Abstract
It has been suggested that atypical amygdala function contributes to the social impairments characteristic of autism spectrum disorders (ASDs). Previous research has demonstrated that adolescents and adults with ASD generate normal response during a fear-potentiated startle paradigm, suggesting this aspect of amygdala function is intact and may not account for the social dysfunction associated with the condition. The amygdala also plays a crucial role in the expression of anxiety and may contribute to high rates of reported anxiety in individuals with ASD. The present study partially replicates prior work by examining the fear-potentiated startle response in adolescents with ASD, and extends this to investigate the relationship between startle response and anxiety. Eyeblink magnitude and latency (electromyographic activity; EMG) were collected from 20 adolescents with ASD and 19 typically developing (TD) age-matched adolescents during a fear-potentiated startle paradigm. Parent report and self-report of anxiety and additional psychiatric symptoms were collected. Parental reports indicated higher rates of associated psychopathology in adolescents with ASD compared with TD adolescents. Consistent with previous results, both groups showed normal potentiated startle response, and no group differences in EMG were found. Symptoms of anxiety and level of social impairment were unrelated to startle response. These findings held for all levels of anxiety, suggesting that within the context of the fear-potentiated startle paradigm, amygdala response is not associated with degree of atypical social or emotional functioning in ASD.
Keywords: autism spectrum disorders, anxiety, startle response, amygdala
Introduction
With recent prevalence of autism spectrum disorders (ASDs) estimated at 1 in 88 youth in the United States, there is growing public health concern for treating and managing the clinical complexities associated with the condition [Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators & Centers for Disease Control and Prevention, 2012]. In addition to the triad of core deficits, psychiatric disturbances with ASD occur at very high rates [e.g. Gjevik, Eldevik, Fjæran-Granum, & Sponheim, 2011]. Comorbid psychiatric symptoms, including anxiety, can be difficult to detect in ASD because of difficulties with communication and lack of standardized measures. Nevertheless, co-occurring anxiety disorders among children and adolescents with ASD are increasingly recognized [e.g. van Steensel, Bögels, & Perrin, 2011].
ASD and Anxiety
Symptoms of anxiety may in fact be the most commonly associated psychiatric concern among individuals with ASD [Skokauskas & Gallagher, 2010], with rates ranging from 40% to 84% [e.g. de Bruin, Ferdinand, Meester, de Nijs, & Verheij, 2006; Muris, Steerneman, Merckelbach, Holdrinet, & Meesters, 1998; van Steensel et al., 2011]. Systematic reviews indicate higher rates of anxiety in older children [van Steensel et al., 2011; White, Oswald, Ollendick, & Scahill, 2009], suggesting that adolescents may be at particularly high risk. Anxiety symptoms can lead to significant functional impairment and exacerbation of core ASD symptomology, or even be the most disabling feature of ASD [Seltzer, Shattuck, Abbeduto, & Greenberg, 2004]. Evidence suggests that parental report of increased anxiety is associated with a number of concerns including deficits in child self-esteem, academic success, social skills, peer relationships, social responsiveness, and potential to be bullied [Bellini, 2004; McPheeters, Davis, Navarre, & Scott, 2011; Reaven, 2009; Sukhodolsky et al., 2008]. Comorbid anxiety is also associated with problem behavior (e.g. conduct, learning, somatic, impulsivity, and hyperactivity) in youth with ASD [Evans, Canavera, Kleinpeter, Maccubbin, & Taga, 2005]. Anxiety may intensify core ASD symptoms, underscoring its clinical impact [Wood & Gadow, 2010].
Determining whether an individual with ASD has comorbid anxiety is challenging, given that symptoms arising from both conditions lead to similar behavioral manifestations. For example, social avoidance might be considered a symptom of social anxiety when attributed to anxious anticipation or distress [American Psychiatric Association, 2000]. But in many children with ASD, this behavior may simply reflect core deficits in social motivation or skill. Although rigorous diagnostic criteria and standardized measures for ASD and anxiety exist, in the absence of a reliable biological marker, diagnostic distinctions often depend on clinical judgment. Moreover, research has yet to clarify whether anxiety symptoms have the same clinical manifestation in ASD as they do in typically developing (TD) individuals. High rates of comorbid anxiety are generally based on interviews and questionnaires designed to assess anxiety in TD individuals, and have not yet been validated in the ASD population. It is unclear the extent to which these methods are measuring the same construct. One potential strategy to address this challenge is to investigate whether the underlying biological mechanisms associated with anxiety in ASD are analogous to mechanisms observed in TD individuals.
The Amygdala and ASD
It has been hypothesized that the amygdala, which plays a major role in emotional response to aversive stimuli [e.g. Adolphs & Tranel, 1999], influences social and emotional functioning in ASD. This is due in part to the observation that experimental lesions of the amygdala lead to a range of social-emotional impairments that resemble ASD. For example, monkeys with Klüver–Bucy syndrome, resulting from bilateral lesions to the anterior temporal lobes, exhibit lack of facial expression and absence of emotional reactions [Klüver & Bucy, 1939]. Bilateral removal of the amygdala in newborn monkeys results in social avoidance, disinterest, unexpressive faces, flattened vocalizations, and diminished eye contact [Bachevalier, 1996; Newman & Bachevalier, 1997]. Similar to individuals with ASD [Uono, Sato, & Toichi, 2011], postencephalic people with damage to the amygdala show impaired recognition of facial expressions of fear [Broks et al., 1998].
In fact, there is evidence of atypical amygdala structure and function in ASD. Postmortem studies have revealed possible microscopic amygdala abnormalities in ASD [e.g. Kemper & Bauman, 1998], although findings are not consistent [Schumann & Amaral, 2006]. Findings from imaging studies are also varied, with evidence suggesting that amygdala development may be atypical in ASD. Specifically, the amygdala is initially larger in young children [e.g. Sparks et al., 2002] but does not undergo the expected age-related increase in volume observed in TD children; the result is that no differences in amygdala volume are found between ASD and typical adolescents, despite the altered growth trajectory [e.g. Schumann et al., 2004]. In toddlers with ASD, larger amygdala volume has been associated with impaired social and communication skills and poorer outcome [Munson et al., 2006; Schumann, Barnes, Lord, & Courchesne, 2009].
