Abstract
Background
Licensing arrangements for nicotine replacement therapy (NRT) in the UK were broadened in 2005 to allow prescribing to pregnant smokers. However, estimates of NRT prescribing in pregnant females in the UK are currently lacking.
Aim
To assess trends in NRT prescribing around pregnancy, and variation in prescribing by maternal characteristics.
Design and setting
Population-based descriptive study using pregnancy data from The Health Improvement Network primary care database, 2001–2012.
Method
NRT prescriptions were identified during pregnancy and in the 9 months before and after. Annual prescribing prevalence was calculated. Logistic regression was used to assess females’ likelihood of receiving prescriptions by maternal characteristics.
Results
Of 388 142 pregnancies studied, NRT was prescribed in 7551 for an average duration of 2 weeks. The prescribing prevalence of NRT increased from 0.03% (0.7% in smokers) in 2001 to 2.6% (11.4% in smokers) in 2005, after which it remained stable. Prescribing prevalence of NRT before and after pregnancy was half the prevalence during pregnancy. The odds of prescribing NRT during pregnancy in smokers increased with socioeconomic deprivation (OR = 1.29, 95% CI = 1.15 to 1.45 in the most compared with the least deprived group). Prescribing was 33% higher in pregnant smokers with asthma (OR = 1.33, 95% CI = 1.22 to 1.45) and mental illness (OR = 1.33, 95% CI = 1.23 to 1.44) compared with smokers without these diagnoses.
Conclusion
NRT prescribing is higher during pregnancy compared with before and after, and is higher in smokers from more socioeconomically deprived groups, those with asthma or those diagnosed mental illness.
Keywords: nicotine replacement therapy, pregnancy, prescribing, smoking cessation
INTRODUCTION
Smoking in pregnancy is related to several adverse outcomes for both mothers and their children.1,2 In the UK, 26% of mothers smoke directly before or during their pregnancy, and 12% continue to smoke throughout.3 Similar prevalence has been reported in Australia and the US (11.7% and 10.7% respectively).4,5 Therefore, reducing smoking in pregnancy is a global public health priority.6
Nicotine replacement therapy (NRT) is a pharmacological smoking cessation aid which became available on prescription from the UK NHS in April 2001.7 It was initially contraindicated during pregnancy because of a lack of evidence for its safety.8 To date there is no conclusive evidence on its effectiveness during pregnancy,9 and studies of NRT safety during pregnancy are inconclusive.2,10–12 Nevertheless, expert consensus is that NRT is likely to be less harmful than smoking during pregnancy and, with various caveats, NRT has been recommended by international guidelines when smoking cessation without NRT is unsuccessful.13–16
Literature describing NRT use in pregnancy is limited to observational studies from the US and Denmark assessing the association of NRT use during pregnancy and adverse birth outcomes. The prevalence of self-reported NRT used in the first 12 weeks of gestation was 0.3%,17 2–2.5% in 17–27 weeks of gestation in the Danish National Birth Cohort,10,11 and in the Pregnancy Risk Assessment Monitoring System (PRAMS) from four US states it was 3.9% (2004).2 Since their publication, new NRT products have been introduced and international guidelines on gestational NRT use have changed. In 2013, the World Health Organization (WHO) recommended an urgent need for studies on the surveillance of current NRT use in pregnancy.16
In December 2005, UK licensing arrangements were changed to allow prescribing of NRT to pregnant smokers.18 As a result, NRT can now be prescribed to pregnant females by GPs, midwives, or other licensed health professionals working in NHS Stop Smoking Services (SSS) after discussing the risks and benefits of using the drug in pregnancy. Although it can also be bought directly from pharmacies or other retailers such as supermarkets, all drug packaging retains warnings against its use in pregnancy without prior GP consultation. Most NRT is probably received via GP prescription, as half of NRT provided by pregnancy SSSs is issued via the patient’s GP.19
Thus far, only two UK studies have assessed NRT use in pregnancy.20,21 One of these studies only presents local data from Tayside, Scotland,21 and the second was only among females attending NHS SSS in England.20 Given that only 3% of all pregnant females attend SSSs, this will have excluded most pregnant smokers.22,23 In this study, UK prescribing of NRT is quantified before, during, and after pregnancy using a nationally representative sample, and the characteristics of females who receive NRT prescriptions are investigated.
How this fits in
Pregnancy is an opportunistic time to offer smoking cessation interventions to females. This study is the first to quantify prescribing of nicotine replacement therapy before, during, and after pregnancy in the UK. Prescribing prevalence of nicotine replacement therapy during pregnancy was 11% among smokers, double the prescribing prevalence before and after pregnancy. However, most females received only 2 weeks of nicotine replacement therapy during pregnancy. Prescribing was higher in pregnant smokers from more deprived areas and in smokers with diagnoses of asthma or mental illness.
