Abstract
Background and Objectives
Thermally mediated modalities of cartilage reshaping utilize localized heating of cartilage combined with mechanical deformation to achieve new geometries. We sought to determine the steady state elastic modulus of thermally modified cartilage without deformation, as this provides a constraint in mechanical models of the shape change process.
Study Design/Materials and Methods
The main objective of this study was to characterize the steady state elastic modulus of porcine septal cartilage after uniform heating with radiofrequency (RF) to peak temperatures of 50 ± 5, 65 ± 5, and 85 ± 5°C. The cartilage was divided into three equally sized regions, designated as anterior, middle and posterior. Each region was then sectioned into two specimens with the proximal component serving as a paired control.
Results
The data confirm that there is high baseline variability in control steady state elastic moduli between animals. Also, the control values confirm a decreasing steady state elastic modulus from anterior to posterior. There is no statistical significance (P > 0.05) found between the elastic moduli of control and treated samples.
Conclusions
Although shape change and retention have been fairly well characterized, little is known about the specific relation between steady state elastic modulus of cartilage and maximum treatment temperature. We determined that the difference of steady state elastic modulus between control and treated porcine septal samples was not statistically significant after uniform heating with RF to peak temperatures of 50 ± 5, 65 ± 5, and 85 ± 5°C. Ultimately, the results of this study do not pertain to the regions of heated cartilage that are shaped to hold a new form; however, it does show that the regions that are not mechanically deformed do return to the original pre-treatment elastic modulus. This is still useful information that may be used in finite element models to predict changes in internal stress distributions of cartilage after laser reshaping.
Keywords: steady state elastic modulus, porcine septal cartilage, radiofrequency heating, laser cartilage reshaping
Introduction
Traditional surgical methods of reshaping cartilage require scoring, carving, and suturing to alter internal forces that resist deformation in order to achieve the desired geometry. Since these methods are limited by variable availability of suitable autogenous tissue, several alternative tissue reshaping methods have been explored. Laser and radiofrequency (RF)-mediated cartilage reshaping both are thermally dependent methods that use heat to create shape change. Laser and RF can produce similar temperature ranges, heating rates and reshaping geometries, though laser heating is non-uniform whereas RF heating is comparatively more uniform [1–10].
Thermally induced shape change is thought to occur at threshold of 60–75°C due to relaxation of internal stresses in mechanically deformed cartilage specimens [8–11]. Of note, the threshold is a range because the specific temperature at which shape change occurs differs with the rate of heating. This has been born out in mechanical studies performed by our group using traditional calorimetry, and in rate process models of tissue injury [11,12]. Previous studies have shown that thermoforming of cartilage can produce significant changes in geometry, but it is still unclear as to what extent the reshaped cartilage retains its mechanical stability [3–7]. Thermally mediated modalities of cartilage reshaping utilize localized heating of cartilage combined with mechanical deformation to achieve a new geometry. Although most of the heat is applied to the area of conformation change, there are some peripheral areas which still receive significant heating during the process, but are not mechanically deformed. It would be valuable to determine the steady state elastic modulus of thermally treated cartilage acutely before wound healing and reparative mechanisms occur, as this would provide information that can aid in the finite element modeling of the shape change process. This would be extremely important in applications such as rhinoplasty where a significant reduction of cartilage steady state elastic modulus could lead to catastrophic results [13,14].
Our previous work used RF needle electrodes to induce shape change in porcine cartilage [3]. This method produces a non-uniform heating profile, similar to applying laser heating in several different spots. Under the assumption that a local change in temperature would affect the local elastic modulus, a non-uniform thermal profile would result in a non-uniform change in mechanical properties. This would complicate mechanical analysis of the sample; therefore, for the purposes of this study, an RF source was configured to generate a relatively more uniform temperature field. One important advantage to using RF to uniformly heat cartilage is that it produces a temperature increase rate similar to laser irradiation. Other methods of uniformly heating cartilage such as hot water baths are slower and less clinically applicable than RF or laser heating.