There are two divergent viewpoints regarding the specificity and consequences of amygdala dysfunction in ASD. First, the “amygdala theory of autism” [Baron-Cohen et al., 2000] suggests that the amygdala is one component of the “social brain,” which represents the underlying neural substrates for social intelligence [Brothers, 1990]; thus, disruption of this system would account for development and maintenance of social impairments in ASD. Alternatively, findings from animal studies suggest that the amygdala may be responsible for appropriate fear response, rather than social functioning [Amaral & Corbett, 2003]. For example, amygdala-lesioned monkeys show decreased fear responses to previously aversive stimuli [Weiskrantz, 1956], reduced cautiousness and inhibition when introduced to unfamiliar monkeys [Emery et al., 2001], and more fear behavior such as grimaces, screams, and general heightened sense of fear during dyadic social interactions [Prather et al., 2001]. These findings have led to the idea that the amygdala may contribute to the ability to detect threats and mobilize an appropriate behavioral response; amygdala dysfunction in ASD would therefore contribute to increased levels of fear and anxiety, rather than fundamentally impacting social impairments [Amaral, Bauman, & Schumann, 2003]. Consistent with this hypothesis, structural evidence from magnetic resonance imaging indicates that symptoms of anxiety, but not cognitive ability or Autism Diagnostic Observation Schedule (ADOS) scores, are correlated with increased amygdala volume in children with ASD [Juranek et al., 2006].
Amygdala and Anxiety
Hyperresponsivity within the amygdala to threat-inducing stimuli mediates physiological symptoms of anxiety, such as autonomic hyperarousal [Rauch, Shin, & Wright, 2003]. As expected, neuroimaging studies have revealed increased amygdala activation, or hyperresponsivity, during anxiety provocation in individuals with social anxiety disorder, specific phobias, and posttraumatic stress disorder (PTSD) [Fredrikson & Furmark, 2003; Liberzon et al., 1999; Tillfors et al., 2001], supporting a neurocognitive model of underlying amygdala dysfunction in anxiety disorders [e.g. Bishop, 2007].
The amygdala is crucially involved in both the acquisition and expression of fear conditioning, making this process particularly informative in understanding amygdala function [e.g. Wilensky, Schafe, Kristensen, & LeDoux, 2006]. In fact, humans with amygdala damage exhibit fear conditioning impairment [Coopens, van Paesschen, Vandenbulcke, & Vansteenwegen, 2010]. Fear conditioning can be assessed via a noninvasive method known as startle response, elicited by a sudden onset of a stimulus. Startle magnitude can be modified or enhanced by exposure to a conditioned stimulus (CS) previously paired with an aversive unconditioned stimulus (US). The CS produces a state of fear that potentiates the startle response and increases reflexive behavior. The fear-potentiated startle (FPS) effect refers to the exaggerated startle reaction potentiated by the learned CS–US association [see Lang, Davis, & Ohman, 2000 for a review]. In essence, the threat cue primes an individual for response, creating a fear state, manifested by an exaggerated startle reflex to any sudden new stimulus. This reflex is viewed as a defensive response that varies with the individual’s emotional state [Lang, Bradley, & Cuthbert, 1990]. The startle response is characterized by a fast series of muscle contractions around the head, neck, and shoulders; the eyeblink is the first, fastest, and most reliable part of this reaction [Dawson, Schell, & Bohmelt, 1999]. Strength of the startle reflex is measured by electromyographic (EMG) activity of the orbicularis muscle during the eyeblink, or blink magnitude.
Potentiated startle response tends to be larger in individuals with higher levels of fear and anxiety, [e.g. Grillon, Ameli, Foot, & Davis, 1993], perhaps reflecting a general startle sensitivity in individuals with high “negative affect” and sustained anxious apprehension and arousal [Lang et al., 2000]. Enhanced potentiated startle has been reported in individuals with PTSD [e.g. Grillon & Morgan, 1999], social anxiety [Lissek et al., 2008; McTeague et al., 2009], and specific phobias [Sabatinelli, Bradley, & Lang, 2001], and in youth with anxiety disorders [Waters, Henry, & Neumann, 2009]. Larger startle responses have also been reported in youth at risk for anxiety, suggesting that startle reactivity may serve as a vulnerability marker for the development of anxiety [Grillon, Dierker, & Merikangas, 1997]. Startle is particularly potentiated by stimuli with negative valence, including aversive airpuff [e.g. Grillon et al., 1999].
Few studies have utilized the startle paradigm to measure eyeblink magnitude with individuals with ASD, despite strong implications for amygdala dysfunction in the disorder. Overall, findings indicate no differences in potentiated startle response compared with control groups. There were no group differences in amplitude of startle response to a conditioned acoustic probe found in 14 children and adolescents with high-functioning autism, ages 8–18, and 18 age-gender-matched control children [Salmond, de Haan, Friston, Gadian, & Vargha-Khadem, 2003]. Similarly, no differences were reported in 14 adolescents and adults with ASD, and 14 matched controls using an acoustic FPS [Bernier, Dawson, Panagiotides, & Webb, 2005]. In sum, both studies conclude that ASD per se does not appear to be associated with increased startle response.
The Current Study
This is the first study to use FPS to elucidate mechanisms of anxiety in addition to social dysfunction in ASD. Startle response could potentially help identify a subset of individuals with ASD with autonomic hyperarousal and propensity for developing anxiety. Adolescents were the focus of the current study given increased risk for anxiety during this phase of development [Kessler, Ruscio, Shear, & Wittchen, 2010], as well as specifically in youth with ASD [e.g. Kuusikko et al., 2008]. The primary aims of the current study were to: (a) determine whether a particular aspect of amygdala function, namely FPS, was impaired in adolescents with ASD and related to social deficits, and (b) assess anxiety symptoms with both child report and parent report, and examining the relationship between anxiety and FPS. Based on normal FPS reported in previous studies, together with the age-related pattern of amygdala function described earlier, it was hypothesized that both adolescents with ASD and TD would exhibit normal FPS response, and as such, FPS response would not be associated with social impairment in ASD. Moreover, it was hypothesized that if manifest anxiety symptoms in individuals with ASD represent the same construct as anxiety in TD, FPS would increase as a function of anxiety severity. If a positive relationship between anxiety and FPS was not found among adolescents with ASD, this would suggest different physiological mechanisms contributing to anxiety, or symptoms that appear on the surface to manifest as anxiety but are actually related to core autism deficits or other underlying causes.
Methods
Participants
Recruitment took place through an ongoing longitudinal study at the University of Washington (UW) Autism Center, yielding 11 participants with ASD and ten TD participants. Additionally, nine adolescents with ASD and ten TD adolescents were recruited through the UW Autism Center, as well as a local network of medical practices and parent advocacy groups and the UW Communications Subject Pool. Because of equipment error during the FPS experiment, data for one TD teen were not included in the final analyses. All aspects of the study, including recruitment, were approved by the UW Institutional Review Board.
