scores on the VABS was similar across diagnostic groups, similar age-equivalent scores can mask underlying differences in social behaviors (Sattler, 2001). For example, young children with ASD may show greater intra-individual scatter on VABS socialization scores (Van Meter, Fein, Morris, Waterhouse, & Allen, 1997). Finally, the present study cannot resolve the extent to which the ERP responses observed in Experiment 2 were influenced by the relatively intensive early intervention received by all children in that group. In the wider study VABS Socialization scores did not differentially change in the Assessment and Monitoring and Early Start Denver Model groups, which may indicate that early intervention did not completely drive effects in the present study; however, it may be that socialization scores were equally affected by intervention in both groups. Further work relating the practices included in early intensive intervention to the development of face processing in young children is warranted.
Summary
In summary, the present ERP data indicate that 18 to 30 month old toddlers with ASD do show differential neural responses to the face of their primary caregiver from a 500 ms stimulus presentation. However, their responses did not match those seen in chronological-age matched children, but instead resembled responses seen in younger typically developing children, indicating that the typical developmental changes related to the influence of facial familiarity on anterior cortical activity and the speed of early neural posterior cortical responses may progress more slowly in ASD. Relations were found in both groups between developmental level of social behavior and ERP responses, consistent with the proposal that self-directed social experiences may impact the development of aspects of face processing. Future research should identify the mechanisms underlying these relations, and their longitudinal developmental trajectory.
Table 2.
Characteristics of the participants in Experiment 1 and 2 (mean, standard error in parentheses, range). Verbal and nonverbal age equivalent scores are taken from the Mullen Scales of Early Learning; Socialization and Communication age equivalent scores are taken from the Vineland Adaptive Behavior Scales.
| Included Trials | Age-equivalent scores (months) | ADOS scores | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Age (mos) | Familiar | Unfamiliar | Verbal | Nonverbal | Socn | Comm | Soc | Comm | |
| TD-12to17 | 14.9 (0.4) |
24.3 (2.9) |
23.3 (3.3) |
NA | NA | 14.0 (0.6) |
15.1 (0.7) |
NA | NA |
| 12-17 | 15-52 | 15-58 | 10-17 | 10-17 | |||||
| TD-18to30 | 23.0 (0.7) |
27.2 (1.5) |
28.9 (1.7) |
24.1 (1.2) |
22.5 (0.7) |
21.1 (1.2) |
22.0 (1.0) |
0.9 (0.2) |
0.9 (0.2) |
| 19-31 | 19-42 | 19-42 | 15-34 | 17-28 | 13-32 | 17-30 | 0-3 | 0-3 | |
| ASD-18to30 | 23.9 (1.0) |
29.6 (2.6) |
30.4 (2.6) |
9.6 (0.7) |
18.1 (0.5) |
12.1 (0.6) |
11.8 (0.6) |
11 (0.7) |
5.2 (0.4) |
| 18-30 | 16-49 | 15-54 | 6-14 | 15-24 | 7-17 | 9-16 | 6-14 | 2-8 | |
| ASD-32to47 | 40.1 (1.3) |
22.6 (2.1) |
23.4 (2.6) |
28.0 (2.5) |
26.9 (2.5) |
18.6 (1.9) |
21.7 (1.8) |
8.3 (0.9) |
4.4 (0.5) |
| 32-47 | 15-38 | 15-39 | 12-42 | 17-43 | 10-28 | 12-31 | 1-14 | 2-7 | |
Note. mos = months; Socn = socialization; Comm = communication; Soc = social
Acknowledgments
This project was supported by the NIH Studies To Advance Autism Research Treatment (Dawson/Aylward U54MH066399); the NIH Autism Center of Excellence (King/Webb P50 HD055782); Autism Speaks Postdoctoral Fellowship (Webb/Jones); and Perry Foundation Fellowship (Murias). The Murdoch Trust provided funding for the electrophysiology system. Additional assistance was provided by the staff and undergraduate students in the UW Psychophysiology and Behavioral Systems lab and the staff of the UW Autism Center Statistics and Database Management core. A special thanks to all of the toddler participants and their families.