Unconfined compression was used to evaluate cartilage properties because it is a replicable and reliable method of determining steady state elastic modulus. Notably, shape change was not induced in this study in order to maintain large, flat, and uniform specimen geometry for mechanical testing. In order to heat the samples uniformly and consistently with RF, while minimizing the confounding variable of irregular geometry, the cartilage specimens needed to be at least 5 mm thick. Therefore, porcine cartilage was used as a model for human cartilage because it is a reliable source of large and flat cartilage samples; its thermal and mechanical properties have been previously characterized; and it is readily available at low cost [2,15,16]. Although using human cartilage samples to determine steady state elastic modulus values would be ideal, human cartilage poses several problems in terms of long-term storage, geometric consistency, transportation, and limited supply.
The main objective of this study was to characterize the steady state elastic modulus of porcine septal cartilage after uniform heating with RF to peak temperatures of 50 ± 5, 65 ± 5, and 85 ± 5°C. These temperatures were chosen because 50°C is considered to be below the threshold of thermally inducible shape change; 65°C is at the threshold; and 85°C is well above the temperature threshold established in previous laser cartilage reshaping studies [1,2,8–11].
Materials and Methods
Specimen Preparation
Fresh porcine crania (n = 45) were acquired from a local packing house. The septa were harvested immediately, the perichondrium was removed, and the cartilage was hydrated in an ambient temperature saline bath for at least 20 minutes prior to use. If samples were not used immediately, they were then refrigerated for less than 24 hours before testing. Based on previous studies and preliminary experiments, there is significant variation in the elastic modulus: (1) between individual animals; (2) decreasing elastic modulus along the anterior–posterior axis (in a given animal); and (3) increasing elastic modulus along the superior–inferior axis of the septa (in a given animal) [4,15]. Hence, to minimize the effects of the spatial variation of septal mechanical properties, a central longitudinal segment of the septum was selected as the region of interest to excise specimens for RF heating and mechanical analysis (Fig. 1). This region was divided into three equally sized sub-regions (20 mm × 30 mm × 5 mm), designated as anterior, middle, and posterior. These three sub-regions were evaluated independently as elastic modulus decreases from anterior to posterior. Each sub-region was then sectioned into two specimens with the proximal component serving as a paired control.
Fig. 1.
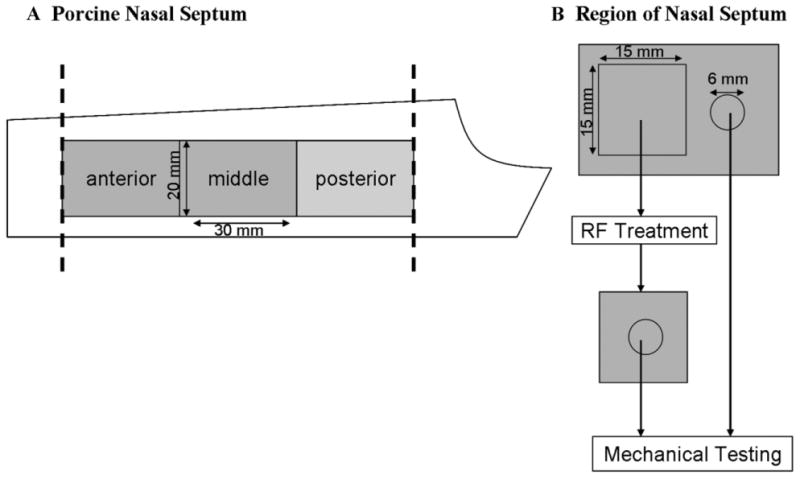
A: A schematic of a porcine nasal septum. The most anterior and posterior sections were removed, and the remaining was divided into three regions. Biopsy punches for mechanical testing were always taken from the longitudinal midline of the samples to minimize spatial variability along the superior–inferior axis. B: Both treatment and control samples were taken from respective regions. A 15 mm × 15 mm × 5 mm sample was trimmed and heated using an RF source, and 6 mm diameter 5 mm thick biopsy punches were used for mechanical testing to determine steady state elastic moduli.