The final sample consisted of 20 high-functioning adolescents with ASD (autistic disorder, n = 8; Asperger’ s disorder, n = 9; pervasive developmental disorder not otherwise specified [PDD-NOS], n = 3) and 19 TD adolescents. Adolescents in the TD group were matched on chronological age to the teens with ASD; all participants were between 13 and 17.5 years of age at the time of assessment (see Table 1 for sample characteristics). Minority representation reflected the distribution of the greater Seattle area, with 20.5% of teens in the total sample being nonwhite. There were three females in the ASD group and six in the TD group.
Table 1.
Participant descriptive characteristics
| Age in months
|
Differential Ability Scales
|
Spatial Mean (SD) | ||||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean (SD) | GCA Mean (SD) | Verbal Mean (SD) | Nonverbal Mean (SD) | ||||
| ASD | 20 | 17 males 3 females |
155–200 | 173.45 (12.29) | 104.60 (14.83) | 102.15 (19.08) | 106.85 (15.11) | 102.00 (13.06) |
| TD | 19 | 13 males 6 females |
156–210 | 168.21 (14.49) | 126.84 (16.95) | 120.26 (11.31) | 122.58 (18.22) | 122.84 (19.67) |
GCA, General Conceptual Ability; TD, typically developing; SD, standard deviation; ASD, autism spectrum disorder.
Exclusionary criteria included neurological disorder of known etiology, significant sensory or motor impairment, major physical abnormalities, birth prematurity, alcohol or drug exposure during the prenatal period, and history of serious head injury and/or neurological disease. In addition, adolescents in the TD group were excluded if there was a family history of ASD, birth or developmental abnormalities, learning or language disability, and current or past history of psychiatric or neurological disorders or that required regular psychoactive medication. Exclusionary criteria were assessed via administration of an initial phone screening, which included administration of the Vineland Adaptive Behavior Scales [Sparrow, Balla, Cicchetti, & Doll, 1984], to ensure that TD individuals were not significantly delayed in adaptive functioning. Teens were also assessed to ensure adequate verbal skills (see cognitive assessment later).
Inclusion criteria included confirmation of an ASD. Participants recruited through the longitudinal study met criteria for an ASD at their first visit (age 3 years) by exceeding cutoffs on the Autism Diagnostic Interview-Revised (ADI-R) and ADOS, and through clinical judgment based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria. Newly recruited participants were diagnosed with an ASD using the ADI-R and ADOS, and clinical judgment based on DSM-IV criteria. All participants in the current study were administered a cognitive assessment (see later) and were required to have a full scale intelligence quotient (IQ) of at least 80.
Measures
Cognitive assessment
IQ was assessed using the Differential Ability Scales, 2nd Edition [DAS-II; Elliott, 2007] at the first visit. Clinical judgment was also used to ensure that participants demonstrated adequate receptive and expressive language abilities to participate in the study, as self-report of psychiatric symptoms and participation in the startle paradigm required adequate verbal and written abilities. Table 1 presents scores for both groups. On average, the TD group yielded significantly higher Differential Ability Scales General Conceptual Ability standard scores (t(37) = −4.37, P < 0.001), Verbal Cluster standard scores (t(37) = −3.58, P = 0.001), Nonverbal Cluster standard scores (t(37) = −2.94, P = 0.006), and Spatial Cluster standard scores (t(37) = −3.92, P < 0.001) than the ASD group.
Diagnostic assessment
Diagnosis of an ASD (autistic disorder, Asperger’s disorder, or PDD-NOS) was based on the ADI-R [Lord, Rutter, & Le Couteur, 1994] and the ADOS-WPS [Lord, Rutter, DiLavore, & Risi, 2002]. In addition, expert clinical judgment of diagnosis was made according to DSM-IV diagnostic criteria.
Child report of psychiatric symptoms was assessed during the second visit. Anxiety was measured using the Revised Children’s Manifest Anxiety Scale Second Edition [RCMAS-2; Reynolds & Richmond, 1997], designed to measure anxiety in youth 6–19 years of age. The measure comprises 37 yes/no questions and provides scores for Total Anxiety as well as four subscales: worry/oversensitivity, physiological anxiety, social concerns/concentration, and lie (defensiveness) scale. Depression was assessed using the Reynolds Adolescent Depression Inventory, Second Edition [RADS-2; Reynolds, 1987], which evaluates the current level of an adolescent’s depressive symptomatology along four basic dimensions of depression: dysphoric mood, anhedonia/negative affect, negative self-evaluation, and somatic complaints, as well as yielding a depression total score representing overall depressive severity.
In order to decrease potential anxiety associated with completing written questionnaires administered by the examiner, the RCMAS-2 and RADS-2 were formatted for use on a laptop computer. All participants utilized the computer to indicate their answers.
Parent report of psychiatric symptoms was assessed using the Child Behavior Checklist [CBCL; Achenbach & Rescorla, 2001]. The CBCL consists of 118 items measuring total behavior problems, broad-band behavior problems (e.g. internalizing behavior problems, externalizing behavior problems), and narrow-band behavior problems (e.g. anxious/depressed, withdrawn/depressed, somatic complaints, social problems, thought problems, attention problems, rule-breaking behavior, and aggressive behavior). For the purposes of the current study, only scores on the internalizing domain and the subscales composing the internalizing domain (i.e. anxious/depressed, withdrawn/depressed, and somatic complaints) are included.
Parent report of social functioning
The Social Skills Rating System [SSRS; Gresham & Elliott, 1990] assesses social competence in terms of cooperation, assertion, responsibility, and self-control using parent report. Based on these subscales, standard scores are created encompassing two scales: social skills and problem behavior. The Social Responsiveness Scale [SRS; Constantino et al., 2003] is a 65-item rating scale of the severity of ASD symptoms, specifically related to social impairment. The SRS yields a total score of ASD impairment and subscale scores based on items assessing social awareness, social cognition/information processing, capacity for reciprocal social responses, social motivation, and autistic mannerisms. Scores on the SRS tend to correspond with the ADI [Constantino et al., 2003].
FPS
Assessment of the FPS experiment took place on the second visit in the UW Integrated Brain Imaging Center. At the first visit, the option for training was provided to familiarize adolescents with the testing room and equipment.