References
- Ackles PK, Cook KG. Attention or memory? Effects of familiarity and novelty on the Nc component of event-related brain potentials in six-month-old infants. International Journal of Neuroscience. 2007;117:837–867. doi: 10.1080/00207450600909970. [DOI] [PubMed] [Google Scholar]
- Ackles PK, Cook KG. Event-related brain potentials and visual attention in six-month-old infants. International Journal of Neuroscience. 2009;119:1446–1468. doi: 10.1080/00207450802330579. [DOI] [PubMed] [Google Scholar]
- Akshoomoff N. Use of the Mullen Scales of Early Learning for the assessment of young children with Autism Spectrum Disorders. Child Neuropsychology. 2006;12:269–277. doi: 10.1080/09297040500473714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- Anderson DK, Oti RS, Lord C, Welch K. Patterns of growth in adaptive social abilities among children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2009;37:1019–1034. doi: 10.1007/s10802-009-9326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: Visual processing in autism. Trends in Cognitive Sciences. 2006;10:258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Frith U, Smith N, Abell F, Cipolotti L. Fractionation of visual memory: Agency detection and its impairment in autism. Neuropsychologia. 2002;40:108–118. doi: 10.1016/s0028-3932(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Carver LJ, Dawson G, Panagiotides H, Meltzoff AN, McPartland J, Gray J, et al. Age-related differences in neural correlates of face recognition during the toddler and preschool years. Developmental Psychobiology. 2003;42:148–159. doi: 10.1002/dev.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Shic F. Looking but not seeing: Atypical visual scanning and recognition of faces in 2- and 4-year-old children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2009;39:1663–1672. doi: 10.1007/s10803-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Volkmar F. Impairments in monkey and human face recognition in two-year-old toddlers with Autism Spectrum Disorder and Developmental Delay. Developmental Science. 2007;10:266–279. doi: 10.1111/j.1467-7687.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: Short-term diagnostic and cognitive outcomes. Journal of Child Psychology and Psychiatry. 2009;50:1235–1245. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C, Leboyer M, Leon F, Jambaque I, Nuttin C, Syrota A. SPECT of the brain in childhood autism: Evidence for a lack of normal hemispheric asymmetry. Developmental Medicine and Child Neurology. 1995;37:849–860. doi: 10.1111/j.1469-8749.1995.tb11938.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Ganz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with Autism Spectrum Disorder, Developmental Delay, and typical development. Child Development. 2002;73:700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125(1):e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27:403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Developmental Science. 2004;7:340–359. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother's face by six-month-old infants: A neurobehavioral study. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35:1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: A review. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2003;51:45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- de Haan M, Pascalis O, Johnson MH. Specialization of neural mechanisms underlying face recognition in human infants. Journal of Cognitive Neuroscience. 2002;14:199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood H, Tucker L, et al. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry. 2009;65:31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W, Black J. Induction of brain structure by experience: Substrate for cognitive development. In: Gunnar MR, Nelson CA, editors. Minnesota symposia on child psychology 24: Developmental behavioral neuroscience. Hillsdale, NJ: Lawrence Erlbaum; 1992. pp. 155–200. [Google Scholar]
- Grelotti DJ, Gauthier I, Schultz RT. Social interest and the development of cortical face specialization: What autism teaches us about face processing. Developmental Psychobiology. 2002;40:213–225. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- Grice SJ, Halit H, Farroni T, Baron-Cohen S, Bolton P, Johnson MH. Neural correlates of eye-gaze detection in young children with autism. Cortex. 2005;41:342–353. doi: 10.1016/s0010-9452(08)70271-5. [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH. Cortical specialization for face processing: Face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage. 2003;19:1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Hauck M, Fein D, Maltby N, Waterhouse L, Feinstein C. Memory for faces in children with autism. Child Neuropsychology. 1998;4:187–198. [Google Scholar]
