Abstract
Aim: During the last decade it became obvious that viruses belonging to Mimiviridae and Marseilleviridae families (order Megavirales), may be potential causative agents of pneumonia. Thus, we have performed a review of the association of Mimiviridae, Marseilleviridae, and virophages with pneumonia, particularly healthcare-associated pneumonia, and other infections of the respiratory tract.
Results and discussion: According to the analysis of the published articles, viruses belonging to Mimiviridae family can be potential agents of both community-acquired and healthcare-associated pneumonia. In particular, these viruses may be associated with poor outcome in patients of intensive care units.
The exact mechanism of their pathogenicity, however, still remains unclear. The discrepancies between the results obtained by serological and genomic methods could be explained by the high polymorphism of nucleotide sequences of Mimiviridae family representatives. Further investigations on the Mimiviridae pathogenicity and on the determination of Mimiviridae-caused pneumonia risk groups are required.
However, the pathogenicity of the viruses belonging to Marseilleviridae family and virophages is unclear up to now.
Keywords: mimivirus, mimiviridae, marseilleviridae, giant viruses, virophages, pneumonia, healthcare-associated infections
Zusammenfassung
Zielsetzung: Im letzten Jahrzehnt wurde vermutet, dass Viren der Familie der Mimiviridae und der Marseilleviridae (sog. Megavirales) Pneumonien verursachen können. Deshalb wurde eine Literaturrecherche zu den möglichen Zusammenhängen von Mimiviridae, Marseilleviridae, Virophagen und Pneumonien mit den Schwerpunkten HA-Infektionen und andere Infektionen der Atemwege durchgeführt.
Ergebnisse und Diskussion: Die Analyse ergab, dass Viren aus der Familie der Mimiviridae potentielle Verursacher sowohl der CA- als auch der HA-Pneumonie sein können. Diese Viren können zu einem verschlechterten Outcome bei Intensivtherapiepatienten führen.
Der genaue Mechanismus ihrer Pathogenität ist jedoch noch immer nicht geklärt. Die unterschiedlichen Ergebnisse zwischen serologischen und Genommethoden sind wahrscheinlich durch den hohen Polymorphismus der Nucleotidsequenzen der Vertreter der Mimiviridae zu erklären. Daher sind weitere Untersuchungen zur Pathogenität der Mimiviridae und ihrer Rolle bei der Pneumonieentstehung in Risikogruppen notwendig.
Im Unterschied dazu ist die Pathogenität der Viren der Familie der Marseilleviridae und der Gruppe der Virophagen noch unklar.
Introduction
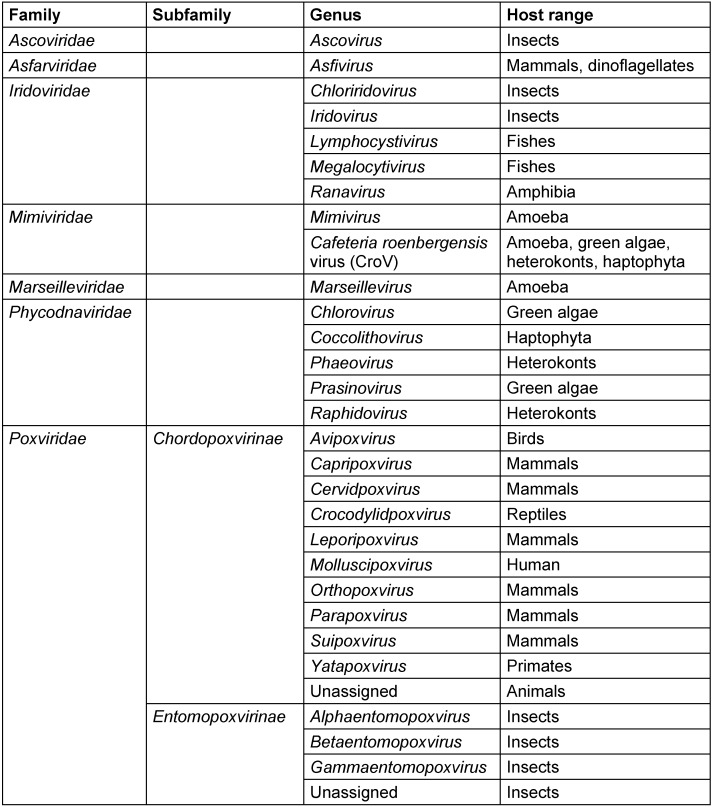
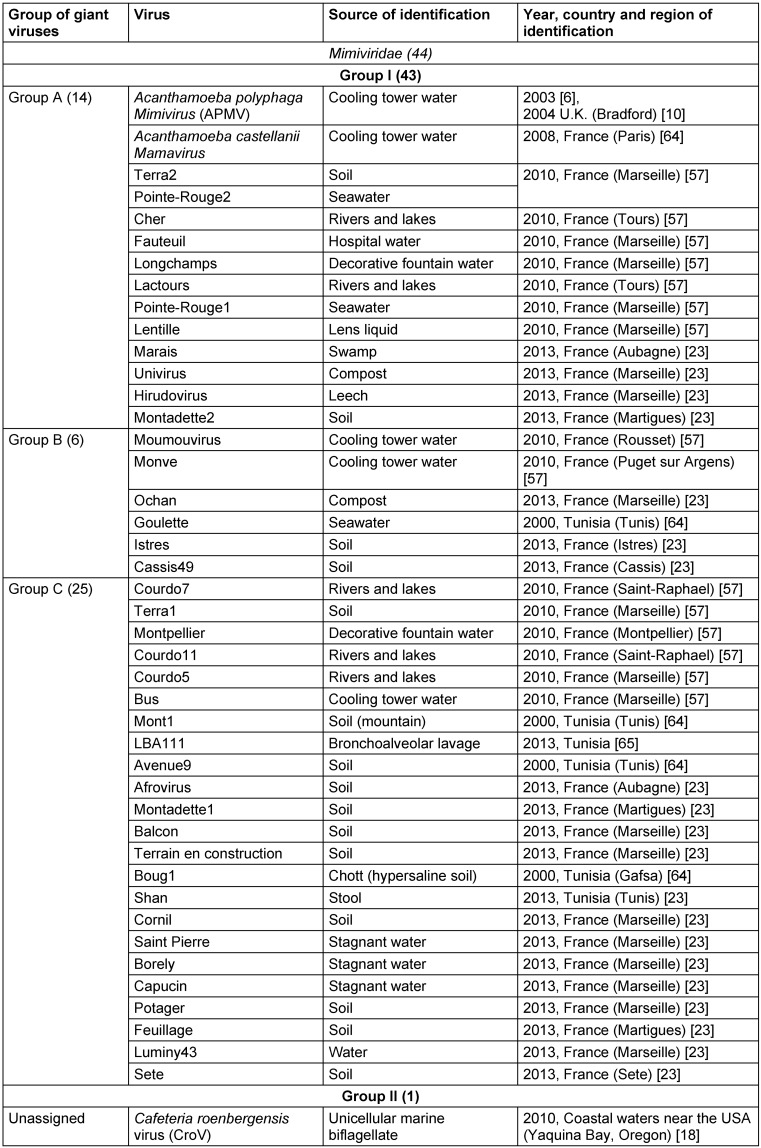
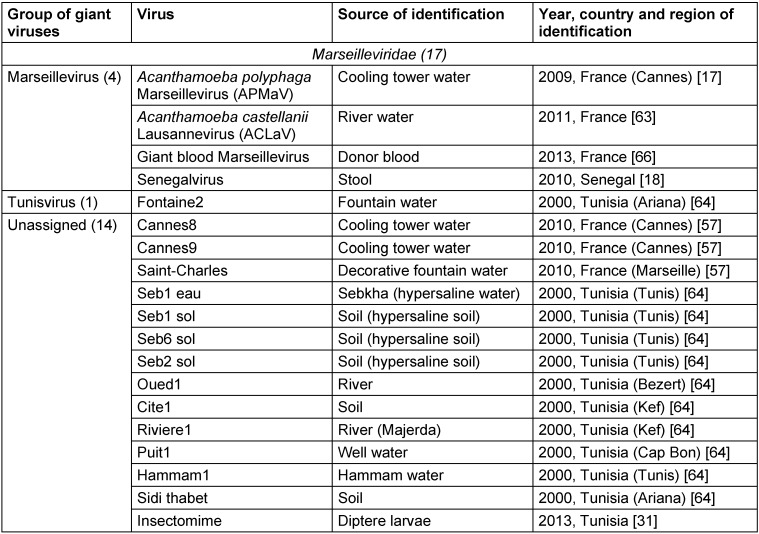
The existence of viruses with extremely large particle and genome sizes has been predicted since the discovery of jumbo bacteriophages in the 1970s and the phycodnaviruses in the early 1980s [1], [2]. An investigation of pneumonia outbreak with no clear causative agent in Bradford, England (1992) by Tim Rowbotham and his team considered water of a cooling tower as a probable origin of the outbreak and amoebae as potential culprits [3] since they were established as Trojan horses due to their ability to harbor multiple agents of human pneumonia that can survive and multiply under the protection from various external physical and chemical agents within the amoebae encyst [4], [5]. This led to an isolation of intra-amoebal pathogenic bacteria, one of which had the appearance of a Gram-positive coccus and was therefore named Bradford coccus [3], [6], [7], [8], [9]. However, it resisted the PCR amplification and 16S ribosomal DNA sequencing. This led to the observation of Bradford coccus using electron microscopy, which revealed that it had a viral icosahedral structure. The viral nature of Bradford coccus was further confirmed by an eclipse phase during its replication cycle [6], and genome sequencing [10]. In 2003, the Bradford coccus was renamed as Acanthamoeba polyphaga Mimivirus (APMV) due to its mimicry of a bacterium by its size and appearance by Gram staining [3]. Raoult et al. [3], [10] reported that APMV genome was the largest among all of viruses (1,181 kb), encoding more than 900 proteins, including those never identified previously in viruses. Overall, the Mimivirus discovery has resulted in a considerable shift in our understanding of the definition, origin, and evolution of viruses [11], [12]. Mimiviruses were classified into a separate group of viruses called nucleocytoplasmic large DNA viruses (NCLDVs) which was first described in 2001 [13] and included the families of Poxviridae, Asfarviridae, Iridoviridae, Ascoviridae, and Phycodnaviridae [14], [15], [16]. In 2007, a first member of the Marseilleviridae family also related to NCLDVs, Acanthamoeba polyphaga marseillevirus (APMaV), was isolated from water collected from a cooling tower in Paris, France, using a method based on Acanthamoeba polyphaga culture [17]. This virus was named in honor of its amoebal host and of the name of the French city, Marseille, where it was discovered [17]. The Marseillevirus was characterized by a 368-kb genome, 457 genes, and a minimum of 49 proteins [17]. Furthermore, the first member of the second branch of Mimiviridae family, Cafeteria roenbergensis virus (CroV), was described in 2010 as an agent infecting marine zooplankton, and its genome consisted of ≈730 kb of double-stranded DNA [18]. Recently, Colson et al. [19], [20] proposed assigning an official taxonomic rank to the NCLDVs as the order Megavirales, due to the large size of the virions and genomes of these viruses, and because of their common ancestral origin [13], [15], [21], [22]. Families and genus belonging to this new tentative order are summarized in Table 1 (Tab. 1), whilst a brief timeline of discoveries in this field is presented in Table 2 (Tab. 2). During the last decade, plenty of other giant virus strains belonging to Mimiviridae and Marseilleviridae families were discovered [23], and their diversity is reflected in Table 3 (Tab. 3) and Table 4 (Tab. 4).
Table 1. Members of the proposed order Megavirales.
Table 2. A brief timeline of discoveries regarding the nucleocytoplasmic large DNA viruses (NCLDVs) superfamily and the Megavirales order.
Table 3. Known members of the Mimiviridae family.
Table 4. Known members of the Marseilleviridae family.
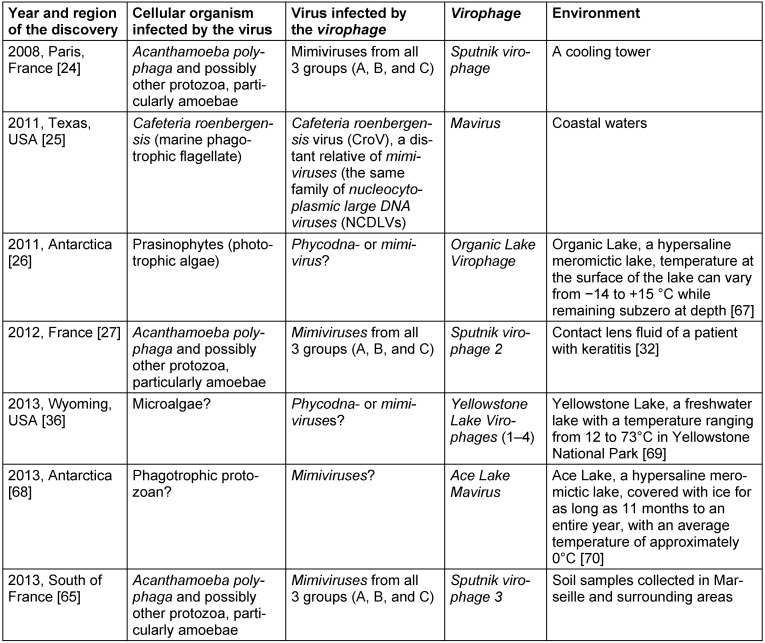
For decades, viruses were considered to be a final level of parasitism in living nature. However, in 2008, La Scola et al. [24] described an icosahedral small virus Sputnik (50 nm in size) which was associated with an APMV. Sputnik did not replicate in Acanthamoeba castellanii but demonstrated a rapid growth in the giant virus factory revealed in amoebae co-infected with APMV. The most incredible fact was that Sputnik life cycle was harmful for APMV and led to the production of abortive forms and abnormal capsid assembly of the host virus. The authors suggested that Sputnik belongs to a new family of viruses and classified it as a virophage [24]. In 2011, Fischer and Suttle [25] described a new virophage called Mavirus, and Yau et al. [26] almost simultaneously reported a discovery of Organic Lake Virophage. Eighteen months later, Desnues et al. [27] revealed a new Sputnik strain, which had 99% identity to the original Sputnik virophage genome sequence. A recent comprehensive investigation carried out by Zhou et al. [6] suggested an existence of five new virophages, namely Yellowstone Lake Virophages 1, 2, 3, 4, and Ace Lake Mavirus. Finally, third Sputnik strain was described in 2013 by Gaia et al. [7]. The discovery of virophages revolutionized our understanding of host-parasite interrelations. Features of known virophages are presented in Table 5 (Tab. 5).
