Abstract
Purpose: Antimicrobial activities of meropenem products on Klebsiella pneumoniae isolates were determined.
Methods: 212 non-duplicated Klebsiella pneumoniae isolates were examined for in vitro meropenem susceptibility test by using the following disks, which were made from Meronem (AstraZeneca, UK), Exipenem (Exir, Iran) and Meroxan (DAANA, Iran) powders. MIC50 and MIC90 for meropenem antibiotics were determined.
Results: Meronem had good activities against most isolates of Klebsiella pneumoniae, and only a few strains had a rather high MIC. Exipenem and Meroxan showed a similar activity with Meronem.
Conclusion: Regarding the comparison of two internal generic meropenem products with the external Meronem product have shown that they are equivalents in terms of microbiological activity, as measured using the disk diffusion and MIC. In developing countries, we suggested preparing disks with antibiotic powders that can be an equivalent function in microbiological activity with standard disks. In addition, since it demonstrated significant antimicrobial activity against the Klebsiella pneumoniae. For use of Exipenem and Meroxan in vivo, it would be better to perform additional testing (activity against different species, stability etc.).
Keywords: Klebsiella pneumoniae, meropenem productions, agar dilution method
Zusammenfassung
Zielsetzung: Bestimmung der antimikrobiellen Wirksamkeit verschiedener Meropenem-Generika gegen Klebsiella pneumoniae-Isolate.
Methode: 212 nicht duplizierte Klebsiella pneumonia-Isolate wurden in vitro auf Empfindlichkeit gegen Meropenem in Form handelsüblicher Plättchen von Meronem (AstraZeneca, UK) und Exipenem (Exir, Iran) bzw. als Meroxan-Puder (DAANA, Iran) untersucht. Bestimmt wurden jeweils die MIC50 und die MIC90.
Ergebnisse: Meronem war gegen die meisten Isolate von Klebsiella pneumoniae gut wirksam, nur einige Stämme hatten eine etwas höhere MIC. Exipenem und Meroxan erwiesen sich als vergleichbar wirksam wie Meronem.
Schlussfolgerung: Der Vergleich zweier interner Meropenem-Generika mit dem externen Standard Meronem ergab, dass alle drei Produkte sowohl im Plättchendiffusionstest als auch bezüglich der MIC in ihrer antimikrobiellen Wirksamkeit gleichwertig waren. Daher empfehlen wir für Entwicklungsländer, Plättchen mit antibiotischem Puder selbst herzustellen, da sie in ihrer antimikrobiellen Aktivität dem Standardplättchen gleichwertig sein können. Meropenem erwies sich als wirksam gegen die Klebsiella pneumonia-Isolate. Für den Einsatz von Exipenem und Meroxan in vivo sollten allerdings ergänzende Tests durchgeführt werden (Wirksamkeit gegen verschiedene Species, Stabilität usw.).
Introduction
Carbapenems are the most potential β-lactam antibiotics, which developed in the 1980s, to oppose to β-lactamases resistance antibiotics. Meropenem is one of the broad-spectrum carbapenems against several clinically relevant Gram-negative aerobes and anaerobes [1], [2]. The bactericidal activity of meropenem is caused by the inhibition of cell wall synthesis through the inactivation of penicillin-binding proteins [2], [3]. Meropenem is approved by FDA for the treatment of bacterial meningitis, complicated skin and soft tissue and intra-abdominal infections. The increasing prevalence of resistance to beta-lactams [4], [5], [6] has prompted carbapenems as one of the cornerstone antibiotic classes remaining a mainstay for the treatment of patients with severe infections due to ESBL-producing Gram-negative bacteria [7], [8], [9]. The high prevalence of antibiotic resistance in Enterobacteriaceae, especially in Klebsiella pneumonia has been achieved in Iran. Resistance often includes new agents such as carbapenems, even before the introduction in Iran [10], [11], [12]. Carbapenems are used extensively in the treatment of Gram-negative bacteria infections in teaching hospitals [13]. To know the susceptibility to meropenem of commonly isolated Klebsiella pneumoniae in Iran, we conducted a study to evaluate in vitro antimicrobial activities of meropenem products against Klebsiella pneumoniae, including those commonly causing nosocomial infections in Tabriz teaching hospitals in Iran.