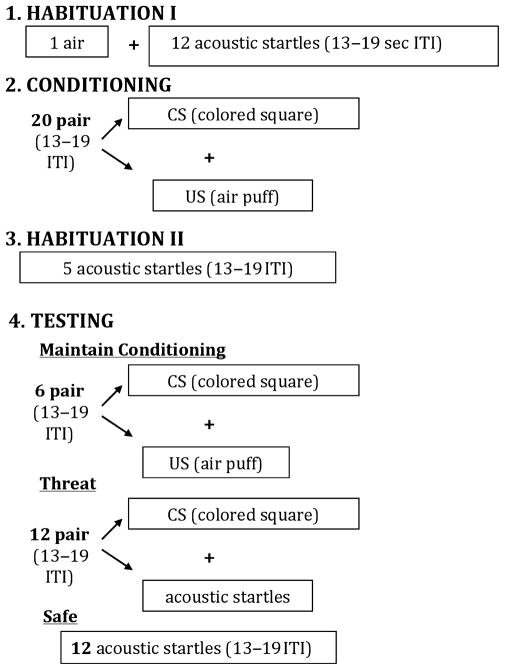
The following procedures are adapted from those described by Bernier et al., [2005]. Participants sat in a comfortable chair facing a blank computer screen and were instructed that they would see different colors appear on the computer screen, hear a startling noise over the headphones, and periodically feel an airpuff from the plastic tubing draped over their neck. The paradigm consisted of four phases: habituation I, conditioning, habituation II, and testing. In order to reduce initial reactivity, the habituation phase began with a single airpuff followed by 12 acoustic startle probes presented between 13 and 19 sec apart, while participants viewed a blank white computer screen. The conditioning phase followed; the CS (a red-colored square) appeared for 2,000 msec on the computer screen. Presentation of the aversive US (the aversive airpuff) overlapped for 50 msec and terminated simultaneously with the CS. Participants received 20 pairs of the CS and the US to facilitate learning of the CS–US association. The conditioning phase was followed by a second habituation phase, consisting of five presentations of the acoustic startle stimulus (SS) alone. Finally, the testing phase consisted of 30 trials presented in randomized order so that participants could not anticipate the next stimulus. Six trials consisted of the CS–US pairing to maintain conditioning. Twelve trials consisted of the CS–SS. These trials served as the “threat condition,” during which the CS was expected to potentiate response to the SS. In the threat condition, the SS was presented after 1,000 msec of the CS onset (i.e. when the participant was most likely in a potentiated state), which lasted for 2,000 msec. The other 12 trials consisted of the SS alone, comprising the “safe” condition (see Fig. 1).
Figure 1.
Fear-potentiated startle paradigm [adapted from Bernier et al., 2005]. ITI, intertrial interval; CS, conditioned stimulus; US, unconditioned stimulus.
Stimuli
The US (airpuff) consisted of a burst of air directed at the throat through a 4-mm internal diameter polyethylene tubing, with 200 msec duration and a pressure of 60 psi. Activation of the airpuff was controlled via a solenoid powered by an AC switch, electronically regulated by Eprime software (••, ••, ••). Compressed air was stored in a cylinder, which was connected to the solenoid using plastic tubing that passes through a regulator. The acoustic SS consisted of a 50-msec burst of white noise presented to both ears through headphones at 100 decibels. Visual and auditory stimuli were presented via Eprime on a data presentation computer.
Data Recording and Preparation
Consistent with procedures outlined in Bernier’s [[2005] published study and following recommendations for electrode placement from Fridlund and Cacioppo [1986], EMG activity was recorded using two Ag/AgCl surface electrodes placed under the left eye (orbicularis oculi) with a ground electrode placed on the forehead. Electrodes were affixed using EC 2 Electrode Cream (••, ••, ••). Placement of electrodes took approximately 10 min for each participant. A Biopac system (Goleta, CA, USA) recorded eyeblink latency and magnitude via the EMG electrodes. Intensity of EMG response was determined in the 20–100 msec following stimulus presentation. Although EMG activity was recorded continuously throughout the experiment, for the purpose of analyses, the responses in the testing phase were of interest. Specifically, the 12 responses to the “threat” and 12 responses to the “safe” conditions were representative of the FPS effect, providing 12 “time points” for each of the two conditions. Theoretically, responses to the “threat” condition should be larger than those to the “safe” if conditioning was successful. Prior to analysis (described in more detail later), the time variable for the EMG measurements reflected the time when the first stimulus was presented as zero. Thus, the first “threat” (e.g. “threat” 1) or “safe” stimulus time was set to zero to create a centered stimulus time.
Results
Psychiatric Symptoms
According to child report, groups did not differ in terms of symptoms of anxiety. However, the adolescents with ASD reported significantly higher levels of total depression than adolescents in the TD group, with significantly more reported symptoms within the domains of dysphoric mood, anhedonia/negative affect, negative self-evaluation, and somatic complaints. See Table 2 for a summary of RCMAS-2 and RADS-2 scores for each group.
Table 2.
Child report of anxiety and depression
| ASD
|
Typical
|
t-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| RCMAS-2 | n = 20 | n = 18 | |
| Total anxiety | 60.65 (11.17) | 65.06 (11.24) | −1.21 |
| Worry/oversensitivity | 58.55 (9.37) | 61.22 (12.59) | −0.75 |
| Physiological anxiety | 60.75 (13.42) | 64.83 (9.17) | −1.08 |
| Social concerns/concentration | 58.45 (10.06) | 64.33 (10.19) | −1.79 |
| RADS-2 | n = 18 | n = 18 | |
| Total depression | 60.11 (7.21) | 50.89 (5.30) | 4.37*** |
| Dysphoric mood | 53.61 (8.93) | 41.17 (6.82) | 4.70*** |
| Anhedonia/negative affect | 48.67 (7.05) | 43.33 (5.24) | 2.58* |
| Negative self-evaluation | 51.33 (11.44) | 42.94 (4.30) | 2.91** |
| Somatic complaints | 51.72 (7.28) | 42.33 (9.80) | 3.26** |
Note. T-scores are reported with mean = 50, SD = 10.
P < 0.05;
P < 0.01;
P < 0.001.
ASD, autism spectrum disorder; SD, standard deviation; RCMAS-2, Revised Children’s Manifest Anxiety Scale Second Edition; RADS-2, Reynolds Adolescent Depression Inventory, Second Edition.
Parents of teens with ASD reported significantly more symptoms and behaviors related to internalizing symptoms (internalizing domain; anxious/depressed, withdrawn/depressed subscales) than parents of TD teens. Parent report of psychiatric symptoms using the CBCL is reported in Table 3.
Table 3.