METHOD
Data source and study population
The Health Improvement Network (THIN), an electronic database containing anonymised patient records from general practices across the UK, was used for this study, covering approximately 6% of the population,24 representative of the UK population in terms of demographics, prevalence of common illnesses, and fertility rates.25,26 Prevalence of smoking and prescribing of smoking cessation medications in the general population in THIN has been validated against national data.27,28 The study population included all pregnancies between January 2001 and December 2012 in females of childbearing age (15–49 years), resulting in a live birth or a stillbirth.
Outcome and covariates
The smoking status of females was determined using Read Codes29 recorded from 27 months before conception up to the end of pregnancy, based on the recording rules in the GP contract,30 which is described in detail elsewhere.31 Multilex Drug Codes for all NRT formulations available in the UK according to the British National Formulary (BNF) were used for NRT prescriptions.32 Code lists are available from the authors on request.
To investigate factors associated with NRT prescribing, data were extracted on females’ age at conception, socioeconomic deprivation (Townsend Index),33 preconception body mass index (BMI), and diagnoses of medical conditions (hypertension, diabetes, asthma, and mental illness, which included depression, anxiety, bipolar disorder, schizophrenia, and other psychoses) during or before pregnancy. These conditions were selected as they are closely related to smoking,3,34–38 and may influence quit attempts.
Statistical analysis
Overall and annual proportions of pregnancies, and pregnancies among smokers, with one or more NRT prescriptions before, during and after pregnancy were determined. There is no evidence of the time before and after pregnancy during which smokers are more likely to attempt to quit, therefore the 9 months before and after pregnancy were used to calculate prescribing prevalence, as these were similar to the average pregnancy length, allowing for comparisons of period prevalence. As smoking behaviours may fluctuate during a 9-month period, 3-month windows were also assessed during and around pregnancy. The use of different forms of NRT (patches, gum, nasal spray, lozenges, sublingual tablets, inhalator cartridges, and combination) was assessed.
Logistic regression was used to calculate ORs for associations between females’ characteristics and prescribing of NRT to smokers during pregnancy, restricting to pregnancies delivered from January 2006, after relaxation of licensing arrangements.18 All covariates reaching statistical significance at the 5% level in univariable models were included in the multivariable analysis and each covariate was sequentially dropped from the model to assess whether it remained statistically significant, retaining only those that were. Some females had more than one pregnancy during the study period and there may be potential clustering of females within practices; this was accounted for by using generalised estimating equations (GEE) with an exchangeable correction structure which provided best estimates of the population-level associations with maternal characteristics despite potential dependence between pregnancy, that is accounting for clustered data.39 Analyses were performed using Stata (version 12.0).
RESULTS
Baseline characteristics
Between 2001 and 2012, 388 142 pregnancies were identified resulting in live births or stillbirths, of which 71 685 (18.5%) were in smokers. Mean age at conception was 29.6 years (SD 5.9). Table 1 describes females’ characteristics for all pregnancies and pregnancies among smokers, and NRT prescribing according to these characteristics.
Table 1.
Baseline characteristics of the study population
| Total pregnancies, n = 388 142 | NRT prescribed, total n = 7551 (% of pregnancies with NRT prescription) | Pregnancies among smokers,an = 71 685 | NRT prescribed, n = 7551 (% of pregnancies among smokers with NRT prescription) | |
|---|---|---|---|---|
| Age at conception, years | ||||
| 5–19 | 27 365 | 930 (3.4) | 9898 | 930 (9.4) |
| 20–24 | 66 484 | 1962 (3.0) | 19 849 | 1962 (9.9) |
| 25–29 | 105 967 | 2004 (1.9) | 19 188 | 2004 (10.4) |
| 30–34 | 118 031 | 1664 (1.4) | 14 534 | 1664 (11.4) |
| 35–39 | 59 541 | 832 (1.4) | 6952 | 832 (12.0) |
| 40–44 | 10 222 | 152 (1.5) | 1203 | 152 (12.6) |
| 45–49 | 532 | 7 (1.3) | 61 | 7 (11.5) |
|
| ||||
| Townsend score in quintilesb | ||||
| Quintile 1 – most affluent | 83 203 | 722 (0.9) | 7970 | 722 (9.1) |
| Quintile 2 | 71 045 | 925 (1.3) | 9511 | 925 (9.7) |
| Quintile 3 | 76 619 | 1432 (1.9) | 14 291 | 1432 (10.0) |
| Quintile 4 | 73 470 | 2068 (2.8) | 18 097 | 2068 (11.4) |
| Quintile 5 – most deprived | 55 653 | 1875 (3.4) | 16 925 | 1875 (11.1) |
| Missing | 28 152 | 529 (1.9) | 4891 | 529 (10.8) |
|
| ||||
| Pre-conception body mass index, kg/m2 | ||||
| Normal (18.0–24.9) | 118 832 | 2266 (1.9) | 22 888 | 2266 (9.9) |
| Underweight (<18.0) | 8614 | 256 (3.0) | 2353 | 256 (10.9) |
| Overweight (25–29.9) | 58 693 | 1237 (2.1) | 11 464 | 1237 (10.8) |
| Obese (≥30) | 42 281 | 992 (2.3) | 9191 | 992 (10.8) |
| Missing | 159 722 | 2800 (1.8) | 25 789 | 2800 (10.9) |
|
| ||||
| Asthma | 33 724 | 1061 (3.1) | 8188 | 1061 (13.0) |
|
| ||||
| Hypertension | 9992 | 154 (1.5) | 1420 | 154 (10.8) |
|
| ||||
| Diabetes | 10 752 | 224 (2.1) | 1798 | 224 (12.5) |
|
| ||||
| Mental illness | 37 055 | 1547 (4.2) | 11 624 | 1547 (13.3) |
Smoker classified as those with a record of current smoking at some point within the 27 months before conception until delivery.
