Abstract
Introduction: Work in hospitals is supported by contributions of life sciences industry representatives (IR) in various ways of fields. Close contact between them, caretakers and patients is unavoidable, even in situations where hygiene is critical.
The present study investigates whether IR display comparable levels of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) contamination after being exposed to a shared environment for a minimum of 4 hours.
Material and methods: An anonymous survey to sample a group of healthcare professionals for traces of fingertip contamination was performed. We used dip slides (S. aureus and MRSA) to evaluate 311 healthcare professionals at the medical exhibition MEDICA. After applying exclusion criteria 298 participants remained valid, they consisted of 208 industry representatives, 49 nurses and 41 physicians.
Results: IR where engaged in hospitals, operating rooms and outpatient clinics (82%, 41.8%, 51.9% respectively). 65.9% of IR (vs. 48.8% physicians and 40.8% nurses) carried a microbiological burden ≥104 CFU (colony forming units). Neither S. aureus (≥104 CFU) in IR (40.9%) did show statistical differences in contamination patterns in comparison to physicians (43.9%, p=0.346) and nurses (36.7%, p=0.878) nor did MRSA (physicians p=0.579, nurses p=0.908). We were unable to differentiate transient from pre-existing permanent colonization.
Conclusion: Exposure to the same environment may result in similar hand contamination patterns of IR when compared caregivers. This supports the concern that industry representatives can cause cross infection between hospitals and hygiene sensitive areas like operation room, intensive care unit and central sterilization units particularly. Further study is required to clarify whether pre-existing bacterial colonization is an influencing factor and how industry is taking care of this to create a safe working environment for their employees, the customers and ultimately the patients.
Keywords: Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, hand hygiene, cross-infection, cross-hospital contamination
Zusammenfassung
Einleitung: Mitarbeiter des Gesundheitswesens sind täglich in einer Vielzahl von Krankenhäusern aktiv. Die meisten begeben sich dabei in Hygiene-sensible Bereiche und arbeiten in unmittelbarer Nähe zu Pflegepersonal und Patienten. In der vorliegenden Studie soll untersucht werden, ob diese Mitarbeiter aus der Industrie nach mindestens 4 Stunden in der gleichen Umgebung mit anderen Angehörigen der Gesundheitsberufe einen vergleichbaren Kontaminationsgrad an Staphylococcus aureus und MRSA (Methicillin-resistenten Staphylococcus aureus) aufweisen.
Material und Methoden: Dazu wurde eine anonymisierte Untersuchung der bakteriellen Besiedlung auf den Fingerflächen verschiedener medizinischer Fachkräfte vorgenommen. Dip-Slides (für S. aureus und MRSA) wurden zur Untersuchung von Teilnehmern der Gesundheitsmesse MEDICA (n=311) herangezogen. 13 Teilnehmer wurden aufgrund der Ausschlusskriterien verworfen, sodass 298 valide Teilnehmer verblieben: Industriebeschäftigte (n=208), Pflegepersonal (n=49) und Ärzte (n=41).
Ergebnisse: Die Untersuchungsteilnehmer besuchten unterschiedliche Krankenhäuser (82%), Operationssäle (41,8%) und Ambulanzen (51,9%). 65,9% der Industriebeschäftigte, 48,8% der Ärzte und 40,8% des Pflegepersonals trugen eine mikrobiologische Belastung von ≥104 KBE.
Für eine S. aureus-Besiedlung von ≥104 KBE ergaben sich bei den Industriebeschäftigte (40,9%) keine statistisch signifikanten Unterschiede im Vergleich zu Ärzten (43,9%, p=0.346) und Krankenschwestern bzw. -pflegern (36,7%, p=0.878), ebenso wenig für die MRSA-Besiedlung der Industriebeschäftigten (gegenüber Ärzten p=0.579 und Pflegepersonal p=0.908) der Fall. Eine Unterscheidung von transienter und existierender permanenter Kolonisation war nicht möglich.
Schlussfolgerung: Die Exposition in der gleichen Umgebung führt zu einer ähnlichen Kontamination der Hände für Mitarbeiter im Gesundheitswesen. Industriebeschäftigte im Gesundheitswesen können daher unwissentlich als Vektor zwischen verschiedenen Krankenhäusern und insbesondere zwischen hygienesensitiven Bereichen wie OP-Saal, Intensivstation und zentrale Sterilisation fungieren. Weitere Studien sind erforderlich, um zu klären, inwieweit eine vorbestehende bakterielle Kolonisation einen Einfluss hat und wie die Industrie eine sichere Arbeitsumgebung für ihre Mitarbeiter, die Kunden und letztlich auch den Patienten sicherstellt.