Materials and methods
Bacterial isolates
In this study, a total of 212 isolates of Klebsiella pneumoniae were collected from hospitalized patients at Tabriz University Hospitals from 2012 to 2013. The obtained isolates were identified by conventional biochemical tests such as oxidase, TSI, SIM, urea, etc. In the next step, the Microgen™ GN-ID kit (Microgen Bioproducts, England) was used for final identification.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing including 12 antibiotics Amoxicillin-clavulanic acid (30 µg), Cefotaxime (30 µg), Ceftriaxone (30 µg), Gentamicin (10 µg), Aztreonam (30 µg), Tetracycline (30 µg), Ceftazidime (30 µg), Cefepime (30 µg), Colistin (10 µg), Ciprofloxacin (5 µg), imipenem (10 µg), and meropenem (10 µg) was done according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [14].
Disk preparation
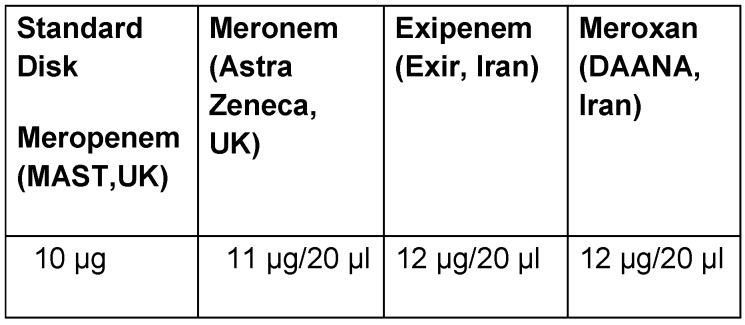
All isolates were examined for in vitro meropenem susceptibility test by using the following disks that were made from Meronem (AstraZeneca, UK), Exipenem (Exir, Iran), and Meroxan (DAANA, Iran) powders; each of these disks was compared with the other kind of meropenem products. Sterile blank diffusion disks were placed in labeled plates for meropenem products. Sterile blank disks were saturated with 20 µl of individual stock meropenem products. After the disks were dried, these were ready to be used for disk diffusion. Commercially available antibiotic disks (meropenem; MAST, UK) were used as standards for comparison.
Agar dilution test
The susceptibilities of all Klebsiella pneumoniae isolates to meropenem products were determined by the agar dilution method as described by the CLSI [14]. The inoculated plates were incubated in ambient air at 35°C for 16 to 18 h. Mueller-Hinton agar was used for susceptibility testing by the agar dilution method. The minimum inhibitory concentration (MIC) of each antimicrobial agent was defined as the lowest concentration that inhibited visible growth of the organism. Control strain, including E. coli ATCC 25922 was included in each set of tests.
Statistical analysis
To compare the in vitro activity of different meropenem products on Klebsiella pneumoniae isolates was determined by chi-square. SPSS, version 16 was used to perform statistical analysis, the chi-square test, when appropriates that p values are less than 0.05 were considered statistically significant.
Results
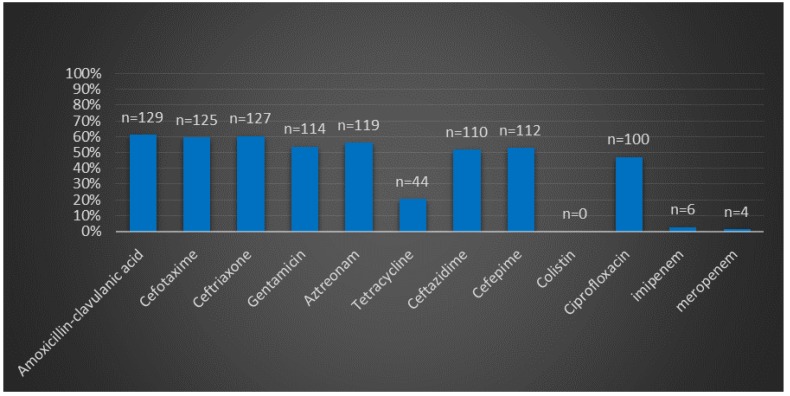