Socioeconomic status. NRT = nicotine replacement therapy.
Patterns of NRT prescribing in and around pregnancy
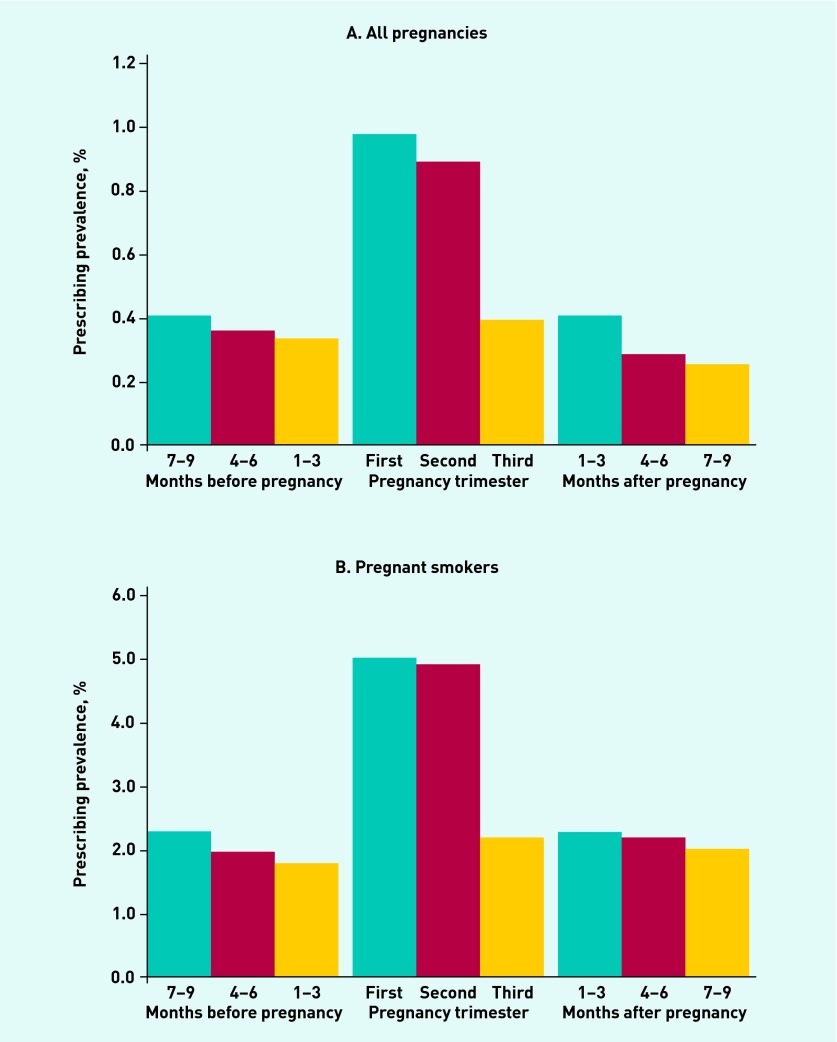
NRT was prescribed in 7551 pregnancies, which represented a prescribing prevalence of 2% of all pregnancies and 11% of pregnancies in smokers. In comparison, the prescribing prevalence was 1% during the 9 months before and after pregnancy overall, and 5% in smokers. Figure 1 shows the prescribing prevalence in 3-month periods outside pregnancy and by trimester. NRT prescribing among smokers was most frequent during the first and second trimesters at just over 5%, compared with 2% in the third trimester.
Figure 1.
Proportion of overall pregnancies and smokers with NRT prescriptions in each 3-month time period, between 2001 and 2012.
Among the pregnancies where NRT was prescribed, over half (55%) had only one prescription issued, 25% had two prescriptions, and 20% had three or more prescriptions. The maximum number of prescriptions issued during pregnancy was 26. On average, females were prescribed a total of 2 weeks’ worth of NRT (interquartile range 1–2 weeks). The prescription frequency and length of NRT issued in the 9 months before and after pregnancy was similar to pregnancy time.
In two-thirds of the pregnancies in which NRT was prescribed, it was initiated only during pregnancy, with no evidence of NRT prescribing prior to the start of pregnancy. The most common form of NRT used during pregnancy was transdermal patches (65% of all prescriptions), followed by inhalator cartridges (17%), gum (8%), lozenges (6%), sublingual tablets (2%), oromucosal spray (0.7%), and nasal spray (0.3%). Combination NRT was used in 14% of pregnancies where NRT was prescribed. The distribution of NRT forms prescribed before and after pregnancy was very similar.