Background
A significant number of deceases, 16 million unnecessary days of hospital stay and large amounts of spending is caused by Healthcare-Associated Infections (HCAI), according to the World Health Organization (WHO) [1]. Major effort has been put into improving hand hygiene standards for healthcare providers (HCP) to manage hospital internal sources of contamination. In recent years strains such as Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) present an increasingly hard challenge to hospital hygiene because their spread cannot be reliably prevented by antibiotics. Life science industry representatives (IR) may escalate this problem: They often work on site in close contact to patients and HCPs are present during surgeries and other treatment. Additionally the IR visit several hospitals in a day and may carry contaminations from one site to another. There is urgent need to elucidate whether these IR exhibit similar contamination levels as HCP [2]. HCP are known to carry a higher risk than the general public, where the prevalence is 20/100 and 2.2/100 for S. aureus and MRSA respectively [3]. If similar levels can be detected revision of procedures and standards is indicated to combat HCAIs and protect patients, hospital workers and external staff [2].
In the present study we analyze and compare the contamination of IRs and HCPs with S. aureus and MRSA after sharing the same environment for more than four hours.
Materials and methods
We selected S. aureus and MRSA as target pathogens because the Robert Koch Institute classified them as pathogens of highest priority [1] and as one of the most frequent causes of surgical site infection, accounting for 24.7% of postoperative wound infections [2]. Their importance is additionally stressed by findings of Gastmeier & Geffers [4] that MRSA in Germany alone accounted for 14,000 HCAI/year.
We asked if the different professional groups would or would not display significant differences in contamination after visiting the same site for at least 4 h. The null hypothesis for dip slide testing was defined as the lack of statistical differences in the contamination of fingers of the different professional categories with regards to:
Total plate count
S. aureus and in a defined subsample MRSA.
Target location & population
This survey was performed anonymously on attendees of the MEDICA medical exhibition, including IR, hospital physicians and nursing staff. The venue was chosen because it provided a large number of participants from each group. Fingertip contamination of participants was examined after leaving the venue. The circumstances of exiting the venue ensured that no objects or gates of any kind had to be touched before participants reached the two distinct interception points, at a minimum distance of 300 m from the exit (equal to 4 minutes walking time. The choice of interception point locations secured that visitors to MEDICA preferring different transportation systems (cars vs. subway/bus) were included. Weather conditions were dry and the temperature at 5°C [5].
Investigator teams
To ensure least possible bias from leaking information to potential MEDICA visitors prior to the study all supporters and suppliers were strictly held under non-disclosure agreements. Investigator teams were chosen from the nursing and geriatric care school of the Diakonie Kaiserswerth. Seventy two candidates, divided in three groups, were invited to participate in a 90 minutes training led by the first author (H.S.) to qualify as investigator.
Eighteen candidates did not pass the qualification for various reasons and were excluded from participation as investigators. Written consent was taken from all remaining investigators. The resulting 18 investigator teams consisted each of
a contact person
a survey specialist and
a specialist to perform the dip-slide testing.
To prevent the investigators from being contaminated or potentially biasing study results physical contact with participants or any other individual during the study was prohibited. In addition all investigators applied alcohol based hand scrub before and after each participant and dip-slide testers additionally wore single use medical gloves. The team members controlled each other to perform all process steps according to standard. Deviation from standard resulted in exclusion of the participant from the survey and microbiological burden testing.
Settings and investigation process
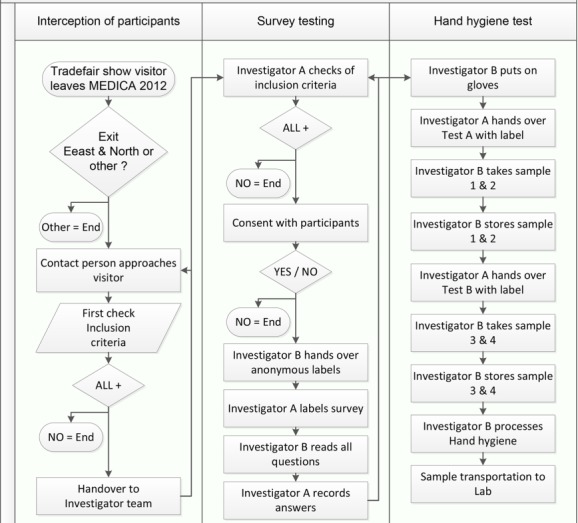
The study was designed as an anonymous non-interventional prospective intercept study (Figure 1 (Fig. 1)) combined with a prospective blinded test of fingertip contamination using the agar dip-slides (Hygiene Monitor and Hygiene Monitor CHROmagar S. aureus double-sided by Transia GmbH, Ober-Mörlen, Germany). Recruiting and data collection was strictly anonymous and executed on one day between 04.30 p.m. and 08.00 p.m. Participants were selected based on the following inclusion criteria:
Figure 1. Study process map including the interception of participant, survey testing and hand hygiene test.