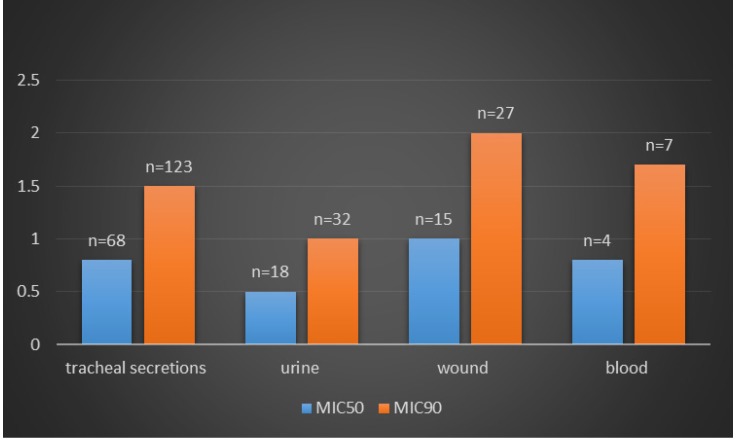
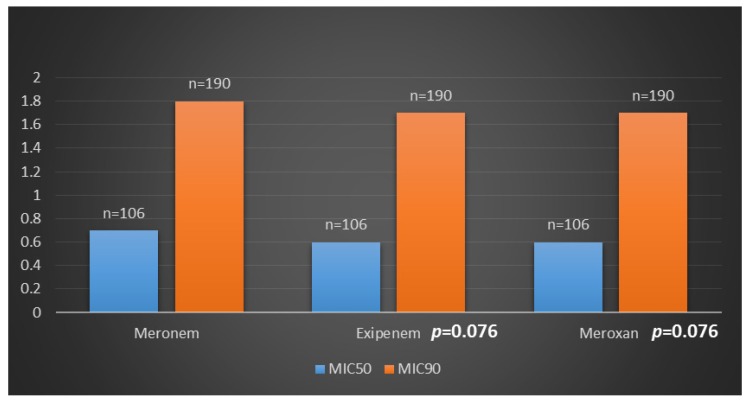
The isolates were collected from different infection sites of patients hospitalized in several wards. Klebsiella pneumoniae isolates were most frequently recovered from tracheal secretions (65%), followed by urine (16.9%), wounds (14.1%) and blood (4%). Frequency of antibiotic resistance in Klebsiella pneumoniae isolates is shown in Figure 1 (Fig. 1). The highest and the lowest resistance were observed in amoxicillin-clavulanic acid (61.3%), imipenem (1.8%), meropenem (0.9%) and colistin (0%) respectively. Meropenem had demonstrated significant in vitro antimicrobial activity against all Klebsiella pneumoniae isolates; 99.1% of the isolates were susceptible to meropenem. Moreover, all isolates were susceptible to colistin. Among the 212 K. pneumoniae isolated from Tabriz hospitalized patients, 128 isolates showed simultaneous resistance to six antibiotics (aztreonam, cefotaxime, amoxicillin-clavulanic acid, gentamicin, ceftriaxone, and ceftazidime). Disks, which were made with three kinds of meropenem products, showed similar antibacterial activity in comparison with the standard meropenem disk (MAST, UK). In Table 1 (Tab. 1) is shown the concentration of different meropenem products (Meronem, Exipenem and Meroxan products compare with meropenem disk (MAST, UK)) that have the similar microbiology activity. Wound infection was the main source of carbapenem-producing K. pneumoniae. K. pneumoniae isolated from wound samples was shown to produce carbapenemase at a significantly different rate (P<0.05) depending on the length of stay in hospital (Figure 2 (Fig. 2)). The MICs of meropenem products against all Klebsiella pneumoniae isolates are shown in Figure 3 (Fig. 3). Meronem (AstraZeneca, UK) had good activities against most isolates of Klebsiella pneumoniae, and only a few strains had a rather high MIC (≥4 µg/mL). Exipenem (Exir, Iran) and Meroxan (DAANA, Iran) showed a similar activity with Meronem (AstraZeneca, UK).
Figure 1. Antibiotic resistances pattern of Klebsiella Pneumoniae in Tabriz hospital, Iran.
Table 1. Comparison of concentration of meropenem products in the disks manually prepared with standard meropenem disk (MAST, UK).
Figure 2. MIC50 and MIC90 of meropenem for Klebsiella pneumoniae isolated from different clinical sources.
Figure 3. Comparative in vitro activity of meropenem products against Klebsiella pneumoniae isolates.