Annual prescribing of NRT before, during, and after pregnancy
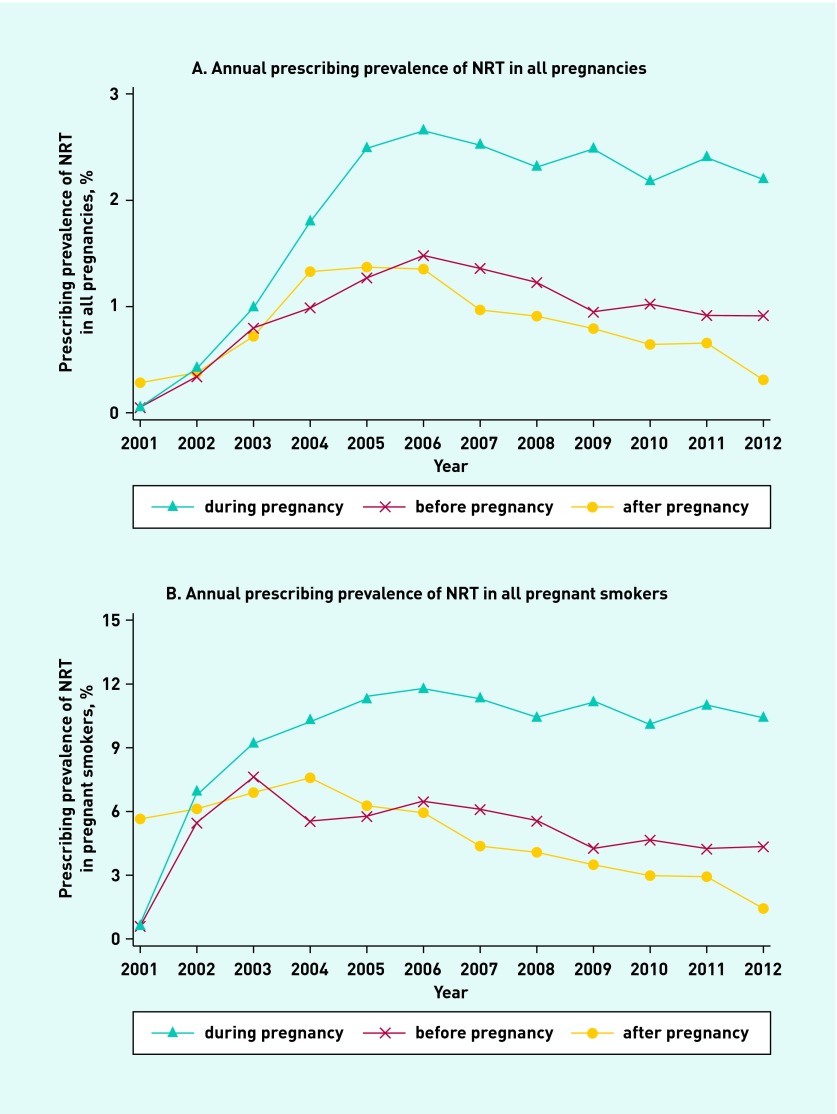
Figure 2 shows the proportion of pregnancies between 2001 and 2012 in which NRT was prescribed before, during, and after pregnancy. In 2001, the prescribing prevalence of NRT in all pregnancies during gestation was 0.03% (0.7% of pregnancies among smokers). This increased to 2.6% (11.4% among smokers) in 2005, after which it remained stable. The proportion of pregnancies with NRT prescriptions issued in the 9 months before and after pregnancy increased until 2004, after which it remained stable at around 1% (6% among smokers) with a gradual decline in prescribing prevalence after 2006.
Figure 2.
Annual prescribing prevalence of NRT between 2001 and 2012.
Prescribing of NRT by maternal characteristics
Table 2 shows the maternal characteristics associated with prescribing in pregnant smokers between 2006 and 2012. Prescribing was higher in older compared with younger age groups (OR for 40–44 years = 1.27, 95% CI = 1.03 to 1.58, compared with the 25–29-year age group). Pregnant smokers from more socioeconomically deprived groups were more likely to receive prescriptions compared with less deprived groups (OR for quintile 5 compared with quintile 1 = 1.29, 95% CI = 1.15 to 1.45). Pregnant smokers with a diagnosis of asthma or mental illness were 33% more likely to be prescribed compared with pregnant smokers without these morbidities.
Table 2.