Table 5. Features of known virophages.
There is a significant lack of knowledge regarding the biology of virophages. Obviously, they have greater importance in living nature then we can suggest at the moment. Virophages perform a control of non-viral host-virus dynamics as the essential regulators of ecological interrelations [26], participate in a transfer of genetic information between various organisms as the mobile genetic elements [25], particularly in an interviral gene transfer [27], may be the pathogens of the multicellular organisms [28], and can possibly be used as a new way to fight with the emerging viral infections. Bacteriophages are being successfully used against bacteria in a clinical practice; presumably, a similar pattern does not seem to be impossible in the case with virophages and human pathogenic viruses. Further research on the phenomenon of virophages will definitely shed light on their role in living nature.
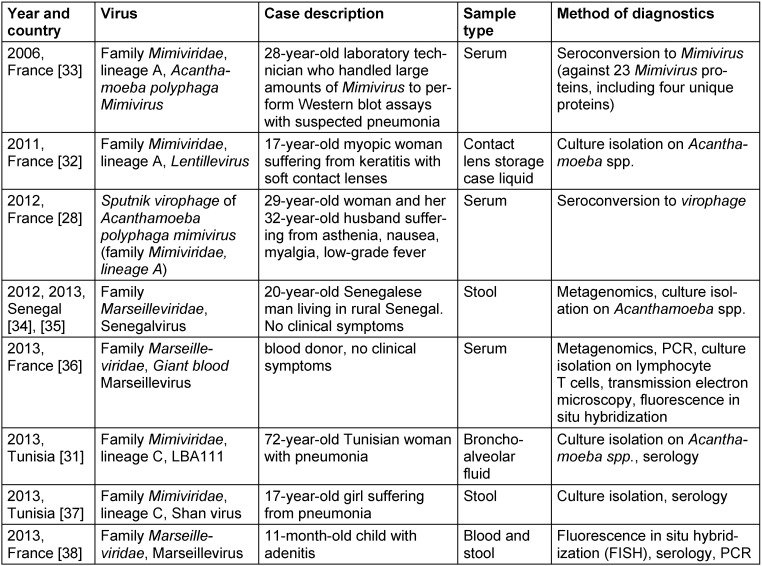
It was suspected that mimiviruses may be potential causative agents of pneumonia due to the setting of its initial discovery and to the involvement of some water-associated amoebae-resistant bacteria, including Legionella pneumophila, in such infections [3], [4]. Experimentally, Mimivirus was found to be capable of inducing pneumonia in mice [29] and infecting macrophages through phagocytosis [30]. A number of case reports showing the pathogenicity of viruses belonging to Mimiviridae and Marseilleviridae families in humans during the last decade (Table 6 (Tab. 6)) [31], [32], [33], [28], [34], [35], [36], [37], [38]). Further, certain reports demonstrated that asymptomatic Marseillevirus infection is not rare in healthy persons and may be transmitted by transfusion [39]. In addition, the results of previous studies identified sequences related to members of families of the order Megavirales in human blood, nasopharyngeal samples, or stools [40], [41], [42], [43], [44], [45], [46].
Table 6. Cases of human infections caused by giant viruses.
Thus, we aimed to perform a review of the association of Mimiviridae, Marseilleviridae, and virophages with pneumonia, particularly healthcare-associated pneumonia (HAP), and other infections of the respiratory tract.
Materials and methods
To the best of our knowledge, all relevant articles published before December of 2013 and available in PubMed database were included in this review. The generation of search queries was performed by combination of words placing at certain positions in the structure of the query, and all feasible variants were browsed:
First position: “mimivirus”, “mimiviruses”, “mimiviridae”, “marseillevirus”, “marseilleviruses”, “marseilleviridae”, “giant virus”, “giant viruses”, “nucleocytoplasmic large DNA virus”, “nucleocytoplasmic large DNA viruses”, or “megavirales”.
Second position: “pneumonia”, “nosocomial”, “community-acquired”, “hospital-acquired”, “ventilator-associated”, “healthcare-associated”, or “respiratory”.
Reference lists of all relevant articles were also screened for the papers which could elude from our search. According to the results of the search, we identified 11 relevant articles in which epidemiological investigations were performed.
Results and discussion
Unfortunately, it was not possible to carry out a meta-analysis since due to significant differences in study design, sample types, and methods of detection used in distinct studies. Thus, we performed only the qualitative comparative analysis.
The results of the first investigation on the association of APMV with pneumonia were published in 2005 by La Scola et al. [47]. The researchers collected serum samples from 376 Canadian patients with community-acquired pneumonia (CAP, 121 ambulatory and 255 hospitalized) and 511 healthy control subjects. Microimmunofluorescence assay revealed that patients with CAP were mimivirus-positive significantly more frequently compared to controls (9.66% vs. 2.30%, P<0.01) (Table 7 (Tab. 7)). Furthermore, the authors identified hospitalization from a nursing home and rehospitalization after discharge as independent risk factors of mimivirus-associated CAP (P<0.05), possibly due to poor efficacy of standard antimicrobial agents against viruses. In addition, immunoelectron microscopy revealed that antibodies of APMV-positive patients specifically recognized mature APMV particles whilst antibody fixation was not found in serum samples from APMV-negative patients. The authors suggested APMV as a particularly hazardous etiologic agent of pneumonia acquired in institutions [47], [48]. In addition, the investigators recruited a second study sample consisted of 26 French patients with intensive care unit (ICU)-acquired pneumonia and 50 healthy controls, showing that APMV can be found in patients with ICU-acquired pneumonia and multiple populations [47]. Moreover, serological positivity to APMV was associated with a higher risk of ICU-acquired pneumonia (19.2% in cases vs. 0.0% in controls, P<0.01) [47] (Table 7 (Tab. 7)). In the third sample (32 French patients with ICU-acquired pneumonia, 21 intubated controls in ICU without ICU-acquired pneumonia), APMV DNA was detected in bronchoalveolar lavage specimen from one of the patients with ICU-acquired pneumonia but in none of the controls, confirming that APMV may reach the respiratory tract of these patients [47] (Table 7 (Tab. 7)). So, La Scola et al. [47] were very first who proposed that APMV should be tested as a possible novel human pathogen, particularly in pneumonia patients.
Table 7. Studies on the association of the giant viruses with infections of the respiratory tract.