MEDICA visitor on 11/15/2012
Minimum visit time 4 h
Age 25–65
Professional category: Life sciences industry employee, hospital physician, hospital nursing staff
Ability to understand the scope & meaning of the survey-consent
Location of occupation: Germany (accounts for variation in MRSA burden in varying countries).
All others were excluded from participation. Oral consent was taken by two independent investigators and documented by attaching the serial number of the sample to the questionnaire as described in the standard protocol of this study. The Ethical Committee of the University Medicine of Rostock, Germany approved the study design under its registration number A2013-0010.
Participants & sample
35% of intercepted MEDICA attendees were included, mainly because only participants with job location in Germany were valid. 30.2% (311 individuals) of the remaining participants agreed to the survey and take part in sampling (Table 1 (Tab. 1)). Thirteen where excluded according to the criteria of the study (see below). Data and microbiological results of those thirteen were dismissed for further analysis.
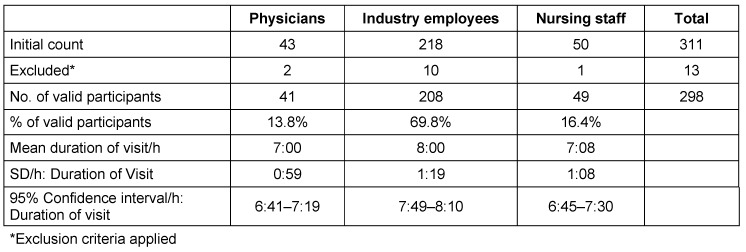
Table 1. A general information overview of participants (physicians; Industry employees; nursing staff) including the initial count, excluded participants, number of valid participants and mean duration of visit/hour.
After applying exclusion criteria 298 participants remained valid, they consisted of 208 (69.8%) industry representatives, 49 (16.4%) nursing staff and 41 (13.8%) physicians. 63 were women and 235 men (21.1% and 78.9% respectively). There was little difference recorded in the median MEDICA visit length for physicians (mean 07:00 h, SD: 00:59 h) and nursing staff (mean 07:08 h, SD: 01:08 h). IR (mean 08:00 h; SD: 01:19 h) stayed on average one additional hour. A total of 113 (37.9%) participants had diurnal contact to domestic animals or livestock. Before sampling all participants were asked about their handedness. Samples were taken from the dominant hand.
All participants were tested on four different fingers using dip-slides: Hygiene Monitor and Hygiene Monitor CHROmagar S. aureus double-sided (both Transia GmbH, Ober-Mörlen, Germany). Incubation and analysis was performed blinded in compliance with manufacturer specifications at Henkelmann GmbH & Co. KG (Volkmarsen, Germany) for (Figure 2 (Fig. 2)):
Figure 2. Dip-slide testing procedure: The participants were investigated on four different fingers using dip-slides. The dip-slides were used for total plate count.