Prescribing of NRT in pregnant smokers by maternal characteristics between January 2006 and December 2012
| Demographic variables | Pregnancies among smokers with one or more NRT prescriptions, n = 5756,an (%) | Unadjusted odds ratio (95% CI) | P- value | Adjusted odds ratio (95% CI)b | P- value |
|---|---|---|---|---|---|
| Age at conception, years | |||||
| 15–19 | 721 (9.9) | 0.92 (0.84 to 1.01) | 0.93 (0.84 to 1.03) | ||
| 20–24 | 1533 (10.2) | 0.94 (0.88 to 1.02) | <0.001 | 0.93 (0.86 to 1.00) | <0.001 |
| 25–29 | 1537 (10.7) | 1.00 | 1.00 | ||
| 30–34 | 1222 (11.9) | 1.12 (1.04 to 1.22) | 1.13 (1.04 to 1.23) | ||
| 35–39 | 616 (12.2) | 1.16 (1.05 to 1.28) | 1.17 (1.05 to 1.30) | ||
| 40–44 | 122 (13.3) | 1.28 (1.05 to 1.56) | 1.27 (1.03 to 1.58) | ||
| 45–49 | 5 (10.9) | 1.01 (0.40 to 2.58) | 1.12 (0.43 to 2.96) | ||
|
| |||||
| Townsend score | |||||
| Quintile 1 (least deprived) | 505 (9.2) | 1.00 | 1.00 | ||
| Quintile 2 | 696 (10.1) | 1.10 (0.98 to 1.25) | <0.001c | 1.09 (0.96 to 1.24) | <0.001c |
| Quintile 3 | 1101 (10.3) | 1.14 (1.01 to 1.27) | 1.19 (1.05 to 1.34) | ||
| Quintile 4 | 1605 (11.8) | 1.35 (1.19 to 1.48) | 1.37 (1.22 to 1.54) | ||
| Quintile 5 (most deprived) | 1428 (11.3) | 1.25 (1.13 to 1.39) | 1.29 (1.15 to 1.45) | ||
| Missing | 421 (11.4) | 1.27 (1.10 to 1.45) | 1.33 (1.15 to 1.54) | ||
|
| |||||
| Pre-conception body mass index, kg/m2 | |||||
| Underweight | 206 (11.0) | 1.12 (0.96 to 1.30) | |||
| Normal | 1786 (10.6) | 1.00 | 0.414 | – | – |
| Overweight | 976 (11.2) | 1.06 (0.97 to 1.14) | |||
| Obese | 806 (11.1) | 1.06 (0.96 to 1.15) | |||
| Missing | 1982 (10.8) | 1.02 (0.95 to 1.09) | |||
|
| |||||
| Diabetes | 193 (13.2) | 1.26 (1.08 to 1.47) | 0.075 | – | – |
|
| |||||
| Hypertension | 114 (11.3) | 1.04 (0.86 to 1.27) | 0.660 | – | – |
|
| |||||
| Asthma | 845 (13.8) | 1.36 (1.26 to 1.48) | <0.001 | 1.33 (1.22 to 1.45) | <0.001 |
|
| |||||
| Mental illness | 1181 (13.7) | 1.38 (1.29 to 1.48) | <0.001 | 1.33 (1.23 to 1.44) | <0.001 |
NRT = nicotine replacement therapy.
Percentages of pregnancies in smokers with NRT prescription.
All covariates mutually adjusted.
P-value for trend.
DISCUSSION
Summary
After NRT was made available on NHS prescription in 2001, prescribing in and around pregnancy increased; by 2005 prescribing was twice as high during pregnancy as in the 9 months immediately before and after pregnancy, despite being contraindicated for pregnant females. The December 2005 licence relaxation to allow prescribing in pregnancy did not further increase these trends and the prescribing prevalence during pregnancy has remained stable at 2% (11% in smokers). Females with asthma or mental illnesses and those from more socioeconomically-deprived areas were more likely to receive prescriptions during pregnancy. Eighty per cent of females received ≤2 prescriptions.
Strengths and limitations
Using a large population-based data source, longitudinal and contemporaneous prescribing estimates are presented; this is the first study of NRT prescribing during pregnancy in the UK and the only study internationally that has assessed prescribing trends. Ascertainment of NRT use is based on prescribing data rather than self-reported NRT use, which females may under-report.11 Prescribing in 9-month periods immediately before and after pregnancy was also assessed, whereas other studies only report NRT use in trimesters one and two.10,11,17 Therefore, the present estimates of NRT prescribing around pregnancy are novel in providing population-level information on smoking cessation attempts pre-conception and postpartum for the first time.
The present study data capture all NRT prescribing to pregnant females in UK primary care in practices registered in THIN. These data may not include NRT prescribing in other settings such as local NHS Stop Smoking Services for Pregnant females (SSSP) and NRT purchased in pharmacies or retailers. A survey of all SSSPs in England conducted between April 2010 and March 2011 reported that almost half of the NRT provided by these services was issued through GPs.40 In terms of self-purchased NRT, the authors believe this will be infrequent for several reasons. Firstly, the prevalence of medication use without health professional consultation is lower during pregnancy than when females are not pregnant.41 Furthermore, all packages of NRT clearly instruct females to consult a doctor before using them if they are pregnant. Lastly, in the UK females are entitled to free NHS prescriptions during pregnancy,42 so they are more likely to get free prescriptions through GPs than paying for NRT. Hence, the authors believe that this study captures most prescriptions of NRT issued and provides valuable information on prescribing patterns during pregnancy.