RF Heating
One 15 mm × 15 mm × 5 mm specimen was cut from each sub-region (e.g., anterior, middle, and posterior) for treatment with RF. Samples were heated with a surgical RF generator (Surgitron FFPF catalog S 2100, 2.8 MHz; Ellman, Inc., Hewlett, NY). A custom jig was made which could adjust the distance between two stainless steel contact plates (5 mm × 20 mm), which served as surface electrodes and held the specimen securely. The samples were held between the two plates, and care was taken to ensure minimal applied compression while maintaining maximum surface contact at the tissue–electrode interface (Fig. 2). Samples were heated using the cut–coag mode at different power settings for 8 seconds to reach three distinct peak temperatures: 50 ± 5; 65 ± 5; or 85 ± 5°C. The specimens were confirmed to be uniformly heated to the peak temperature because spatial variation was ≤3°C for a given sample (Fig. 3). Of note, the time dependence of RF heating is most similar to the heating rate achieved at the center of a laser spot (Fig. 3C and D). The power-setting on the RF generator ranges from “1 to 11” in arbitrary units (“1” being lowest and “11” being highest power), and the settings of 2, 2.5, and 3 were used. During heating, the specimens were filmed with a single element HgCd infrared thermal imaging system incorporating a 3× telescopic lens (Inframetrics 600, FLIR systems, Inc., Boston, MA). Temperature data were collected as described in previous publications [10,17]. During heating at settings of 2 and 2.5, there was no warping of the samples noted. However, heating at the setting of 3 did cause slight warping of the tissue. After heating, the samples were rehydrated in a normal saline bath at ambient temperature for at least 15 minutes.
Fig. 2.
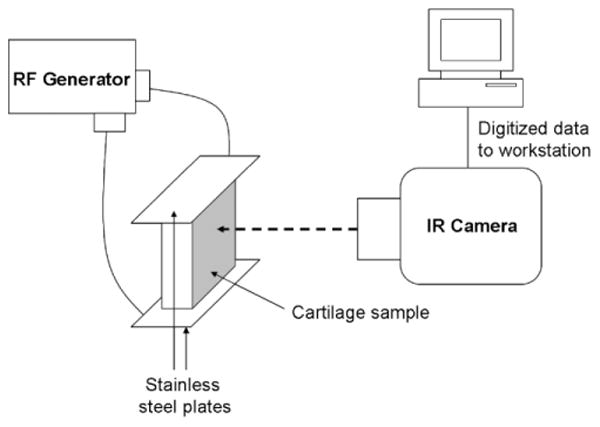
Diagram of experimental setup. Stainless steel plates were used to achieve relatively uniform heating of the 15 mm × 15 mm × 5 mm cartilage sample. An IR camera recorded surface temperature, and data was transferred to a workstation for analysis.
Fig. 3.
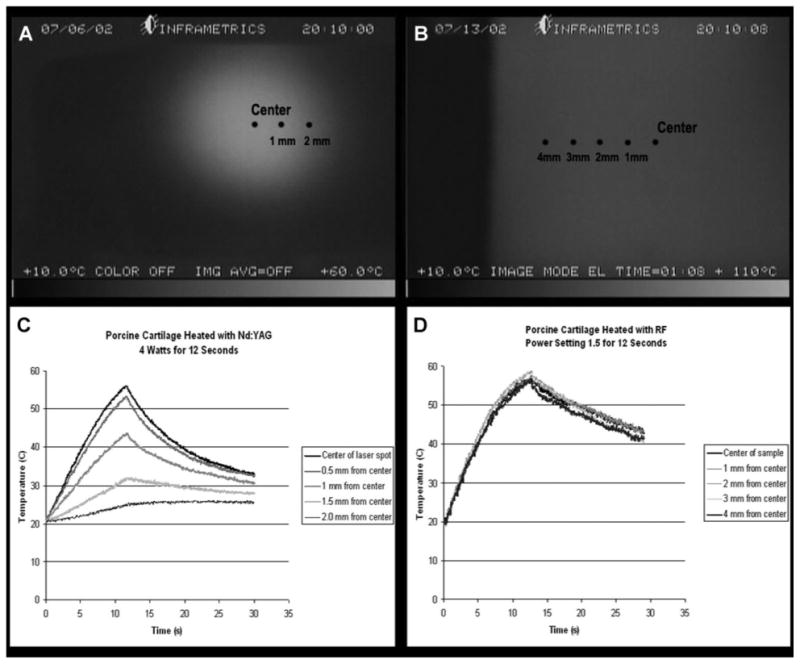
A: Spatial dependence of non-uniform temperature profile of Nd:YAG laser-irradiated porcine septal cartilage. B: Spatial dependence of relatively uniform temperature profile of RF heated porcine septal cartilage. C: Representative example of the time dependence of temperature of laser heated cartilage. D: Representative example of time dependence of temperature of IR heated cartilage.