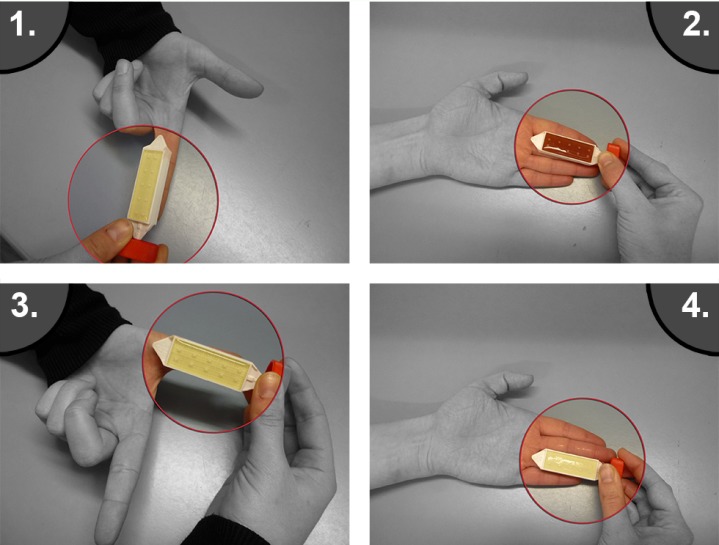
Total plate count,
S. aureus.
The count results varied from 102 to 107. From the initial raw sample we drew a blinded subsample of 99 dip-plates. After primary incubation and analysis those dip-slides were transported to the G+S Laboratory of Bacteriology and Food Hygiene GmbH; (Rheda-Wiedenbrück; Germany). They were inoculated on Baird-Parker-Agar to isolate gram-positive Staphylococci species. Consequently all Baird Parker positive colonies were inoculated on Brilliance MRSA2-Agar (Oxoid) and if found positive for MRSA validated with agglutination Latex test PBP2 (Oxoid).
Data analysis and statistics
Excel 2010 and Minitab 16 were used to register and to analyze the data, supported by the Institute for Biostatistics and Informatics in Medicine and Ageing Research, University Medicine of Rostock. Qualitative parameters were tested, using the Chi-square test. KS-test and Anderson-Darling were applied to test quantitative data sets for normality. U-Test from Mann/Whitney was used to test for significance. Statistical significance was assumed for p-values below 0.05.
Results
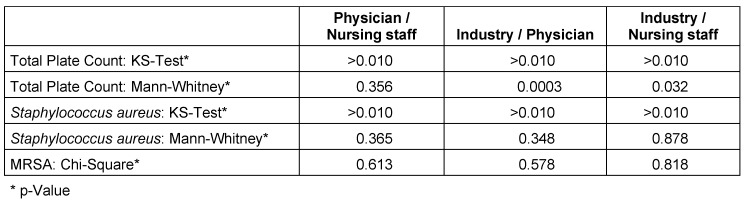
IR where engaged in hospitals, operating rooms and outpatient clinics (82%, 41.8%, 51.9% respectively). 65.9% of IR (vs. 48.8% physicians and 40.8% nurses) carried a microbiological burden ≥104 CFU (colony forming units) (Table 2 (Tab. 2)). Neither S. aureus (≥104 CFU) in IR (40.9%) did show statistical differences in contamination patterns in comparison to physicians (43.9%, p=0.346) and nurses (36.7%, p=0.878) nor did MRSA (physicians p=0.579, nurses p=0.908), see Figure 3 (Fig. 3).
Table 2. Microbiological burden per professional group – Total plate count / S. aureus.
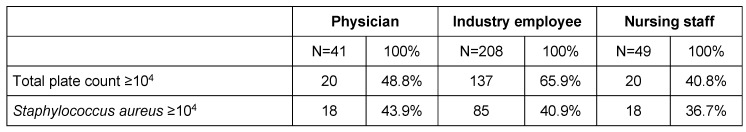
Figure 3. Bioburden on fingertips – There are no significant difference in S. aureus contamination of the different professional groups. S. aureus burden was carried by 40.9% of IR, 43.9% of physicians, and 36.7% of nursing staff ≥104.
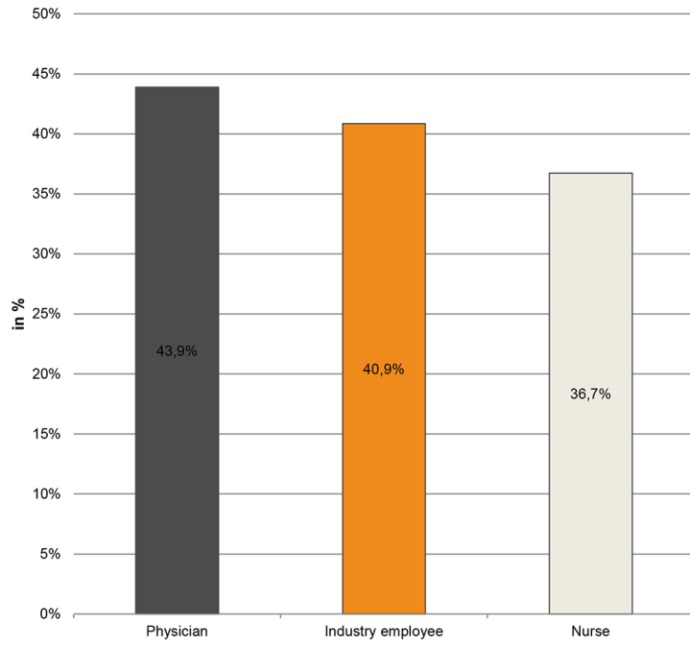
Total plate count resulted in a statistically significant higher proportion of IR (65.9%) carrying a microbiological burden ≥104 CFU when compared to physicians (p=0.0003) and nursing staff (p=0.032). Physicians and nursing staff did not return a statistical difference in total plate count (p=0.356).