Discussion
Bacterial resistance to antibiotic therapy is an increasing public health problem around the world [15]. Moreover, the resistance pattern of the microorganisms could be different in various populations and therefore, each of them needed to be specially planned for reduction of resistance to antibiotics especially those most commonly used for treatment [16]. Most studies demonstrated that meropenem were one of the most effective agents for the treatment of infection due to Enterobacteriaceae. Meropenem has been used for severe nosocomial infections, often in hospital units. Their value lies in their broad spectrum and in overcoming most resistance in Gram-negative bacilli. Exposure and use of carbapenems for the treatment of diverse infectious disease appear to be a prerequisite to the development of resistance [17]. The emergence of carbapenem resistance among clinical isolates of Klebsiella pneumoniae has recently raised fears that effective antimicrobial treatment options for these isolates may soon be severely limited [18]. Comparison of the results of our study and other similar studies in other countries shows that meropenem is highly active in vitro against all the clinical isolates of Klebsiella pneumoniae [19]. An excellent level of concordance between the two internal generic meropenem products company (Exipenem and Meroxan) and the Meronem (AstraZeneca, UK) has been demonstrated only for Klebsiella pneumoniae isolates.
The level of essential agreement by Meronem (AstraZeneca, UK) is over 90%, achieved for all Klebsiella pneumoniae isolates. Antimicrobial susceptibility test for all Klebsiella pneumoniae isolates was performed by Mast company provided disk and similar results were achieved. Our studies show that Exipenem and Meroxan are effective in the same spectrums. The disks were impregnated by various meropenem products used for disk diffusion after drying because these disks do not have enough stability.
Antibiotics behaviors must be evaluated in vitro and in vivo to confirm their suitability for therapeutic use. Pharmaceutical equivalence or MIC values of any generic products are not useful criteria for granting therapeutic equivalence [20]. Because MIC breakpoints of meropenem products (Exipenem, Meroxan and Meronem) have not yet been obtained for Gram-negative and Gram-positive bacteria, it remains unclear whether the in vitro activity of antibacterial drugs is predictive of the clinical outcome. In order to do it, all generic products of meropenem should be tested in vivo. For in vivo use of Exipenem and Meroxan, it would be better to perform additional testing (activity against different species, stability etc.).
Conclusions
Regarding the comparison of two internal generic meropenem products with the external Meronem product has shown that they are equivalents in terms of microbiological activity, as measured using the disk diffusion and MIC. In developing countries, we suggested preparing disks with antibiotic powders that can be an equivalent function in microbiological activity with standard disks. In addition, in order to better validate of these generics (Exipenem and Meroxan) be equivalent to Meronem (AstraZeneca, UK), these in vitro findings must be further investigated (activity against different species, stability etc.) and confirmed in vivo.
Notes
Acknowledgments
The Research Center of Infectious and Tropical Diseases of Tabriz University of Medical Sciences, Tabriz, Iran, financially supported this work (grant No.10687/7 Jan. 2012), and the manuscript was written based on a dataset of Ph.D. thesis, registered in Tabriz University of Medical Sciences. The authors also would like to thank Sina hospital Staff for their help. The Ethic Commission of Tabriz University of Medical Sciences approved this study (Number: 329011/12 Mar. 2012).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Baldwin CM, Lyseng-Williamson KA, Keam SJ. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs. 2008;68(6):803–838. doi: 10.2165/00003495-200868060-00006. Available from: http://dx.doi.org/10.2165/00003495-200868060-00006. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JR. Meropenem: a microbiological overview. J Antimicrob Chemother. 1995 Jul;36 Suppl A:1–17. doi: 10.1093/jac/36.suppl_A.1. Available from: http://dx.doi.org/10.1093/jac/36.suppl_A.1. [DOI] [PubMed] [Google Scholar]