Potential changes in smoking habits were not accounted for over the study, and therefore this study has also presented proportions for all pregnancies. Some females may quit or relapse after delivery consequently leading to changes in the baseline smokers; NRT estimates could therefore be overestimated if more females relapse than are recorded, and underestimated if more females quit. In the present data, however, over 75% of pregnant females who were classified as smokers during pregnancy and who had a recording of smoking status within the 9 months after delivery were still recorded as smokers. Therefore, a substantial overestimation or underestimation is unlikely.
Comparison with existing literature
The present study data suggest that UK prescribing of NRT during pregnancy increased between 2001 and 2005, after which it plateaued. Despite NRT use being recommended in smoking cessation guidelines for pregnant females in several countries,14,15,18 no other studies thus far have assessed the annual prescribing prevalence of NRT during pregnancy for comparison. Studies from the US, Denmark, and Scotland report an overall prescribing prevalence of between 0.3% and 4%.2,10,11,21 NRT use in pregnant smokers attending English SSSs is reported to be 85%20 and considering that only 3% of pregnant females attend these services, this equates to 2.5%, which is similar to the present findings.
The National Institute for Health and Care Excellence (NICE) recommends that pregnant females should initially be prescribed 2 weeks of NRT from their agreed stop date with further NRT after re-assessment.7 The average duration of prescription for females in the present study was 2 weeks and most females (80%) received two or fewer prescriptions. One reason for this may be that compliance was low and females did not quit or use it to quit, in which case no further NRT was prescribed. Some females may have bought NRT independently after the first prescription; however, considering that females are entitled to free prescriptions during pregnancy and NRT from retailers is reasonably expensive, this is unlikely. Studies in other populations have not reported the duration of NRT use in pregnancy. However, 8–12 weeks’ use is recommended for optimal effectiveness in the general population,43 so it is unlikely that 2 weeks’ use is effective for smoking cessation in pregnancy.
A study including 5716 pregnant females from the US showed NRT prescribing to be lower in pregnant smokers aged <35 years compared with pregnant smokers aged ≥35 years,2 which is similar to the present findings. Low socioeconomic status is associated with a higher prevalence of chronic disease44 and higher risk of adverse pregnancy outcomes,45 which could explain why pregnant smokers in the deprived group in this study were prescribed NRT more often than affluent groups. Asthma and mental illness are the most common medical conditions encountered during pregnancy,46,47 and are closely related to smoking, which may explain the significant association with NRT prescribing compared with other conditions.
The English SSSPs study reported that 55% of all pregnant smokers (65% of pregnant NRT users) used combination NRT,20 which is high compared with the present estimate of 14%. This is mostly likely related to different baseline populations. Females voluntarily attending these specialist services likely have a higher motivation to quit, which may result in more quit attempts and more NRT being prescribed compared with females attending primary care.
It is unfortunate that NRT prescribing prevalence outside pregnancy began to decline considering the demonstrated effectiveness of NRT;48 however, this may be related to the licensing of varenicline for smoking cessation in the non-pregnant population in December 2006, after which a reduction in NRT and bupropion prescribing was seen in the general population.49
Implications for practice
Pregnancy offers a strategic opportunity for health professionals to promote smoking cessation as females are generally more receptive to cessation interventions and are more likely to attempt to quit smoking because of the potential foetal harm associated with smoking during pregnancy.50 The study findings give insight into the prescribing in and around pregnancy, which is important for policy makers and GPs to monitor and promote smoking cessation in females of childbearing age. The study shows that NRT was prescribed for an average of only 2 weeks during pregnancy, which is unlikely to be effective considering that NRT use in the general population for smoking cessation is recommended for at least 8–12 weeks. It is also highlighted that only 1% of smokers who are not yet pregnant receive NRT, which indicates missed opportunities to assist young females to quit, despite the reported effectiveness of NRT outside pregnancy. Although interactions between health professionals and females during pregnancy should be used to discuss and offer interventions to promote smoking cessation, greater potential benefit would result from starting before pregnancy which should be a prioritised focus for females and healthcare providers.