Parent report of psychiatric symptoms and social functioning
| ASD
|
Typical
|
t-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| CBCL | n = 19 | n = 18 | |
| Internalizing T | 59.89 (7.62) | 40.28 (6.92) | 8.19*** |
| I. Anxious/depressed T | 58.68 (8.17) | 50.50 (1.65) | 4.17*** |
| II. Withdrawn/depressed T | 60.26 (8.75) | 50.61 (1.29) | 4.63*** |
| III. Somatic complaints T | 58.26 (6.62) | 52.11 (3.38) | 3.53** |
| SSRS | n = 17 | n = 18 | |
| Social skills | 11.53 (3.81) | 16.78 (3.06) | −4.51*** |
| Problem behavior | 5.41 (2.40) | 1.89 (1.28) | 5.46*** |
| SRS | n = 18 | n = 16 | |
| Total score | 77.17 (10.67) | 41.25 (6.79) | 11.54*** |
| Social awareness | 70.72 (10.54) | 44.50 (11.41) | 6.97*** |
| Social cognition | 72.33 (9.71) | 40.62 (7.12) | 10.74*** |
| Reciprocal social communication | 76.50 (12.78) | 41.62 (6.72) | 9.77*** |
| Social motivation | 67.72 (11.78) | 44.62 (6.97) | 6.84*** |
| Autistic mannerisms | 77.06 (10.83) | 42.75 (4.17) | 11.89*** |
Note. CBCL and SRS T-scores are reported with mean = 50, SD = 10; SSRS clinical cutoff = 75 [Constantino et al., 2003].
P < 0.05;
P < 0.01;
P < 0.001.
ASD, autism spectrum disorder; SD, standard deviation; CBCL, Child Behavior Checklist; SSRS, Social Skills Rating System; SRS, Social Responsiveness Scale.
Social Skills
Parents of adolescents with ASD reported significantly more social impairment on the SRS and SSRS than parents of TD adolescents. Results from these parent measures of social skills are presented in Table 3.
FPS Response
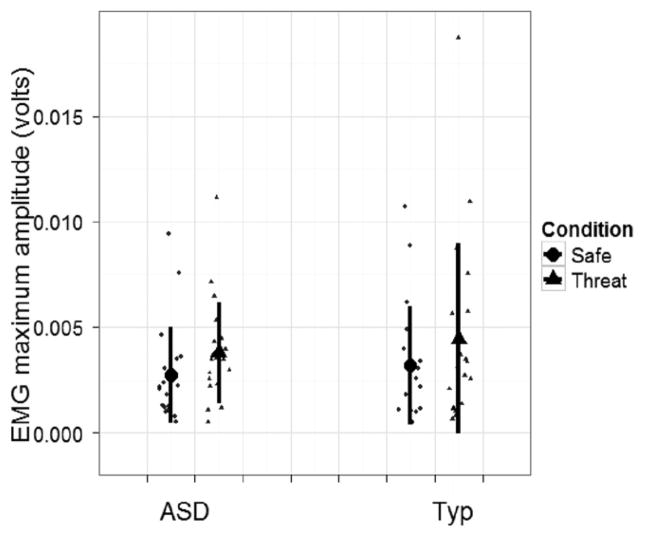
A linear mixed model was conducted to compare the maximum amplitude of the startle response (EMG) for each condition (threat, safe). As illustrated in Figure 2, both groups exhibited significantly greater startle magnitude for the threat versus the safe condition (t(893) = 5.80, P < 0.001). No differences in magnitude were found between groups, indicating that both groups demonstrated potentiated response during the threat condition, but did not differ from each other in terms of mean amplitude of EMG response.
Figure 2.
Mean of maximum electromyographic (EMG) amplitude for safe and threat conditions for autism spectrum disorder (ASD) (n = 20) and typical (n = 19) groups.
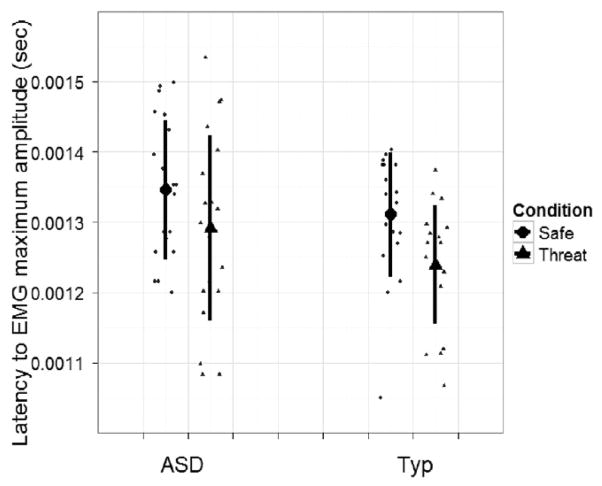
Latency of startle response was also examined using a linear mixed model (see Fig. 3). Both groups exhibited shorter latency (i.e. faster response) to the threat condition versus the safe condition, (t(893) = −2.40, P < 0.05), but did not differ from each other in terms of latency of response.
Figure 3.
Mean latency of electromyographic (EMG) response for safe and threat conditions for autism spectrum disorder (ASD) (n = 20) and typical (n = 19) groups.
Next, the relationship between parent- (CBCL) and child-reported (RCMAS) anxiety and FPS, and parent-rated social skills (SSRS, SRS) and FPS were examined for the ASD group. No significant correlations emerged between the CBCL internalizing domain, the associated subscales that comprise the internalizing domain (anxiety/depressed, withdrawn/depressed, and somatic complaints), RCMAS subscales or total score, SSRS sub-scales and total score, SRS subscales and total score, and EMG amplitude during the threat condition (rs = −0.18 to 0.30, ps > 0.19). The same correlations were conducted between measures of anxiety and social skills and EMG latency of response for the ASD group; there were no significant relationships (rs = −0.46 to 0.32, ps > 0.13, with the exception of problem behavior, P = 0.062). In light of high rates of reported depression discussed earlier, for exploratory purposes, we examined the correlations between depressive symptoms (RADS-2) and EMG amplitude and latency; no significant correlations were revealed (rs = −0.39 to 0.37, ps > 0.11).
Discussion
Consistent with previous findings [e.g. Mazurek & Kanne, 2010], teens with ASD exhibited more symptoms associated with internalizing conditions compared with their TD peers, based on both parent and child report. This was especially evident in ASD teens’ self-report of depressive symptoms on the RADS-2, which revealed significant elevations across all domains compared with TD teens. Contrary to expectations, adolescents with ASD did not report more symptoms of anxiety than their TD peers. On the other hand, parents of teens with ASD reported greater overall symptom severity across all internalizing domains of the CBCL (anxious/depressed, withdrawn/depressed, and somatic) compared with parents of TD teens.