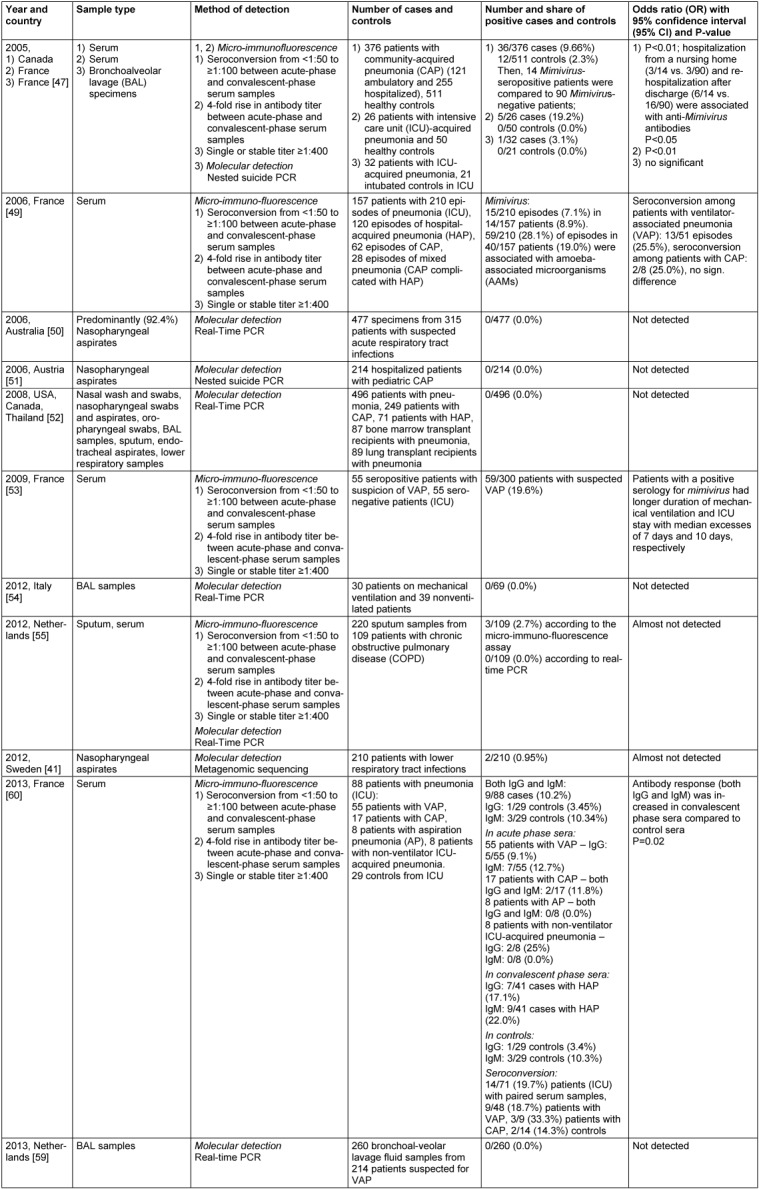
The second study devoted to this issue was carried out by Berger et al. [49]. This investigation included 157 French ICU patients with 210 episodes of pneumonia (120 episodes of healthcare-associated pneumonia (HAP), 62 episodes of CAP, and 28 episodes of mixed pneumonia (CAP complicated with HAP) [49]. Using the similar microimmunofluorescence approach, the authors showed that 15/210 episodes (7.1%) in 14/157 patients (8.9%) were associated with APMV [49]. In addition, laboratory investigations for amoeba-associated microorganisms (AAMs) revealed 59/210 (28.1%) diagnoses in 40/157 (19.0%) patients [49]. Seroconversion among patients with ventilator-associated pneumonia (VAP) was observed in 13/51 episodes (25.5%), whereas seroconversion among patients with CAP was detected in 2/8 episodes (25.0%) that may demonstrate the possible pathogenic role of APMV in both VAP and CAP [49] (Table 7 (Tab. 7)). However, there was no control group in this study; therefore the prevalence of APMV in a general population remains unknown [49]. In the same year, Arden et al. [50] and Larcher et al. [51] failed to find any APMV-positive individuals among 315 Australian patients with suspected acute respiratory tract infections and 214 Austrian patients with pediatric CAP, respectively; nonetheless, the authors used predominantly nasopharyngeal aspirates as a study material and real-time [50] or nested suicide [51] PCR instead of microimmunofluorescence as a method of APMV detection (Table 7 (Tab. 7)). Similar results were further obtained by Dare et al. [52] who were unable to detect APMV using real-time PCR in 496 patients with pneumonia (249 patients with CAP, 71 patient with HAP, 87 bone marrow transplant recipients with pneumonia, and 89 lung transplant recipients with pneumonia) from 9 epidemiologically varied pneumonia patient populations (Thailand, Canada, USA) (Table 7 (Tab. 7)). The authors suggested that seropositivity may reflect chronic exposure to APMV antigen rather than active infection, and the potential for nonspecific cross-reactions with the serologic assays used may have inflated the true prevalence of APMV colonization/infection [52]. Nevertheless, most of the specimens examined in this study were obtained from the upper respiratory tract but not from the lower respiratory tract where the only reported APMV PCR-positive sample was identified [47]. Distinct composition of the studies' populations could also play a role in the differences between the studies [52]. However, in 2009 Vincent et al. [53] found 59/300 (19.6%) French patients with suspected VAP to be APMV-positive, detected by microimmunofluorescence (Table 7 (Tab. 7)). In addition, APMV-positive patients had longer duration of mechanical ventilation and ICU stay with median excesses of 7 days and 10 days, respectively, so a positive serology for mimivirus was associated with a poorer outcome in mechanically ventilated ICU patients [53].
Three years later, Costa et al. [54] investigated the prevalence of APMV in bronchoalveolar lavage (BAL) specimens from 30 ventilated compared to 39 nonventilated Italian patients using real-time PCR but could not detect any signs of APMV colonization/infection among this population (Table 7 (Tab. 7)). Then, Lysholm et al. [41] investigated nasopharyngeal aspirates from 210 Sweden patients with lower respiratory tract infections through metagenomic sequencing approach but detected APMV only in 2/210 (0.95%) cases. Furthermore, Vanspauwen et al. [55] collected 220 sputum and serum samples from 109 Dutch patients with chronic obstructive pulmonary disease (COPD) and analyzed them using the microimmunofluorescence assay and real-time PCR; however, only 3/210 (2.7%) patients were APMV-seropositive and none of the patients were positive for APMV DNA (Table 7 (Tab. 7)). The authors suggested that the low seropositivity might be explained by either a low abundance of the virus or the presence of the virus may depend on the spatiotemporal regional variation as in the case with Legionella-caused infections [55], [56]. Negative PCR results could be explained by a number of reasons [55]. The viral load of the samples may have been below the detection limit of the PCR (12 copies/reaction), or a polymorphism in the area of amplification could occur [55]. A wide genomic diversity of Mimiviridae family may support the latter suggestion [19], [57], [55], [58], so patients could be positive for other viruses of the Mimiviridae family [55]. In addition, sputum is possibly not an appropriate study material compared to BAL sample [55]. In the second study performed by this research group with 260 bronchoalveolar lavage fluid samples from 214 Dutch patients suspected for VAP, no APMV DNA was detected in all of the samples using real-time PCR [59] (Table 7 (Tab. 7)). Finally, in the recent study of Bousbia et al. [60], seroconversion to APMV was observed in 14/71 (19.7%) French pneumonia patients (ICU) with paired serum samples (Table 7 (Tab. 7)). The authors also observed an elevation of the antibody response (both IgG and IgM) to APMV antigens in the convalescent-phase sera in comparison with the admission sera (16/41 (39.0%) patients with HAP and 4/29 (13.8%) controls (ICU patients without pneumonia), respectively, P=0.02) [60].