- 3.Wiseman LR, Wagstaff AJ, Brogden RN, Bryson HM. Meropenem. A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs. 1995 Jul;50(1):73–101. doi: 10.2165/00003495-199550010-00007. Available from: http://dx.doi.org/10.2165/00003495-199550010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Pitout JD. Infections with extended-spectrum beta-lactamase-producing enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010 Feb;70(3):313–333. doi: 10.2165/11533040-000000000-00000. Available from: http://dx.doi.org/10.2165/11533040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Rahal JJ. Antimicrobial resistance among and therapeutic options against gram-negative pathogens. Clin Infect Dis. 2009 Aug;49 Suppl 1:S4–S10. doi: 10.1086/599810. Available from: http://dx.doi.org/10.1086/599810. [DOI] [PubMed] [Google Scholar]
- 6.Coque TM, Baquero F, Canton R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 2008 Nov;13(47) Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19044. [PubMed] [Google Scholar]
- 7.Nicolau DP. Carbapenems: a potent class of antibiotics. Expert Opin Pharmacother. 2008 Jan;9(1):23–37. doi: 10.1517/14656566.9.1.23. Available from: http://dx.doi.org/10.1517/14656566.9.1.23. [DOI] [PubMed] [Google Scholar]
- 8.Baughman RP. The use of carbapenems in the treatment of serious infections. J Intensive Care Med. 2009 Jul-Aug;24(4):230–241. doi: 10.1177/0885066609335660. Available from: http://dx.doi.org/10.1177/0885066609335660. [DOI] [PubMed] [Google Scholar]
- 9.Hawkey PM, Livermore DM. Carbapenem antibiotics for serious infections. BMJ. 2012;344:e3236. doi: 10.1136/bmj.e3236. Available from: http://dx.doi.org/10.1136/bmj.e3236. [DOI] [PubMed] [Google Scholar]
- 10.Bazzaz BS, Naderinasab M, Mohamadpoor AH, Farshadzadeh Z, Ahmadi S, Yousefi F. The prevalence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae among clinical isolates from a general hospital in Iran. Acta Microbiol Immunol Hung. 2009 Mar;56(1):89–99. doi: 10.1556/AMicr.56.2009.1.7. Available from: http://dx.doi.org/10.1556/AMicr.56.2009.1.7. [DOI] [PubMed] [Google Scholar]
- 11.Ramazanzadeh R, Chitsaz M, Bahmani N. Prevalence and antimicrobial susceptibility of extended-spectrum beta-lactamase-producing bacteria in intensive care units of Sanandaj general hospitals (Kurdistan, Iran) Chemotherapy. 2009;55(4):287–292. doi: 10.1159/000224656. Available from: http://dx.doi.org/10.1159/000224656. [DOI] [PubMed] [Google Scholar]
- 12.Mehrgan H, Rahbar M, Arab-Halvaii Z. High prevalence of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a tertiary care hospital in Tehran, Iran. J Infect Dev Ctries. 2010 Mar;4(3):132–138. doi: 10.3855/jidc.488. Available from: http://dx.doi.org/10.3855/jidc.488. [DOI] [PubMed] [Google Scholar]
- 13.Rahbar M, Kabeh-Monnavar M, Vatan KK, Fadaei-haqi A, Shakerian F. Carbapenem resistance in gram-negavtive bacilli isolates in an Iranian 1000 bed tertiary care hospital. Pak J Med Sci. 2008;24(4):537–540. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute (CLSI) M100-S23 performance standards for antimicrobial susceptibility testing; twenty-third informational supplement. Wayne: CLSI; 2013. [Google Scholar]
- 15.Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005 Nov-Dec;36(6):697–705. doi: 10.1016/j.arcmed.2005.06.009. Available from: http://dx.doi.org/10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Namvar AE, Asghari B, Zadeh S, Lari AR. Comparison of in vitro activity of imipenem productions on bacterial isolates from Hashemi Nezhad Tehran hospitalized patients. J Bacteriol Res. 2010;2(5):51–54. [Google Scholar]
- 17.Drusano GL, Lode H, Edwards JR. Meropenem: clinical response in relation to in vitro susceptibility. Clin Microbiol Infect. 2000 Apr;6(4):185–194. doi: 10.1046/j.1469-0691.2000.00062.x. Available from: http://dx.doi.org/10.1046/j.1469-0691.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- 18.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009 Apr;9(4):228–236. doi: 10.1016/S1473-3099(09)70054-4. Available from: http://dx.doi.org/10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 19.Gesser RM, McCarroll K, Teppler H, Woods GL. Efficacy of ertapenem in the treatment of serious infections caused by Enterobacteriaceae: analysis of pooled clinical trial data. J Antimicrob Chemother. 2003 May;51(5):1253–1260. doi: 10.1093/jac/dkg237. Available from: http://dx.doi.org/10.1093/jac/dkg237. [DOI] [PubMed] [Google Scholar]
- 20.Jones RN, Fritsche TR, Moet GJ. In vitro potency evaluations of various piperacillin/tazobactam generic products compared with the contemporary branded (Zosyn, Wyeth) formulation. Diagn Microbiol Infect Dis. 2008 May;61(1):76–79. doi: 10.1016/j.diagmicrobio.2007.12.010. Available from: http://dx.doi.org/10.1016/j.diagmicrobio.2007.12.010. [DOI] [PubMed] [Google Scholar]