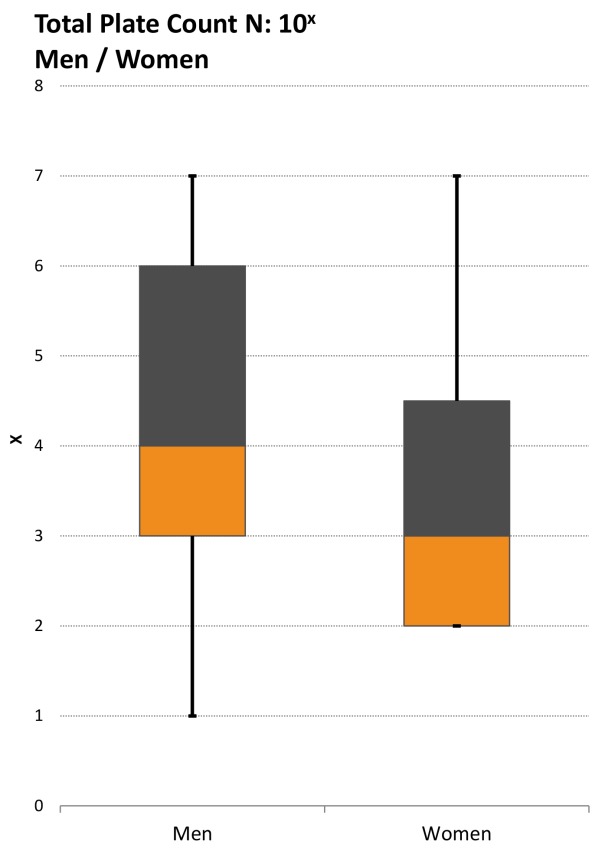
We found total plate count median for women (103) being lower than for men (104) (Figure 4 (Fig. 4)) and we observed no significant difference in S. aureus contamination of the different professional groups consistently returning p-values >0.05. As for S. aureus burden, 40.9% of IR, 43.9% of physicians, and 36.7% of nursing staff carried ≥104 (Figure 3 (Fig. 3) and Figure 4 (Fig. 4)).
Figure 4. Boxplot: Comparison of total plate count, men vs. woman: the total plate count median for women is being lower than for men.
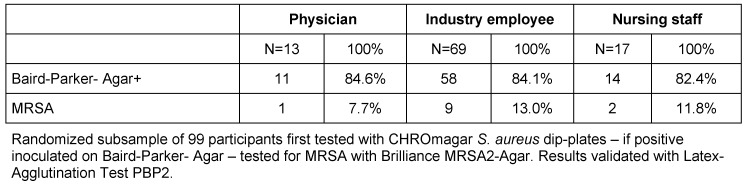
Furthermore, the microbiological burden test data of randomized subsample of 99 participants tested with CHROmagar S. aureus dip-plates is shown in Table 3 (Tab. 3).
Table 3. Microbiological burden per professional group – Baird Parker-Agar+ / MRSA.
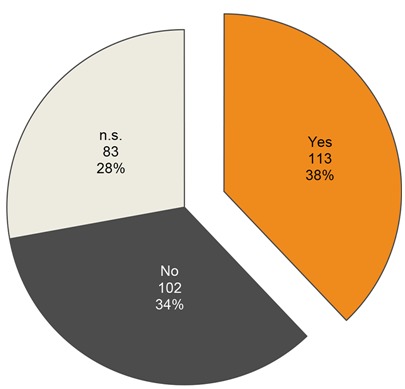
Finally our analysis of daily contact to livestock or domestic animals (37.9%) did not return a positive statistical correlation for S. aureus (p=0.6993) – a possible explanation is the likely lack of professional farmers in the study data (Figure 5 (Fig. 5)).
Figure 5. Diurnal contact to domestic animal or productive livestock shown in a pie chart. The participants were asked during hand-hygiene testing procedure.
The analysis of MRSA positive tests in the subgroup analysis with Chi-Square test returned p-values <0.05 suggesting no significant difference in MRSA colonization between the different professional groups (see Table 4 (Tab. 4)).