It is important to note that although adolescents with ASD self-reported significantly higher levels of depression than the TD group based on the RADS-2, the only scale that was actually elevated within the ASD group was total depression (t-score = 60.11). For all other scales, the t-scores fell close to the population mean of 50. However, ASD scores were relatively elevated compared with the average t-scores for the TD group, which fell below the mean, ranging from 41.17 to 43.33.
With regard to the RCMAS-2, the adolescents with ASD did not report more symptoms of anxiety than their TD peers. However, the scores for the ASD group converge around 1 standard deviation above the population mean, suggesting mild, subclinical anxiety in the group. Interestingly, the TD group reported symptoms 1–1.5 standard deviations above the population mean as well, suggesting the TD group may also be exhibiting subclinical levels of anxiety. The parents of the TD group did not report elevated anxiety in their children, suggesting that TD teens in this study may be exhibiting underlying internalizing symptoms not apparent to their caregivers. It is also possible that the startle session itself elicited anxiety, which could be reflected in the self-report measures completed during this visit. This may have been more salient for the TD versus ASD group, given that many of the participants in the ASD group had prior experience with a variety of psychophysiology measures at the laboratory.
Parent and child report of psychiatric symptoms did not correspond with each other. Previous findings suggest that an association between adult and child ratings of internalizing symptoms is often low because adults do not have access to the internalizing experiences of the children [Achenbach, McConaughy, & Howell, 1987]. Within ASD, evidence suggests elevated rates of depression and anxiety in children with ASD when assessed via parent, but not child, report; child report of depression has been shown to correlate with parent report of depression, whereas measures of anxiety do not [Lopata et al., 2010]. Results from the current study support this pattern of findings. Elevated CBCL but not RCMAS-2 scores may be a reflection of more accurate report of anxiety symptoms by parents versus children with ASD. Examination of individual items on the CBCL suggests parents of adolescents with ASD endorsed symptoms related to anxiety at higher rates than parents of adolescents with TD (e.g. fears he/she might do something bad; nervous, high strung, or tense; too fearful or anxious), suggesting that anxiety may be present in this sample of teens with ASD, but not detected using self-report. Alternatively, parents may misinterpret behavioral manifestations of core ASD symptoms as anxiety, thereby inflating CBCL scores.
Moreover, consistent with present findings, Lopata et al. [2010] found that depression was more prominent than anxiety in their sample of 40 children with ASD. The correspondence between child and parent report may be an indication that depressive symptoms are more common in youth with ASD, or more easily detected and better understood by children and their parents. Behaviors and emotions indicative of anxiety may be more difficult to recognize and interpret given their overlap with ASD core symptoms or their variable and atypical expression in ASD. In addition, although the current sample was expected to exhibit high levels of anxiety based on rates previously reported in the literature, this sample was not recruited specifically for clinical anxiety, thereby reducing the overall anxiety symptomatology and variability within the group; the size of the ASD sample may also contribute to these limitations.
The current study utilized child report as a primary anxiety measure, whereas previous studies yielding high rates tended to rely on parent report [e.g. Sukhodolksy et al., 2007]. Lack of insight and ability to recognize and report emotional states and mood fluctuations may have contributed to inaccurate report of anxiety by teens with ASD [Hill, Berthoz, & Frith, 2004]. The fact that adolescents in the study were able to recognize and report their depressive symptoms may challenge this notion; however, the interpretation of anxiety may be reliant on a more sophisticated cognitive process and thus more difficult to recognize than depression [e.g. Beck & Clark, 1988]. This is consistent with previous reports suggesting that higher functioning individuals with ASD are capable of self-report of depressive symptoms [Berthoz & Hill, 2005].
Finally, the RCMAS-2 and CBCL are not validated for use with individuals with ASD. The CBCL has previously yielded elevated rates in large samples of youth with ASD, which may actually be driven by the core impairments of ASD itself rather than comorbid symptoms [e.g. Mazefsky, Anderson, Conner, & Minshew, 2011]. Such findings call into question the utility of this measure in identifying and quantifying coexisting psychopathology in ASD. The lack of validated assessments is especially noteworthy given that clinical observation indicated that several of the teens had significant symptoms of anxiety, requiring accommodations during the assessments (e.g. requiring frequent explanations of what to expect, and familiarization with the psychophysiology materials and settings before commencing with tasks). Granted, these needs could be conceptualized as reflections of autism symptoms (e.g. need for routine and difficulty transitioning), yet one would still expect that such behaviors would be identified by measures of anxiety. A more in-depth diagnostic interview for anxiety may have been informative, but this was not feasible given the limitations of the current study. Lack of a clinically validated measure of anxiety provides further incentive for identification of a biomarker for such symptoms.
Consistent with previous results, the present study demonstrated that adolescents with ASD and TD potentiated the startle response, as demonstrated by larger EMG magnitude and faster response to the threat condition. Further, there were no group differences in magnitude or latency of the EMG startle response. Within the ASD group, parent-reported social functioning and parent- and self-reported symptoms of anxiety were not related to EMG magnitude or latency.
A discussion of the ASD and TD sample features is worth including, given the potential for individual characteristics to impact startle response. For example, the TD group is composed of three additional females than the ASD group. Evidence suggests that there are sex differences in eyeblink conditioning; in particular, females may learn to elicit the eyeblink response sooner than males, perhaps because they are anticipating the onset of the US [Dalla & Shors, 2009]. It is conceivable that the startle magnitude was inflated or the latency was reduced in the TD group, given the three additional females. These sex differences appear to emerge after puberty [Dalla & Shors, 2009], and puberty status was not available on the current sample. The TD group also exhibits higher average cognitive scores (mean = 126.84) than the ASD group (mean = 104.60), as measured by the DAS. The impact of IQ is expected to be negligible given that fear circuitry depends on primary sensory systems rather than more complex structures supporting language or multi-faceted social behavior, and does not require reportable perception of the fear-eliciting stimulus [e.g. Lang et al., 2000]. In fact, this reliance on more basic systems makes startle response ideal for use with populations with limited communication, providing a nonverbal indication of underlying neural mechanisms contributing to pathology.
These results suggest that within the context of the FPS paradigm, amygdala function is intact in adolescents with ASD. Despite a large body of evidence implicating amygdala dysfunction in ASD, participants in the current study were able to acquire the conditioning (i.e. the CS–US association), evidenced by heightened response to the threat condition. The formation of the CS–US association is thought to take place in the basal and lateral nuclei of the amygdala, with the central nucleus projecting to various autonomic and somatomotor networks involved in mediating the fear response [see Kim & Jung, 2006 for review]. Findings from the present study suggest that social symptoms associated with ASD, assessed using the SRS and SRSS, are not linked directly to these components of amygdala function, at least as measured by FPS. It is reasonable to hypothesize that symptoms of ASD may be related to the functions of other amygdala nuclei not measured by FPS.