- Johnson MH, Griffin R, Csibra G, Halit H, Farroni T, de Haan M, et al. The emergence of the social brain network: Evidence from typical and atypical development. Development and Psychopathology. 2005;17:599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007;18:1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, Stone W, Williams SM. What do infants see in faces? ERP evidence of different roles of eyes and mouth for face perception in 9-month-old infants. Infant and Child Development. 2009;18:149–162. doi: 10.1002/icd.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in Autism Spectrum Disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Kuefner D, de Heering A, Jacques C, Palmero-Soler E, Rossion B. Early visually evoked electrophysiological responses over the human brain (P1, N170) show stable patterns of face-sensitivity from 4 years to adulthood. Human Neuroscience. 2010;67(3):1–22. doi: 10.3389/neuro.09.067.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM. Foreign-language experience in infancy: Effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences; USA. 2003. pp. 9096–9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mareschal D, Johnson MH, Sirois S, Spratling M, Thomas M, Westermann G. Neuroconstructivism. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- McCleery JP, Akshoomoff N, Dobkins KR, Carver LJ. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry. 2009;66:950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, Shyu V, Dunn W. Overview of the short sensory profile. In: Dunn W, editor. The sensory profile: Examiner's manual. San Antonio, TX: The Psychological Corporation; 1999. pp. 59–73. [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45:1235–45. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Mills D, Sheehan EA. Experience and developmental changes in the organization of language relevant brain activity. In: Coch D, Fischer KW, Dawson G, editors. Human behavior, learning and the developing brain: Typical development. 2007. [Google Scholar]
- Milne E, Scope A, Pascalis O, Buckley D, Makeig S. Independent component analysis reveals atypical electroencephalographic activity during visual perception in individuals with autism. Biological Psychiatry. 2009;65:22–30. doi: 10.1016/j.biopsych.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Moulson MC, Westerlund A, Fox NA, Zeanah CH, Nelson CA. The effects of early experience on face recognition: an event-related potential study of institutionalized children in Romania. Child Development. 2009;80:1039–1056. doi: 10.1111/j.1467-8624.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: AGS; 1995. [Google Scholar]
- Nelson CA. The development and neural bases of face recognition. Infant and Child Development. 2001;10:3–18. [Google Scholar]
- Nelson CA, Collins PF. Event-related potential and looking-time analysis of infants' responses to familiar and novel events: Implications for visual recognition memory. Developmental Psychology. 1991;27:50–58. [Google Scholar]
- Nelson CA, DeRegnier RA. Neural correlates of attention and memory in the first year of life. Developmental Neuropsychology. 1992;8:119–134. [Google Scholar]
- Nelson CA. Neural correlates of recognition memory in the first postnatal year of life. In: Dawson G, Fischer K, editors. Human Behavior and the Developing Brain. Guilford Press; New York: 1994. pp. 269–313. [Google Scholar]
- Nelson CA. Electrophysiological correlates of early memory development. In: Reese HW, Franzen MD, editors. Thirteenth West Virginia University Conference on Life Span Developmental Psychology: Biological and Neuropsychological Mechanisms. Lawrence Erlbaum; New Jersey: 1996. pp. 95–131. [Google Scholar]
- Nunez P, Srinivasan R. Electric fields of the brain: The neurophysics of EEG. Second. Oxford: Oxford University Press; 2006. [Google Scholar]