There are several explanations for the inconsistency observed between the results of the above-mentioned studies. The most evident explanation is that Mimiviridae family DNA detection could possibly have been hampered in studies that used genomic methods of detection. PCR primers used in these studies targeted only APMV genome, whilst a number of APMV relatives exhibiting considerable genetic diversity have been described (Table 3 (Tab. 3)). Recently developed modern real-time PCR systems targeting giant viruses and their virophages are able to accurately detect all or most of the members of the currently delineated lineages of giant viruses infecting Acanthamoebae as well as the virophages [61]; hence, this obstacle may be overcome in the near future. Other reasons for the disparities may include differences in prevalence of giant viruses in distinct populations along with spatiotemporal regional variation as well as the use of inappropriate study material (for instance, samples from upper respiratory tract instead of bronchoalveolar lavage (BAL) specimens). In some cases, giant viruses possibly could not be identified due to the too small viral load for the PCR detection in some cases, insufficient amount of specimens. In addition, APMV was shown to contain up to 23 proteins that may cause immune response resulting in a production of antibodies [33], so proteins of other Mimiviridae family members may cross-react with those of APMV [71]. In this case, molecular detection of APMV could be negative [62]. Exposure to APMV, since it is an AAM, most likely occurs from environmental sources such as contaminated hospital water supplies [52], which is the case in ICU patients. The high rate of seroconversion in pneumonia patients in certain studies [47], [49], [53] suggests that they may have had a contact with APMV or a cross-reacting agent which could possibly be other member of Mimiviridae family.
Conclusion
Viruses belonging to Mimiviridae family can be potential agents of both CAP and HAP. In particular, these viruses may be associated with poorer outcome in ICU patients. The exact mechanism of their pathogenicity, however, still remains unclear. The discrepancies between the results obtained by serological and genomic methods could be explained by the high polymorphism of nucleotide sequences of Mimiviridae family representatives. Further investigations on the Mimiviridae pathogenicity and on the determination of Mimiviridae-caused pneumonia risk groups are required. However, pathogenicity of the viruses belonging to Marseilleviridae family is unclear.
Notes
Competing interests
The authors declare that they have no competing interests.
Funding
There was no funding for this paper.
References
- 1.Donelli G, Dore E, Frontali C, Grandolfo ME. Structure and physico-chemical properties of bacteriophage G. III. A homogeneous DNA of molecular weight 5 times 10(8) J Mol Biol. 1975 Jun 5;94(4):555–565. doi: 10.1016/0022-2836(75)90321-6. Available from: http://dx.doi.org/10.1016/0022-2836(75)90321-6. [DOI] [PubMed] [Google Scholar]
- 2.Van Etten JL, Meints RH, Kuczmarski D, Burbank DE, Lee K. Viruses of symbiotic Chlorella-like algae isolated from Paramecium bursaria and Hydra viridis. Proc Natl Acad Sci USA. 1982 Jun;79(12):3867–3871. doi: 10.1073/pnas.79.12.3867. Available from: http://dx.doi.org/10.1073/pnas.79.12.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raoult D, La Scola B, Birtles R. The discovery and characterization of Mimivirus, the largest known virus and putative pneumonia agent. Clin Infect Dis. 2007 Jul;45(1):95–102. doi: 10.1086/518608. Available from: http://dx.doi.org/10.1086/518608. [DOI] [PubMed] [Google Scholar]
- 4.Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004 Apr;17(2):413–433. doi: 10.1128/CMR.17.2.413-433.2004. Available from: http://dx.doi.org/10.1128/CMR.17.2.413-433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker J, Brown MR. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology (Reading, Engl) 1994 Jun;140(Pt 6):1253–1259. doi: 10.1099/00221287-140-6-1253. Available from: http://dx.doi.org/10.1099/00221287-140-6-1253. [DOI] [PubMed] [Google Scholar]
- 6.La Scola B, Audic S, Robert C, Jungang L, de Lamballerie X, Drancourt M, Birtles R, Claverie JM, Raoult D. A giant virus in amoebae. Science. 2003 Mar;299(5615):2033. doi: 10.1126/science.1081867. Available from: http://dx.doi.org/10.1126/science.1081867. [DOI] [PubMed] [Google Scholar]
- 7.Raoult D. Giant viruses from amoeba in a post-Darwinist viral world. Intervirology. 2010;53(5):251–253. doi: 10.1159/000312909. Available from: http://dx.doi.org/10.1159/000312909. [DOI] [PubMed] [Google Scholar]
- 8.La Scola B, Birtles RJ, Greub G, Harrison TJ, Ratcliff RM, Raoult D. Legionella drancourtii sp. nov., a strictly intracellular amoebal pathogen. Int J Syst Evol Microbiol. 2004 May;54(Pt 3):699–703. doi: 10.1099/ijs.0.02455-0. Available from: http://dx.doi.org/10.1099/ijs.0.02455-0. [DOI] [PubMed] [Google Scholar]
- 9.Adeleke AA, Fields BS, Benson RF, Daneshvar MI, Pruckler JM, Ratcliff RM, Harrison TG, Weyant RS, Birtles RJ, Raoult D, Halablab MA. Legionella drozanskii sp. nov., Legionella rowbothamii sp. nov. and Legionella fallonii sp. nov.: three unusual new Legionella species. Int J Syst Evol Microbiol. 2001 May;51(Pt 3):1151–1160. doi: 10.1099/00207713-51-3-1151. Available from: http://dx.doi.org/10.1099/00207713-51-3-1151. [DOI] [PubMed] [Google Scholar]
- 10.Raoult D, Audic S, Robert C, Abergel C, Renesto P, Ogata H, La Scola B, Suzan M, Claverie JM. The 1.2-megabase genome sequence of Mimivirus. Science. 2004 Nov;306(5700):1344–1350. doi: 10.1126/science.1101485. Available from: http://dx.doi.org/10.1126/science.1101485. [DOI] [PubMed] [Google Scholar]
- 11.Raoult D, Forterre P. Redefining viruses: lessons from Mimivirus. Nat Rev Microbiol. 2008 Apr;6(4):315–319. doi: 10.1038/nrmicro1858. Available from: http://dx.doi.org/10.1038/nrmicro1858. [DOI] [PubMed] [Google Scholar]
- 12.Forterre P. Giant viruses: conflicts in revisiting the virus concept. Intervirology. 2010;53(5):362–378. doi: 10.1159/000312921. Available from: http://dx.doi.org/10.1159/000312921. [DOI] [PubMed] [Google Scholar]