The lack of association between symptoms of anxiety and EMG response among the teens with ASD contradicts the pattern reported in the TD literature of larger startle response associated with greater anxiety symptomology. For example, in a study of over 100 adolescents, participants with high levels of behavioral inhibition and a lifetime occurrence of anxiety disorders exhibited increased startle reactivity, suggesting that this reflex could actually be a vulnerability marker for the development of anxiety in teens [Reeb-Sutherland et al., 2009]. There are several possible explanations for the absence of a significant relationship in the current sample of adolescents. First, it is possible that symptoms that appear on the surface as manifest anxiety are not actually tied to the same physiological underpinnings as those linked to true clinical anxiety. Parents and teens used measures (RCMAS-2, CBCL) that were validated for the evaluation of psychopathology in the TD population. It is conceivable that the reported anxiety symptoms among the teens with ASD in this study are reflections of other behaviors or symptoms related to ASD. As such, physiological mechanisms that represent anxiety would not be detected. It is also possible that individuals with ASD experience atypical symptoms of anxiety that are not mediated by the same underlying physiological processes as those observed in TD individuals. The lack of validated measures of comorbidity in ASD constrains proper interpretation of reported symptoms, limiting their utility in detecting a relationship with physiological mechanisms. Second, while a few parents reported clinical levels of anxiety in their child with ASD, this sample generally had limited variability in symptoms, making it difficult to examine the relationship between varying levels of anxiety and physiological response; recruiting specifically for individuals with ASD who have known difficulties with anxiety may help address this issue for future studies. Third, there may have been insufficient power to detect a relationship between anxiety and EMG response given the relatively small sample size of 20 adolescents with ASD. Future research would benefit from exploring the association between anxiety and physiological response in larger groups of individuals with ASD to confirm whether this was a limiting factor for the current study. Finally, self-report indicated that the current sample of teens with ASD suffered from significant depression. Depression and anxiety are difficult to tease apart and rarely occur as distinctly unique conditions among adolescents [e.g. van Lang, Ferdinand, Ormel, & Verhulst, 2006]; this makes interpretation of findings, particularly the relationship between anxiety and EMG, difficult. In light of these challenges, the development of a standardized and validated tool for measuring anxiety (and depression) in ASD would facilitate proper assessment of symptoms, elucidation of biological mechanisms, and provision of appropriate treatment; this should be an imminent focus of future work.
Acknowledgments
Funding for this project was provided by the UW Autism Center of Excellence (P50 HD055782, King) and an Autism Speaks Predoctoral Fellowship (Sterling).
References
- Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: Implications of cross-informant correlations for situational specificity. Psychological Bulletin. 1987;101:213–232. [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Adolphs R, Tranel D. Preferences for visual stimuli following amygdala damage. Journal of Cognitive Neuroscience. 1999;11:610–616. doi: 10.1162/089892999563670. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: Implications from non-human primate studies. Genes, Brain and Behavior. 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Corbett BA. The amygdala, autism and anxiety. Novartis Foundation Symposium. 2003;251:177–187. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators, & Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2008. Surveillance Summaries. 2012;61:1–19. [PubMed] [Google Scholar]
- Bachevalier J. Brief report: Medial temporal lobe and autism: A putative animal model in primates. Journal of Autism and Developmental Disorders. 1996;26:217–220. doi: 10.1007/BF02172015. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SCR. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Beck AT, Clark DA. Anxiety and depression: An information processing perspective. Anxiety Research. 1988;1:23–36. [Google Scholar]
- Bellini S. Social skills deficits and anxiety in high-functioning adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities. 2004;19:78–86. [Google Scholar]
- Bernier R, Dawson G, Panagiotides H, Webb S. Individuals with autism spectrum disorder show normal responses to a gear potential startle paradigm. Journal of Autism and Developmental Disorders. 2005;35:575–583. doi: 10.1007/s10803-005-0002-0. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Hill EL. The validity of using self-reports to assess emotion regulation abilities in adults with autism spectrum disorder. European Psychiatry. 2005;20:291–298. doi: 10.1016/j.eurpsy.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: An integrative account. Trends in Cognitive Sciences. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- Constantino J, Davis SA, Todd RD, Schindler MK, Gross MM, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the Autism Diagnostic Interview—Revised. Journal of Autism & Developmental Disorders. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiology & Behavior. 2009;25:229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Bohmelt AH. Startle modification: Introduction and overview. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification. Cambridge, UK: Cambridge University Press; 1999. pp. 6–18. [Google Scholar]
- de Bruin EI, Ferdinand RF, Meester S, de Nijs PFA, Verheij F. High rates of psychaitric co-morbidity in PDD-NOS. Journal of Autism and Developmental Disabilities. 2006;37:877–886. doi: 10.1007/s10803-006-0215-x. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential ability scales. 2. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115:515–544. [PubMed] [Google Scholar]
- Evans DW, Canavera K, Kleinpeter FL, Maccubbin E, Taga K. The fears, phobias and anxieties of children with autism spectrum disorders and Down syndrome: Comparisons with developmentally and chronologically age matched children. Child Psychology and Human Development. 2005;36:3–26. doi: 10.1007/s10578-004-3619-x. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Furmark T. Amygdaloid regional cerebral blood flow and subjective fear during symptom provocation in anxiety disorders. Annals of the New York Academy of Sciences. 2003;985:341–347. doi: 10.1111/j.1749-6632.2003.tb07092.x. [DOI] [PubMed] [Google Scholar]
- Fridlund A, Cacioppo J. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Gjevik E, Eldevik S, Fjæran-Granum T, Sponheim E. Kiddie-SADS reveals high rates of DSM-IV disorders in children and adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2011;41:761–769. doi: 10.1007/s10803-010-1095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham FM, Elliott SN. Social skills rating system. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Grillon C, Ameli R, Foot M, Davis M. Fear-potentiated startle: Relationship to the level of state/trait anxiety in healthy subjects. Biological Psychiatry. 1993;33:566–574. doi: 10.1016/0006-3223(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Startle modulation in children at risk for anxiety disorders and/or alcoholism. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:925–932. doi: 10.1097/00004583-199707000-00014. [DOI] [PubMed] [Google Scholar]