- O'Connor K, Hamm JP, Kirk IJ. The neurophysiological correlates of face processing in adults and children with Asperger's syndrome. Brain and Cognition. 2005;59:82–95. doi: 10.1016/j.bandc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Parker SW, Nelson CA Bucharest Early Intervention Project Core Group. The impact of early institutional rearing on the ability to discriminate facial expressions of emotion: An event-related potential study. Child Development. 2005;76:54–72. doi: 10.1111/j.1467-8624.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;5571:1321–1322. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, Coleman M, et al. Plasticity of face processing in infancy. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of “who”. Biological Psychiatry. 2008;64:552–60. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: Findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An event-related potential and cortical source localization study. Developmental Psychology. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Developmental Science. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: Cognitive applications. San Diego: J.M. Sattler; 2001. [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57:331–343. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Scott LS, Monesson A. The origin of biases in face perception. Psychological Science. 2009;20:676–80. doi: 10.1111/j.1467-9280.2009.02348.x. [DOI] [PubMed] [Google Scholar]
- Scott L, Shannon R, Nelson C. Neural correlates of human and monkey face processing in 9-month-old infants. Infancy. 2006;10:171–186. doi: 10.1207/s15327078in1002_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K, Webb SJ, Nelson CA. Theoretical and methodological implications of variability in infant brain response during a recognition memory paradigm. Infant Behavior and Development. 2002;25:466–494. [Google Scholar]
- Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants perception of others actions. Cognition. 2005;96:B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales II. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV, Doll EA. Vineland adaptive behavior scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, Elam M, et al. Abnormal EEG lateralization in boys with autism. Clinical Neurophysiology. 2007;118:1842–1854. doi: 10.1016/j.clinph.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Swingler MM, Sweet MA, Carver L. Brain-behavior correlations: Relationships between mother-stranger face processing and infants' behavioral responses to a separation from mother. Developmental Psychology. 2007;46:669–680. doi: 10.1037/a0018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler MM, Sweet MA, Carver L. Relations between mother-child interactions and the neural correlates of face processing in 6-month-olds. Infancy. 2007;11:63–86. [Google Scholar]
- Thomas MC, Annaz D, Ansari D, Scerif G, Jarrold C, Karmiloff-Smith A. Using developmental trajectories to study developmental disorders. Journal of Speech, Language and Hearing Research. 2009;52:336–358. doi: 10.1044/1092-4388(2009/07-0144). [DOI] [PubMed] [Google Scholar]
- VanMeter L, Fein D, Morris R, Waterhouse L, Allen D. Delay versus deviance in autistic social behavior. Journal of Autism and Developmental Disorders. 1997;27:557–70. doi: 10.1023/a:1025830110640. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Dawson G, Bernier R, Panagiotides H. ERP evidence of atypical face processing in young children with autism. Journal of Autism and Developmental Disorders. 2006;36:881–890. doi: 10.1007/s10803-006-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Long JD, Nelson CA. A longitudinal investigation of visual event-related potentials in the first year of life. Developmental Science. 2005;8:605–16. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Merkle K, Murias M, Richards T, Aylward E, Dawson G. ERP responses differentiate inverted but not upright face processing in adults with ASD. Social Cognitive and Affective Neuroscience. 2009 doi: 10.1093/scan/nsp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Faja S, Dawson G. Face processing in autism. In: Calder A, Rhodes G, Haxby J, Johnson M, editors. Handbook of Face Perception. Oxford University Press; 2009. [Google Scholar]
- Webb SJ, Jones EJH, Merkle KM, Murias M, Greenson J, Richards T, Aylward E, Dawson G. Response to familiar faces, newly familiar faces, and novel faces as assessed by ERPs is intact in adults with autism spectrum disorders. International Journal of Psychophysiology. 2010;77:106–117. doi: 10.1016/j.ijpsycho.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle KM, Namkung J, Toth K, Greenson J, et al. Increased attention to faces during habituation in toddlers with elevated autism symptoms. Child Neuropsychology. 2010;16:255–278. doi: 10.1080/09297041003601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Pascalis O, Blades M. Familiar face recognition in children with autism: The differential use of inner and outer face parts. Journal of Autism and Developmental Disorders. 2007;37:314–320. doi: 10.1007/s10803-006-0169-z. [DOI] [PubMed] [Google Scholar]