Table 4. Comparison of the microbiological tests including total plate count, CFU of Staphylococcus aureus and CFU of MRSA with regard to Physician vs. Nursing staff, Industry vs. Physician and Industry vs. Nursing staff.
Discussion
Our findings of total plate counts (≥104) are consistent with previous findings [6]. The statistically higher total plate count (≥104) we found on IR compared to physicians and nursing staff could be explained by the additional hour those employees were exposed on average to the MEDICA environment. This assumption is backed by findings of other authors that median length of exposure is associated to bacterial contamination of HCP hands [7] and “prolonged contact with health care environments is a known risk factor for acquisition of HCAI’s” [8].
Comparison of prevalence of S. aureus and MRSA in the general German public (20/100 and 2.2/100 respectively) to MEDICA participants, leaves the conclusion that IR and HCP either acquired contamination at the venue or are permanent carriers thereby posing an even increased contamination risk [3]. There is also evidence that physicians and nursing staff acquire transient pathogen flora from contact with inanimate objects [9], [10] or touching intact areas of skin [11]. It is known that this transient contamination can turn into a persistent colonization [12]. In the presence of inconsistent HCP hand hygiene compliance rates, the transfer of pathogens from contaminated IR to patients seems possible. This is additionally backed by findings that pathogens can be transmitted from out-of-hospital sources to patients via the hands of HCPs [13].
Pittet found S. aureus fingertip contamination in 11% of HCP [7] using agar fingertip impression plates. Our findings of S. aureus colonization are higher (≥104) and uniformly distributed between physicians (43.9%, n=10), IR (40.9%, n=85) and nursing staff (36.7%, n=18). Comparing IR with physicians and nursing staff, no statistical significant difference in S. aureus (IR vs. physician: p=0.348 / IR vs. nursing staff: p=0.878) or MRSA (IR vs. physician: p=0.579 / IR vs. nursing staff: p=0.908) contamination was identified. This supports our hypothesis that different professional groups sharing the same work environment (MEDICA) may acquire similar transient contamination patterns, though there is a potential that our methods documented permanent colonization instead.
Our findings are in line with the findings of Kampf, Löffler and Gastmeier [14] who published that S. aureus was present in up to 78% of health care workers hands. Other investigators have found MRSA to account for up to 18 to 20% of clinical S. aureus samples [15]. Similarly, we found MRSA on the fingertips of 7.7% (1 physician), 13.0% (9 IR) and 11.8% (2 nursing staff) in the subsample. One study raised the assumption that there might be a fixed risk associated with each patient to HCP contact to acquire HCAI. According to this study this risk accumulates during the course of a hospital stay [16]. We need to ask if this might be true for each IR employee and HCP contact as well. According to Henkelmann GmbH & Co. KG, the measured S. aureus (≥104) microbiological burden would be considered intolerable for German food processing industry employees.
David and Daum [17] described community-associated methicillin-resistant S. aureus as “new clones of MRSA not being confined to healthcare environments and spreading rapidly among healthy people in the community”. The propagation of MRSA from agricultural livestock to humans is known to be frequent [15]. The relationship of nasal MRSA colonization in German veterinarians is 2–45%, and their related livestock (pig) farmers 86% [18] has been described in previous studies. In addition to this there are studies that suggest that 10–40% of MRSA colonization in the Netherlands are in patients that had exposure to hospitals outside the Netherlands [19] suggesting that MRSA root causes may well lie outside of the individual hospital. In the Netherlands, this led to the banning of hospital staff from work until three negative tests for MRSA have been performed over a period of three weeks, if the staff member had worked in a hospital outside the Netherlands [20]. Given the fact that we did all our sampling outdoors and that MRSA has the ability to persist for up to 7 months [21] on inmate surfaces, MRSA may not be restricted to hospital environments but also be spread to IR automobiles and homes.
We made significant effort to reduce bias and increase data quality through standard processes:
Separation/blinding of data gathering, dip-slide test and data analysis
-
Investigator training including
SOP’s incl. guides for contact, interview and sample
4 eyes principle during consent, survey and sample procedure
Same day data and sample collection. Avoids double contact and potential bias due to raised awareness on next day
2 distinct interception points (Exit North and Exit East)
18 independent investigators teams
Adequate storage/unbroken cooling chain of samples in the entire process.