Funding
This work is supported by a University of Nottingham International Research Excellence Scholarship and the National Institute for Health Research (NIHR). This article presents independent research funded by the NIHR under its Programme Grants for Applied Research Programme (reference RP-PG 0109-10020). The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. Nafeesa N Dhalwani, Lisa Szatkowski and Tim Coleman are members of the UK Centre for Tobacco and Alcohol Studies (UKCTAS) (http://www.ukctas.ac.uk). Funding from the BHF, Cancer Research UK, the Economic and Social Research Council, the Medical Research Council and the NIHR, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
Ethical approval for this study was obtained from the THIN Scientific Review Committee (reference number 11-047).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Tim Coleman was paid to attend a symposium arranged by Pierre Fabre Laboratories, France in 2012. The other authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: www.bjgp.org/letters
REFERENCES
- 1.Neuman A, Hohmann C, Orsini N, et al. Maternal smoking in pregnancy and asthma in preschool children. Am J Respir Crit Care Med. 2012;186(10):1037–1043. doi: 10.1164/rccm.201203-0501OC. [DOI] [PubMed] [Google Scholar]
- 2.Gaither KH, Brunner Huber LR, Thompson ME, et al. Does the use of nicotine replacement therapy during pregnancy affect pregnancy outcomes? Matern Child Health J. 2009;13(4):497–504. doi: 10.1007/s10995-008-0361-1. [DOI] [PubMed] [Google Scholar]
- 3.The infant feeding survey 2010: early results. York: The NHS Information Centre; 2011. Health and Social Care Information Centre. [Google Scholar]
- 4.Scollo M, Winstanlet M. Tobacco in Australia: facts and issues. Melbourne: Cancer Council Victoria; 2012. [Google Scholar]
- 5.Centers for Disease Control and Prevention Prevalence of Smoking during the last 3 months of Pregnancy, PRAMS, 2000–2008. http://www.cdc.gov/prams/DATA-TobaccoTables.htm#table2 (accessed 9 Jul 2014)
- 6.World Health Organization . Report on the Global Tobacco Epidemic 2008 — the mpower package. Geneva: World Health Organization; 2008. [Google Scholar]
- 7.National Institute for Health and Care Excellence Quitting smoking in pregnancy and following childbirth. 2010 NICE guidelines [PH26]. http://guidance.nice.org.uk/PH26/Guidance/pdf/English (accessed 9 Jul 2014) [Google Scholar]
- 8.Coleman T, Chamberlain C, Cooper S, et al. Efficacy and safety of nicotine replacement therapy for smoking cessation in pregnancy: systematic review and meta-analysis. Addiction. 2011;106(1):52–61. doi: 10.1111/j.1360-0443.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 9.Coleman T, Cooper S, Thornton JG, et al. A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med. 2012;366(9):808–818. doi: 10.1056/NEJMoa1109582. [DOI] [PubMed] [Google Scholar]
- 10.Lassen TH, Madsen M, Skovgaard LT, et al. Maternal use of nicotine replacement therapy during pregnancy and offspring birthweight: a study within the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2010;24(3):272–281. doi: 10.1111/j.1365-3016.2010.01104.x. [DOI] [PubMed] [Google Scholar]
- 11.Strandberg-Larsen K, Tinggaard M, Andersen AN, et al. Use of nicotine replacement therapy during pregnancy and stillbirth: a cohort study. Br J Obstet Gynaecol. 2008;115(11):1405–1410. doi: 10.1111/j.1471-0528.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 12.Coleman T, Chamberlain C, Davey M-A, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;9:CD010078. doi: 10.1002/14651858.CD010078. [DOI] [PubMed] [Google Scholar]
- 13.Report of the committee on safety of medicines working group on nicotine replacement therapy. London: Medicines and Healthcare products Regulatory Authority; 2005. Medicines and Healthcare products Regulatory Agency. [Google Scholar]
- 14.European Smoking Cessation Guidelines: the authoritative guide to a comprehensive understanding of the implications and implementation of treatments and strategies to treat tobacco dependence. Brussels: European Network for Smoking and Tobacco Prevention aisbl (ENSP); 2011. European Network for Smoking and Tobacco Prevention. [Google Scholar]
- 15.Zwar N, Richmond R, Borland R, et al. Supporting smoking cessation: a guide for health professionals. Melbourne: The Royal Australian College of General Practitioners; 2011. [Google Scholar]
- 16.World Health Organization . WHO recommendations for the prevention and management of tobacco use and second-hand exposure in pregnancy. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 17.Morales-Suarez-Varela MM, Bille C, Christensen K, et al. Smoking habits, nicotine use, and congenital malformations. Obstet Gynecol. 2006;107(1):51–57. doi: 10.1097/01.AOG.0000194079.66834.d5. [DOI] [PubMed] [Google Scholar]
- 18.Nicotine replacement therapy — guidance for health professionals on changes in the licensing arrangements for nicotine replacement therapy. London: ASH; 2005. Action on Smoking and Health. [Google Scholar]
- 19.Fahy SJ, Cooper S, Coleman T, et al. Provision of smoking cessation support for pregnant women in England: results from an online survey of NHS stop smoking services for pregnant women. BMC Health Serv Res. 2014;14(1):107. doi: 10.1186/1472-6963-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brose LS, McEwen A, West R. Association between nicotine replacement therapy use in pregnancy and smoking cessation. Drug Alcohol Depend. 2013;132(3):660–664. doi: 10.1016/j.drugalcdep.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Irvine L, Flynn RW, Libby G, et al. Drugs dispensed in primary care during pregnancy. Drug Saf. 2010;33(7):593–604. doi: 10.2165/11532330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.National Audit Office, Department of Health . Maternity services in England. London: The Stationery Office; 2013. [Google Scholar]