- Grillon C, Merikangas KR, Dierker L, Snidman N, Arriaga RI, et al. Startle potentiation by threat of aversive stimuli and darkness in adolescents: A multi-site study. International Journal of Psychophysiology. 1999;32:63–73. doi: 10.1016/s0167-8760(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Hill E, Berthoz S, Frith U. Brief report: Cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. Journal of Autism and Developmental Disorders. 2004;34:229–235. doi: 10.1023/b:jadd.0000022613.41399.14. [DOI] [PubMed] [Google Scholar]
- Juranek J, Filipek PA, Berenji GR, Modahl C, Osann K, Spence MA. Association between amygdala volume and anxiety level: Magnetic resonance imaging (MRI) study in autistic children. Journal of Child Neurology. 2006;21:1051–1058. doi: 10.1177/7010.2006.00237. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. Journal of neuropathology and experimental neurology. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ruscio AM, Shear K, Wittchen HU. Epidemiology of anxiety disorders. Current Topics in Behavioral Neurosciences. 2010;2:21–35. [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience and Biobehavioral Reviews. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Kuusikko S, Pollock-Wurman R, Jussila K, Carter AS, Mattila ML, et al. Social anxiety in high-functioning children and adolescents with Autism and Asperger syndrome. Journal of Autism and Developmental Disorders. 2008;38:1697–1709. doi: 10.1007/s10803-008-0555-9. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, et al. Brain activation in PTSD in response to trauma-related stimuli. Biological Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, et al. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. The American Journal of Psychiatry. 2008;165:124–132. doi: 10.1176/appi.ajp.2007.06091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata C, Toomey JA, Fox JD, Volker MA, Chow SY, et al. Anxiety and depression in children with HFASDs: Symptom levels and source differences. Journal of Abnormal Child Psychology. 2010 doi: 10.1007/s10802-010-9406-1. ••, ••–••. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, Anderson R, Conner CM, Minshew N. Child behavior checklist scores for school-aged children with autism: Preliminary evidence of patterns suggesting the need for referral. Journal of Psychopathology and Behavioral Assessment. 2011;33:31–37. doi: 10.1007/s10862-010-9198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek MO, Kanne SM. Friendship and internalizing symptoms among children and adolescents with ASD. Journal of Autism and Developmental Disorders. 2010 doi: 10.1007/s10803-010-1014-y. ••, ••–••. [DOI] [PubMed] [Google Scholar]
- Mcpheeters ML, Davis A, Navarre JR, Scott TA. Family report of ASD concomitant with depression or anxiety among US children. Journal of Autism and Developmental Disorders. 2011;41:646–653. doi: 10.1007/s10803-010-1085-9. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante M, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: Generalization, comorbidity, and physiological reactivity. Biological Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J, Dawson G, Abbott R, Faja S, Webb SJ, et al. Amygdalar volume and behavioral development in autism. Archives of General Psychiatry. 2006;63:686–693. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- Muris P, Steerneman P, Merckelbach H, Holdrinet I, Meesters C. Comorbid anxiety symptoms in children with pervasive developmental disorders. Journal of Anxiety Disorders. 1998;12:397–393. doi: 10.1016/s0887-6185(98)00022-x. [DOI] [PubMed] [Google Scholar]
- Newman JD, Bachevalier J. Neonatal ablations of the amygdala and inferior temporal cortex alter the vocal response to social separation in rhesus macaques. Brain Research. 1997;758:180–186. doi: 10.1016/s0006-8993(97)00212-6. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Reaven J. Children with high-functioning autism spectrum disorders and co-occurring anxiety symptoms: Implications for assessment and treatment. Journal for Specialists in Pediatric Nursing. 2009;14:192–199. doi: 10.1111/j.1744-6155.2009.00197.x. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Helfinstein BA, Degnan KA, Perez-Edgar K, Henderson HA, et al. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:610–617. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. What I think and feel: A revised measure of children’s manifest anxiety. Journal of Abnormal Child Psychology. 1997;25:15–20. doi: 10.1023/a:1025751206600. [DOI] [PubMed] [Google Scholar]
- Reynolds WR. Reynolds adolescent depression inventory. Lutz, FL: PAR Inc; 1987. [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ. Affective startle modulation in anticipation and perception. Psychophysiology. 2001;38:719–722. [PubMed] [Google Scholar]
- Salmond CH, de Haan M, Friston KJ, Gadian DG, Vargha-Khadem F. Investigating individual differences in brain abnormalities in autism. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2003;358:405–413. doi: 10.1098/rstb.2002.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. The Journal of Neuroscience. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biological Psychiatry. 2009;15:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. The Journal of Neuroscience. 2004;24:6392–6402. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Mental Retardation and Developmental Disabilities. 2004;10:234–247. doi: 10.1002/mrdd.20038. [DOI] [PubMed] [Google Scholar]
- Skokauskas N, Gallagher L. Psychosis, affective disorders and anxiety in autistic spectrum disorder: Prevalence and nosological considerations. Psychopathology. 2010;43:8–16. doi: 10.1159/000255958. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV, Doll EA. Vineland adaptive behavior scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Sukhodolsky DG, Seahill L, Gadow KD, Arnold LE, Aman MG, et al. Parent-rated anxiety symptoms in children with pervasive developmental disorders: Frequency and association with core autism symptoms and cognitive functioning. Journal of Abnormal Child Psychology. 2008;36:117–128. doi: 10.1007/s10802-007-9165-9. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: A PET study. The American Journal of Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- Uono S, Sato W, Toichi M. The specific impairment of fearful expression recognition and its atypical development in pervasive developmental disorder. Social Neuroscience. 2011;6:452–463. doi: 10.1080/17470919.2011.605593. [DOI] [PubMed] [Google Scholar]
- van Lang ND, Ferdinand RF, Ormel J, Verhulst FC. Latent class analysis of anxiety and depressive symptoms of the Youth Self-Report in a general population sample of young adolescents. Behaviour Research and Therapy. 2006;44:849–860. doi: 10.1016/j.brat.2005.06.004. [DOI] [PubMed] [Google Scholar]
- van Steensel FJ, Bögels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: A meta-analysis. Clinical Child and Family Psychology Review. 2011;14:302–317. doi: 10.1007/s10567-011-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Henry J, Neumann DL. Aversive Pavlovian conditioning in childhood anxiety disorders: Impaired response inhibition and resistance to extinction. Journal of Abnormal Psychology. 2009;118:311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative and Physiological Psychology. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescence with autism spectrum disorders. Clinical Psychology Review. 2009;29:216–229. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. The Journal of Neuroscience. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, Gadow KD. Exploring the nature and function of anxiety in youth with autis autism spectrum disorders. Clinical Psychology: Science and Practice. 2010;17:281–292. [Google Scholar]