As usual intercept surveys cannot provide a cause and effect relationship – leaving the primary source of contamination for all individuals unknown - this will have to be the focus of future investigations. It is possible that due to the inoculation and double validation process of both S. aureus and MRSA in the subgroup, we found a reduced number of MRSA positive samples. A medical trade fare location was chosen instead of a hospital environment because it allowed us to reduce bias from artificially increased compliance due to leaking information. It allowed us to recruit a high number of participants in a short period of time. We acknowledge that the investigation site may have an impact on findings – more in depth research will be required on hospital sites to verify this. Confounding for gender may have had some impact on the study results. Bias may have occurred when employees of the different professions thought that their employers or industry may expect them to answer question in a specific direction – or not answer at all. We did not measure the contamination status of the different professional populations prior accessing MEDICA and are aware that there might be a confounding of transient with permanent colonization due to the nature of the professionals – but this would make our findings only more worrisome. The fact that we had >50% of participants travelling to MEDICA by car reduces the likelihood of confounding for public transportation as the primary contamination source.
Conclusion
Our findings suggest that industry representatives (IR) provide a potential reservoir for direct or indirect cross-transmission to patients. They show a comparable contamination risk as HCP and patients for S. aureus and MRSA. It can be assumed, that the same applies for other types of pathogens, but especially for other multi-resistance species, further research is needed to support this. In comparison to the general public the increased risk of IR is characterized by their access to critical sections in hospitals, roaming between several hospitals per day, a high frequency of contacts with HCP, the work environment and medical gear. Also, they are often neither sufficiently instructed in standard operating procedures nor sufficiently protected by vaccination.
It follows that hospitals and IRs have to revise their methods and procedures to prevent these risks in the short-term. Furthermore, to avoid cross-infection inter and intra hospitals, the same hygienic standards and procedures that apply to HCP have to be applied to IR. Standards according to EUCOMED suggestions could be a starting point to improve the situation.
Notes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The authors wish it to be known that in their opinion, the first two authors should be regarded as joint First Authors.
All authors planned and developed the study and performed analysis of the data. HS and SZ drafted the manuscript; all authors revised and approved the final version of the manuscript.
Acknowledgement
The authors want to express special thanks to the nursing students and teachers of Kaiserswerther Diakonie; Düsseldorf, Germany as well as Marc Dreseler and his team from Henkelmann GmbH & Co. KG and Hatice Gül-Özüberk who performed the survey hands-on. Sincere thanks to Kelly Carden, MD, MBA for her help. Prof. Dr. Günter Kundt, Institute for Biostatistics and Informatics in Medicine and Ageing Research, University Medicine of Rostock contributed to the statistical analysis. Finally, kind thanks to David E. Halliday, who provided equipment and single use materials.
References
- 1.Robert Koch-Institut. Priorisierung übertragbarer Infektionserreger unter dem Aspekt der Surveillance und epidemiologischen Forschung. Epidemiol Bull. 2011;(26):397–408. [Google Scholar]
- 2.Robert Koch-Institut. Deutsche Daten im Rahmen der ersten europäischen Prävalenzerhebung zum Vorkommen nosokomialer Infektionen und zur Antibiotikaanwendung. Epidemiol Bull. 2012;(44):239–248. [Google Scholar]
- 3.Herrmann M, Petit C, Dawson A, Biechele J, Halfmann A, von Müller L, Gräber S, Wagenpfeil S, Klein R, Gärtner B. Methicillin-resistant Staphylococcus aureus in Saarland, Germany: a statewide admission prevalence screening study. PLoS ONE. 2013;8(9):e73876. doi: 10.1371/journal.pone.0073876. Available from: http://dx.doi.org/10.1371/journal.pone.0073876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gastmeier P, Geffers C. Nosokomiale Infektionen in Deutschland: Wie viele gibt es wirklich. [Nosocomial infections in Germany. What are the numbers, based on the estimates for 2006?]. Dtsch Med Wochenschr. 2008 May;133(21):1111–1115. doi: 10.1055/s-2008-1077224. (Ger). Available from: http://dx.doi.org/10.1055/s-2008-1077224. [DOI] [PubMed] [Google Scholar]
- 5.WetterKontor. Rückblick – Höchst- und Tiefstwerte am 15.11.2012 (Karte) [Internet] 2012. [cited 7 Jan 201]. Available from: http://www.wetterkontor.de/de/deutschland_extremwerte_karte.asp?id=20121115&p=1.