- 23.Health and Social Care Information Centre NHS Stop Smoking Services: England, April 2012 to December 2012 (Q3– Quarterly report) http://www.hscic.gov.uk/catalogue/PUB10693 (accessed 29 Jul 2014)
- 24.CSD Medical Research UK THIN Data Guide for Researchers. 2011 [Google Scholar]
- 25.Blak BT, Thompson M, Dattani H, et al. Generalisability of The Health Improvement etwork (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19:251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 26.Tata LJ, Hubbard RB, McKeever TM, et al. Fertility rates in women with asthma, eczema, and hay fever: a general population-based cohort study. Am J Epidemiol. 2007;165(9):1023–1030. doi: 10.1093/aje/kwk092. [DOI] [PubMed] [Google Scholar]
- 27.Szatkowski L, Lewis S, McNeill A, et al. Can data from primary care medical records be used to monitor national smoking prevalence? J Epidemiol Community Health. 2012;66(9):791–795. doi: 10.1136/jech.2010.120154. [DOI] [PubMed] [Google Scholar]
- 28.Langley TE, Szatkowski L, Wythe S, et al. Can primary care data be used to monitor regional smoking prevalence? An analysis of The Health Improvement Network primary care data. BMC Public Health. 2011;11(773) doi: 10.1186/1471-2458-11-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Health and Social Care Information Centre Read Codes. http://systems.hscic.gov.uk/data/uktc/readcodes (accessed 1 Aug 2014) [Google Scholar]
- 30.Primary Care Commissioning http://www.pcc-cic.org.uk/search/site/general%20practice%20contracts?f%255b0%255d=im_field_topics%253A52&f%255b1%255d=im_field_topics%253A68 (accessed 1 Aug 2014)
- 31.Dhalwani NN, Tata LJ, Coleman T, et al. Completeness of maternal smoking status recording during pregnancy in United Kingdom primary care data. PLoS ONE. 2013;8(9):e72218. doi: 10.1371/journal.pone.0072218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joint Formulary Committee . British National Formulary (BNF) 62nd edn. London: BMJ Group and Pharmaceutical Press; 2011. [Google Scholar]
- 33.Townsend P, Phillimore P, Beattie A. Health and deprivation: inequality and the North. London: Croom Helm; 1988. [Google Scholar]
- 34.Primatesta P, Falaschetti E, Gupta S, et al. Association between smoking and blood pressure evidence from the health survey for England. Hypertension. 2001;37:187–193. doi: 10.1161/01.hyp.37.2.187. [DOI] [PubMed] [Google Scholar]
- 35.Chiolero A, Faeh D, Paccaud F, et al. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 36.Siroux V, Pin I, Oryszczyn M, et al. Relationships of active smoking to asthma and asthma severity in the EGEA study. Epidemiological study on the Genetics and Environment of Asthma. Eur Respir J. 2000;15(3):470–477. doi: 10.1034/j.1399-3003.2000.15.08.x. [DOI] [PubMed] [Google Scholar]
- 37.Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness. JAMA. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 38.Will JC, Galuska DA, Ford ES, et al. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int J Epidemiol. 2001;30(3):540–546. doi: 10.1093/ije/30.3.540. [DOI] [PubMed] [Google Scholar]
- 39.Hanley JA, Negassa A, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–75. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 40.Fahy SJ, Cooper S, Coleman T, et al. Provision of smoking cessation support for pregnant women in England: results from an online survey of NHS stop smoking services for pregnant women. BMC Health Serv Res. 2014;14:107. doi: 10.1186/1472-6963-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Afolabi AO. Public health — social and behavioural health. Croatia: Intech; 2012. Self medication, drug dependence and self-managed health care — a review; pp. 223–243. [Google Scholar]
- 42.NHS Choices Are pregnant women entitled to free NHS prescriptions? http://www.nhs.uk/chq/Pages/941.aspx?CategoryID=68&SubCategoryID=161#close. (accessed 9 Jul 2014) [Google Scholar]
- 43.McRobbie H, Maniapoto M. Getting the most out of nicotine replacement therapy. Best Practice Journal. 2009;20:58–62. [Google Scholar]
- 44.Eachus J, Williams M, Chan P, et al. Deprivation and cause specific morbidity: evidence from the Somerset and Avon survey of health. BMJ. 1996;312:287–292. doi: 10.1136/bmj.312.7026.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng G, Thompson ME, Hall GB. Pathways of neighbourhood-level socioeconomic determinants of adverse birth outcomes. Int J Health Geographics. 2013;12(1):32. doi: 10.1186/1476-072X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson-Piercy C. Asthma in pregnancy. BMJ. 2001;56:325–8. doi: 10.1136/thorax.56.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett HA, Einarson A, Taddio A, et al. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103(4):698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 48.Stead LF, Perera R, Bullen C, et al. Nicotine Replacement Therapy for smoking cessation (Review) Cochrane Database Syst Rev. 2008;(1):CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 49.National Institute for Health and Care Excellence NICE implementation uptake report: smoking cessation drugs. https://www.nice.org.uk/proxy/?sourceUrl=http%3a%2f%2fwww.nice.org.uk%2fmedia%2fD79%2f42%2fUptakeReportSmokingCessationPublication.pdf (accessed 1 Aug 2014)
- 50.Klesges LM, Johnson KC, Ward KD, et al. Smoking cessation in pregnant women. Obstet Gynecol Clin North Am. 2001;28(2):269–282. doi: 10.1016/s0889-8545(05)70200-x. [DOI] [PubMed] [Google Scholar]