- 13.Iyer LM, Aravind L, Koonin EV. Common origin of four diverse families of large eukaryotic DNA viruses. J Virol. 2001 Dec;75(23):11720–11734. doi: 10.1128/JVI.75.23.11720-11734.2001. Available from: http://dx.doi.org/10.1128/JVI.75.23.11720-11734.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyer LM, Balaji S, Koonin EV, Aravind L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res. 2006 Apr;117(1):156–184. doi: 10.1016/j.virusres.2006.01.009. Available from: http://dx.doi.org/10.1016/j.virusres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Yutin N, Wolf YI, Raoult D, Koonin EV. Eukaryotic large nucleo-cytoplasmic DNA viruses: clusters of orthologous genes and reconstruction of viral genome evolution. Virol J. 2009;6:223. doi: 10.1186/1743-422X-6-223. Available from: http://dx.doi.org/10.1186/1743-422X-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koonin EV, Yutin N. Origin and evolution of eukaryotic large nucleo-cytoplasmic DNA viruses. Intervirology. 2010;53(5):284–292. doi: 10.1159/000312913. Available from: http://dx.doi.org/10.1159/000312913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyer M, Yutin N, Pagnier I, Barrassi L, Fournous G, Espinosa L, Robert C, Azza S, Sun S, Rossmann MG, Suzan-Monti M, La Scola B, Koonin EV, Raoult D. Giant Marseillevirus highlights the role of amoebae as a melting pot in emergence of chimeric microorganisms. Proc Natl Acad Sci USA. 2009 Dec;106(51):21848–21853. doi: 10.1073/pnas.0911354106. Available from: http://dx.doi.org/10.1073/pnas.0911354106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer MG, Allen MJ, Wilson WH, Suttle CA. Giant virus with a remarkable complement of genes infects marine zooplankton. Proc Natl Acad Sci USA. 2010 Nov;107(45):19508–19513. doi: 10.1073/pnas.1007615107. Available from: http://dx.doi.org/10.1073/pnas.1007615107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colson P, de Lamballerie X, Fournous G, Raoult D. Reclassification of giant viruses composing a fourth domain of life in the new order Megavirales. Intervirology. 2012;55(5):321–332. doi: 10.1159/000336562. Available from: http://dx.doi.org/10.1159/000336562. [DOI] [PubMed] [Google Scholar]
- 20.Colson P, De Lamballerie X, Yutin N, Asgari S, Bigot Y, Bideshi DK, Cheng XW, Federici BA, Van Etten JL, Koonin EV, La Scola B, Raoult D. “Megavirales”, a proposed new order for eukaryotic nucleocytoplasmic large DNA viruses. Arch Virol. 2013 Dec;158(12):2517–2521. doi: 10.1007/s00705-013-1768-6. Available from: http://dx.doi.org/10.1007/s00705-013-1768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyer M, Madoui MA, Gimenez G, La Scola B, Raoult D. Phylogenetic and phyletic studies of informational genes in genomes highlight existence of a 4 domain of life including giant viruses. PLoS ONE. 2010;5(12):e15530. doi: 10.1371/journal.pone.0015530. Available from: http://dx.doi.org/10.1371/journal.pone.0015530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koonin EV, Senkevich TG, Dolja VV. The ancient Virus World and evolution of cells. Biol Direct. 2006;1:29. doi: 10.1186/1745-6150-1-29. Available from: http://dx.doi.org/10.1186/1745-6150-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagnier I, Reteno DG, Saadi H, Boughalmi M, Gaia M, Slimani M, Ngounga T, Bekliz M, Colson P, Raoult D, La Scola B. A decade of improvements in Mimiviridae and Marseilleviridae isolation from amoeba. Intervirology. 2013;56(6):354–363. doi: 10.1159/000354556. Available from: http://dx.doi.org/10.1159/000354556. [DOI] [PubMed] [Google Scholar]
- 24.La Scola B, Desnues C, Pagnier I, Robert C, Barrassi L, Fournous G, Merchat M, Suzan-Monti M, Forterre P, Koonin E, Raoult D. The virophage as a unique parasite of the giant mimivirus. Nature. 2008 Sep;455(7209):100–104. doi: 10.1038/nature07218. Available from: http://dx.doi.org/10.1038/nature07218. [DOI] [PubMed] [Google Scholar]
- 25.Fischer MG, Suttle CA. A virophage at the origin of large DNA transposons. Science. 2011 Apr;332(6026):231–234. doi: 10.1126/science.1199412. Available from: http://dx.doi.org/10.1126/science.1199412. [DOI] [PubMed] [Google Scholar]
- 26.Yau S, Lauro FM, DeMaere MZ, Brown MV, Thomas T, Raftery MJ, Andrews-Pfannkoch C, Lewis M, Hoffman JM, Gibson JA, Cavicchioli R. Virophage control of antarctic algal host-virus dynamics. Proc Natl Acad Sci USA. 2011 Apr;108(15):6163–6168. doi: 10.1073/pnas.1018221108. Available from: http://dx.doi.org/10.1073/pnas.1018221108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desnues C, La Scola B, Yutin N, Fournous G, Robert C, Azza S, Jardot P, Monteil S, Campocasso A, Koonin EV, Raoult D. Provirophages and transpovirons as the diverse mobilome of giant viruses. Proc Natl Acad Sci USA. 2012 Oct;109(44):18078–18083. doi: 10.1073/pnas.1208835109. Available from: http://dx.doi.org/10.1073/pnas.1208835109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parola P, Renvoisé A, Botelho-Nevers E, La Scola B, Desnues C, Raoult D. Acanthamoeba polyphaga mimivirus virophage seroconversion in travelers returning from Laos. Emerging Infect Dis. 2012 Sep;18(9):1500–1502. doi: 10.3201/eid1809.120099. Available from: http://dx.doi.org/10.3201/eid1809.120099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan M, La Scola B, Lepidi H, Raoult D. Pneumonia in mice inoculated experimentally with Acanthamoeba polyphaga mimivirus. Microb Pathog. 2007 Feb-Mar;42(2-3):56–61. doi: 10.1016/j.micpath.2006.08.004. Available from: http://dx.doi.org/10.1016/j.micpath.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Ghigo E, Kartenbeck J, Lien P, Pelkmans L, Capo C, Mege JL, Raoult D. Ameobal pathogen mimivirus infects macrophages through phagocytosis. PLoS Pathog. 2008 Jun;4(6):e1000087. doi: 10.1371/journal.ppat.1000087. Available from: http://dx.doi.org/10.1371/journal.ppat.1000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saadi H, Pagnier I, Colson P, Cherif JK, Beji M, Boughalmi M, Azza S, Armstrong N, Robert C, Fournous G, La Scola B, Raoult D. First isolation of Mimivirus in a patient with pneumonia. Clin Infect Dis. 2013 Aug;57(4):e127–e134. doi: 10.1093/cid/cit354. Available from: http://dx.doi.org/10.1093/cid/cit354. [DOI] [PubMed] [Google Scholar]