- 6.Boyce JM, Pittet D Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm Rep. 2002 Oct 25;51(RR-16):1–45. Available from: http://www.cdc.gov/mmwr/PDF/rr/rr5116.pdf. [PubMed] [Google Scholar]
- 7.Pittet D, Dharan S, Touveneau S, Sauvan V, Perneger TV. Bacterial contamination of the hands of hospital staff during routine patient care. Arch Intern Med. 1999 Apr 26;159(8):821–826. doi: 10.1001/archinte.159.8.821. Available from: http://dx.doi.org/10.1001/archinte.159.8.821. [DOI] [PubMed] [Google Scholar]
- 8.Tess BH, Glenister HM, Rodrigues LC, Wagner MB. Incidence of hospital-acquired infection and length of hospital stay. Eur J Clin Microbiol Infect Dis. 1993 Feb;12(2):81–86. doi: 10.1007/BF01967579. Available from: http://dx.doi.org/10.1007/BF01967579. [DOI] [PubMed] [Google Scholar]
- 9.Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997 Sep;18(9):622–627. doi: 10.2307/30141488. Available from: http://dx.doi.org/10.2307/30141488. [DOI] [PubMed] [Google Scholar]
- 10.Duckro AN, Blom DW, Lyle EA, Weinstein RA, Hayden MK. Transfer of vancomycin-resistant enterococci via health care worker hands. Arch Intern Med. 2005 Feb 14;165(3):302–307. doi: 10.1001/archinte.165.3.302. Available from: http://dx.doi.org/10.1001/archinte.165.3.302. [DOI] [PubMed] [Google Scholar]
- 11.Pessoa-Silva CL, Dharan S, Hugonnet S, Touveneau S, Posfay-Barbe K, Pfister R, Pittet D. Dynamics of bacterial hand contamination during routine neonatal care. Infect Control Hosp Epidemiol. 2004 Mar;25(3):192–197. doi: 10.1086/502376. Available from: http://dx.doi.org/10.1086/502376. [DOI] [PubMed] [Google Scholar]
- 12.Adams BG, Marrie TJ. Hand carriage of aerobic gram-negative rods may not be transient. J Hyg (Lond) 1982 Aug;89(1):33–46. doi: 10.1017/S0022172400070510. Available from: http://dx.doi.org/10.1017/S0022172400070510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passaro DJ, Waring L, Armstrong R, Bolding F, Bouvier B, Rosenberg J, Reingold AW, McQuitty M, Philpott SM, Jarvis WR, Werner SB, Tompkins LS, Vugia DJ. Postoperative Serratia marcescens wound infections traced to an out-of-hospital source. J Infect Dis. 1997 Apr;175(4):992–995. doi: 10.1086/514008. Available from: http://dx.doi.org/10.1086/514008. [DOI] [PubMed] [Google Scholar]
- 14.Kampf G, Löffler H, Gastmeier P. Hand hygiene for the prevention of nosocomial infections. Dtsch Arztebl Int. 2009 Oct;106(40):649–655. doi: 10.3238/arztebl.2009.0649. Available from: http://dx.doi.org/10.3238/arztebl.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Köck R, Mellmann A, Schaumburg F, Friedrich AW, Kipp F, Becker K. The epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in Germany. Dtsch Arztebl Int. 2011 Nov;108(45):761–767. doi: 10.3238/arztebl.2011.0761. Available from: http://dx.doi.org/10.3238/arztebl.2011.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen B, Hyman S, Rosenberg L, Larson E. Frequency of patient contact with health care personnel and visitors: implications for infection prevention. Jt Comm J Qual Patient Saf. 2012 Dec;38(12):560–565. doi: 10.1016/s1553-7250(12)38073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010 Jul;23(3):616–687. doi: 10.1128/CMR.00081-09. Available from: http://dx.doi.org/10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuny C, Nathaus R, Layer F, Strommenger B, Altmann D, Witte W. Nasal colonization of humans with methicillin-resistant Staphylococcus aureus (MRSA) CC398 with and without exposure to pigs. PLoS One. 2009 Aug 27;4(8):e6800. doi: 10.1371/journal.pone.0006800. Available from: http://dx.doi.org/10.1371/journal.pone.0006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrich AW. Häufig gestellte Fragen (FAQ) über MRSA - Euregioprojekt [Internet] 2012.
- 20.Friedrich AW. MRSA - Epidemiologie und Strategien in Europa [Internet] Institut für Hygiene Universitätsklinikum Münster; 2010. [cited 7 Jan 2013]. Available from: http://www.gesunde.sachsen.de/download/Download_Gesundheit/Dr._Friedrich_Muenster.pdf. [Google Scholar]
- 21.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. Available from: http://dx.doi.org/10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]