- 32.Cohen G, Hoffart L, La Scola B, Raoult D, Drancourt M. Ameba-associated Keratitis, France. Emerging Infect Dis. 2011 Jul;17(7):1306–1308. doi: 10.3201/eid1707.100826. Available from: http://dx.doi.org/10.3201/eid1707.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raoult D, Renesto P, Brouqui P. Laboratory infection of a technician by mimivirus. Ann Intern Med. 2006 May;144(9):702–703. doi: 10.7326/0003-4819-144-9-200605020-00025. [DOI] [PubMed] [Google Scholar]
- 34.Lagier JC, Armougom F, Million M, Hugon P, Pagnier I, Robert C, Bittar F, Fournous G, Gimenez G, Maraninchi M, Trape JF, Koonin EV, La Scola B, Raoult D. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect. 2012 Dec;18(12):1185–1193. doi: 10.1111/1469-0691.12023. Available from: http://dx.doi.org/10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 35.Colson P, Fancello L, Gimenez G, Armougom F, Desnues C, Fournous G, Yoosuf N, Million M, La Scola B, Raoult D. Evidence of the megavirome in humans. J Clin Virol. 2013 Jul;57(3):191–200. doi: 10.1016/j.jcv.2013.03.018. Available from: http://dx.doi.org/10.1016/j.jcv.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Popgeorgiev N, Boyer M, Fancello L, Monteil S, Robert C, Rivet R, Nappez C, Azza S, Chiaroni J, Raoult D, Desnues C. Marseillevirus-like virus recovered from blood donated by asymptomatic humans. J Infect Dis. 2013 Oct;208(7):1042–1050. doi: 10.1093/infdis/jit292. Available from: http://dx.doi.org/10.1093/infdis/jit292. [DOI] [PubMed] [Google Scholar]
- 37.Saadi H, Reteno DG, Colson P, Aherfi S, Minodier P, Pagnier I, Raoult D, La Scola B. Shan virus: a new mimivirus isolated from the stool of a Tunisian patient with pneumonia. Intervirology. 2013;56(6):424–429. doi: 10.1159/000354564. Available from: http://dx.doi.org/10.1159/000354564. [DOI] [PubMed] [Google Scholar]
- 38.Popgeorgiev N, Michel G, Lepidi H, Raoult D, Desnues C. Marseillevirus adenitis in an 11-month-old child. J Clin Microbiol. 2013 Dec;51(12):4102–4105. doi: 10.1128/JCM.01918-13. Available from: http://dx.doi.org/10.1128/JCM.01918-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popgeorgiev N, Colson P, Thuret I, Chiarioni J, Gallian P, Raoult D, Desnues C. Marseillevirus prevalence in multitransfused patients suggests blood transmission. J Clin Virol. 2013 Dec;58(4):722–725. doi: 10.1016/j.jcv.2013.10.001. Available from: http://dx.doi.org/10.1016/j.jcv.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Finkbeiner SR, Allred AF, Tarr PI, Klein EJ, Kirkwood CD, Wang D. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog. 2008 Feb;4(2):e1000011. doi: 10.1371/journal.ppat.1000011. Available from: http://dx.doi.org/10.1371/journal.ppat.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lysholm F, Wetterbom A, Lindau C, Darban H, Bjerkner A, Fahlander K, Lindberg AM, Persson B, Allander T, Andersson B. Characterization of the viral microbiome in patients with severe lower respiratory tract infections, using metagenomic sequencing. PLoS ONE. 2012;7(2):e30875. doi: 10.1371/journal.pone.0030875. Available from: http://dx.doi.org/10.1371/journal.pone.0030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loh J, Zhao G, Presti RM, Holtz LR, Finkbeiner SR, Droit L, Villasana Z, Todd C, Pipas JM, Calgua B, Girones R, Wang D, Virgin HW. Detection of novel sequences related to african Swine Fever virus in human serum and sewage. J Virol. 2009 Dec;83(24):13019–13025. doi: 10.1128/JVI.00638-09. Available from: http://dx.doi.org/10.1128/JVI.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yozwiak NL, Skewes-Cox P, Stenglein MD, Balmaseda A, Harris E, DeRisi JL. Virus identification in unknown tropical febrile illness cases using deep sequencing. PLoS Negl Trop Dis. 2012;6(2):e1485. doi: 10.1371/journal.pntd.0001485. Available from: http://dx.doi.org/10.1371/journal.pntd.0001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wylie KM, Mihindukulasuriya KA, Sodergren E, Weinstock GM, Storch GA. Sequence analysis of the human virome in febrile and afebrile children. PLoS ONE. 2012;7(6):e27735. doi: 10.1371/journal.pone.0027735. Available from: http://dx.doi.org/10.1371/journal.pone.0027735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan PF, Allander T, Lysholm F, Goh S, Persson B, Jacks A, Evengård B, Pedersen NL, Andersson B. An unbiased metagenomic search for infectious agents using monozygotic twins discordant for chronic fatigue. BMC Microbiol. 2011;11:2. doi: 10.1186/1471-2180-11-2. Available from: http://dx.doi.org/10.1186/1471-2180-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breitbart M, Rohwer F. Method for discovering novel DNA viruses in blood using viral particle selection and shotgun sequencing. Biotechniques. 2005 Nov;39(5):729–736. doi: 10.2144/000112019. [DOI] [PubMed] [Google Scholar]
- 47.La Scola B, Marrie TJ, Auffray JP, Raoult D. Mimivirus in pneumonia patients. Emerging Infect Dis. 2005 Mar;11(3):449–452. doi: 10.3201/eid1103.040538. Available from: http://dx.doi.org/10.3201/eid1103.040538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001 Mar;163(3 Pt 1):645–651. doi: 10.1164/ajrccm.163.3.2005075. Available from: http://dx.doi.org/10.1164/ajrccm.163.3.2005075. [DOI] [PubMed] [Google Scholar]
- 49.Berger P, Papazian L, Drancourt M, La Scola B, Auffray JP, Raoult D. Ameba-associated microorganisms and diagnosis of nosocomial pneumonia. Emerging Infect Dis. 2006 Feb;12(2):248–255. doi: 10.3201/eid1202.050434. Available from: http://dx.doi.org/10.3201/eid1202.050434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006 Sep;78(9):1232–1240. doi: 10.1002/jmv.20689. Available from: http://dx.doi.org/10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larcher C, Jeller V, Fischer H, Huemer HP. Prevalence of respiratory viruses, including newly identified viruses, in hospitalised children in Austria. Eur J Clin Microbiol Infect Dis. 2006 Nov;25(11):681–686. doi: 10.1007/s10096-006-0214-z. Available from: http://dx.doi.org/10.1007/s10096-006-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dare RK, Chittaganpitch M, Erdman DD. Screening pneumonia patients for mimivirus. Emerging Infect Dis. 2008 Mar;14(3):465–467. doi: 10.3201/eid1403.071027. Available from: http://dx.doi.org/10.3201/eid1403.071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vincent A, La Scola B, Forel JM, Pauly V, Raoult D, Papazian L. Clinical significance of a positive serology for mimivirus in patients presenting a suspicion of ventilator-associated pneumonia. Crit Care Med. 2009 Jan;37(1):111–118. doi: 10.1097/CCM.0b013e318192fa8b. Available from: http://dx.doi.org/10.1097/CCM.0b013e318192fa8b. [DOI] [PubMed] [Google Scholar]
- 54.Costa C, Bergallo M, Astegiano S, Terlizzi ME, Sidoti F, Solidoro P, Cavallo R. Detection of Mimivirus in bronchoalveolar lavage of ventilated and nonventilated patients. Intervirology. 2012;55(4):303–305. doi: 10.1159/000329088. Available from: http://dx.doi.org/10.1159/000329088. [DOI] [PubMed] [Google Scholar]
- 55.Vanspauwen MJ, Franssen FM, Raoult D, Wouters EF, Bruggeman CA, Linssen CF. Infections with mimivirus in patients with chronic obstructive pulmonary disease. Respir Med. 2012 Dec;106(12):1690–1694. doi: 10.1016/j.rmed.2012.08.019. Available from: http://dx.doi.org/10.1016/j.rmed.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 56.Ricketts KD, Joseph CA European Working Group for Legionella Infections. Legionnaires’ disease in Europe 2003–2004. Euro Surveill. 2005 Dec;10(12):256–259. [Google Scholar]
- 57.La Scola B, Campocasso A, N’Dong R, Fournous G, Barrassi L, Flaudrops C, Raoult D. Tentative characterization of new environmental giant viruses by MALDI-TOF mass spectrometry. Intervirology. 2010;53(5):344–353. doi: 10.1159/000312919. Available from: http://dx.doi.org/10.1159/000312919. [DOI] [PubMed] [Google Scholar]
- 58.Colson P, Yutin N, Shabalina SA, Robert C, Fournous G, La Scola B, Raoult D, Koonin EV. Viruses with more than 1,000 genes: Mamavirus, a new Acanthamoeba polyphaga mimivirus strain, and reannotation of Mimivirus genes. Genome Biol Evol. 2011;3:737–742. doi: 10.1093/gbe/evr048. Available from: http://dx.doi.org/10.1093/gbe/evr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanspauwen MJ, Schnabel RM, Bruggeman CA, Drent M, van Mook WN, Bergmans DC, Linssen CF. Mimivirus is not a frequent cause of ventilator-associated pneumonia in critically ill patients. J Med Virol. 2013 Oct;85(10):1836–1841. doi: 10.1002/jmv.23655. Available from: http://dx.doi.org/10.1002/jmv.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bousbia S, Papazian L, Saux P, Forel JM, Auffray JP, Martin C, Raoult D, La Scola B. Serologic prevalence of amoeba-associated microorganisms in intensive care unit pneumonia patients. PLoS ONE. 2013;8(3):e58111. doi: 10.1371/journal.pone.0058111. Available from: http://dx.doi.org/10.1371/journal.pone.0058111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ngounga T, Pagnier I, Reteno DG, Raoult D, La Scola B, Colson P. Real-time PCR systems targeting giant viruses of amoebae and their virophages. Intervirology. 2013;56(6):413–423. doi: 10.1159/000354563. Available from: http://dx.doi.org/10.1159/000354563. [DOI] [PubMed] [Google Scholar]
- 62.Vincent A, La Scola B, Papazian L. Advances in Mimivirus pathogenicity. Intervirology. 2010;53(5):304–309. doi: 10.1159/000312915. Available from: http://dx.doi.org/10.1159/000312915. [DOI] [PubMed] [Google Scholar]
- 63.Thomas V, Bertelli C, Collyn F, Casson N, Telenti A, Goesmann A, Croxatto A, Greub G. Lausannevirus, a giant amoebal virus encoding histone doublets. Environ Microbiol. 2011 Jun;13(6):1454–1466. doi: 10.1111/j.1462-2920.2011.02446.x. Available from: http://dx.doi.org/10.1111/j.1462-2920.2011.02446.x. [DOI] [PubMed] [Google Scholar]
- 64.La Scola B, Barrassi L, Raoult D. Isolation of new fastidious alpha Proteobacteria and Afipia felis from hospital water supplies by direct plating and amoebal co-culture procedures. FEMS Microbiol Ecol. 2000 Dec;34(2):129–137. doi: 10.1016/s0168-6496(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 65.Gaia M, Pagnier I, Campocasso A, Fournous G, Raoult D, La Scola B. Broad spectrum of mimiviridae virophage allows its isolation using a mimivirus reporter. PLoS ONE. 2013;8(4):e61912. doi: 10.1371/journal.pone.0061912. Available from: http://dx.doi.org/10.1371/journal.pone.0061912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Popgeorgiev N, Boyer M, Fancello L, Monteil S, Robert C, Rivet R, Nappez C, Azza S, Chiaroni J, Raoult D, Desnues C. Marseillevirus-like virus recovered from blood donated by asymptomatic humans. J Infect Dis. 2013 Oct;208(7):1042–1050. doi: 10.1093/infdis/jit292. Available from: http://dx.doi.org/10.1093/infdis/jit292. [DOI] [PubMed] [Google Scholar]
- 67.Franzman PD, Deprez PP, Burton HR, van den Hoff J. Limnology of Organic Lake, Antarctica, a meromictic lake that contains high concentrations of dimethyl sulfide. Aust J Mar Freshw Res. 1987;38(3):409–17. doi: 10.1071/MF9870409. Available from: http://dx.doi.org/10.1071/MF9870409. [DOI] [Google Scholar]
- 68.Zhou J, Zhang W, Yan S, Xiao J, Zhang Y, Li B, Pan Y, Wang Y. Diversity of virophages in metagenomic data sets. J Virol. 2013 Apr;87(8):4225–4236. doi: 10.1128/JVI.03398-12. Available from: http://dx.doi.org/10.1128/JVI.03398-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clingenpeel S, Macur RE, Kan J, Inskeep WP, Lovalvo D, Varley J, Mathur E, Nealson K, Gorby Y, Jiang H, LaFracois T, McDermott TR. Yellowstone Lake: high-energy geochemistry and rich bacterial diversity. Environ Microbiol. 2011 Aug;13(8):2172–2185. doi: 10.1111/j.1462-2920.2011.02466.x. Available from: http://dx.doi.org/10.1111/j.1462-2920.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- 70.Coolen MJL, Hopmans EC, Rijpstra WIC, Muyzer G, Schouten S, Volkman JK, Sinninghe Damsté JS. Evolution of the methane cycle in Ace Lake (Antarctica) during the Holocene: response of methanogens and methanotrophs to environmental change. Org Geochem. 2004 Oct;35(10):1151–67. doi: 10.1016/j.orggeochem.2004.06.009. Available from: http://dx.doi.org/10.1016/j.orggeochem.2004.06.009. [DOI] [Google Scholar]
- 71.Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000 Aug;31(2):347–382. doi: 10.1086/313954. Available from: http://dx.doi.org/10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]