Mechanical Testing
After rehydration, irregular surface features caused by warping of the treated sample at maximum temperatures of 85°C were trimmed, and a biopsy punch was taken from the center of the 15 mm × 15 mm × 5 mm RF heated sample to produce a cylinder 6 mm in diameter and 5 mm in thickness. The steady state elastic modulus was determined in unconfined compression using a mechanical testing platform (Enduratec Electroforce 3200, Bose Corp., Eden Prairie, MN). During mechanical analysis, every effort was made to reduce dehydration, and samples were carefully hydrated with a drop (1/10 ml) of saline to prevent dehydration. Displacement was applied to achieve 0.10 ± 0.02 strain at a rate of 0.05 mm/second, and was sustained at 0.10 strain for 10 minutes, which was sufficient time for the cartilage samples to reach stable stress–relaxation, as indicated by preliminary data and previous experiments [10]. Paired controls taken from respective adjacent proximal regions were also evaluated in the described method.
Results
Matched control and post-treatment steady state elastic modulus values were compared for each heating parameter (Figs. 4–6). The data confirm that there is high baseline variability in control steady state elastic moduli between animals. Also, the control values confirm baseline spatial variability, that is, a decreasing steady state elastic modulus from anterior to posterior. As indicated by the matched samples, there is no clear trend of change in elastic modulus after treatment. Furthermore, trends were not clear in matched anterior, middle, and posterior pre- and post-treatment data obtained from individual animals (data not shown). The percent change between each matched control and treatment sample was calculated. The average of the percent change for each parameter is shown Figure 7. Dependent t-test was used to determine statistical significance between matched control and treatment samples (Table 1). The resultant data indicate that there is no statistical significance found between the elastic moduli of control and treated samples.
Fig. 4.
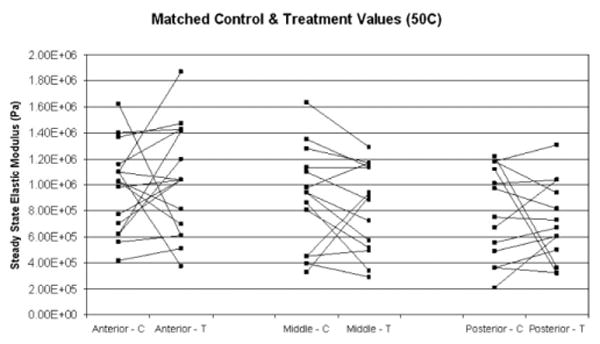
Matched control (C) and post-treatment (T) steady state elastic modulus values for samples treated at 50°C, for anterior, middle, and posterior regions of porcine septum.
Fig. 6.
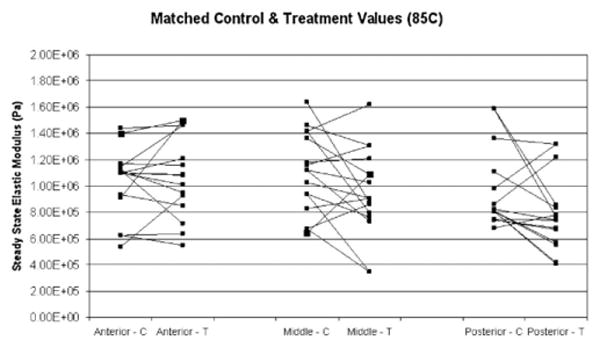
Matched control (C) and post-treatment (T) steady state elastic modulus values for samples treated at 85°C, for anterior, middle, and posterior regions of porcine septum.
Fig. 7.
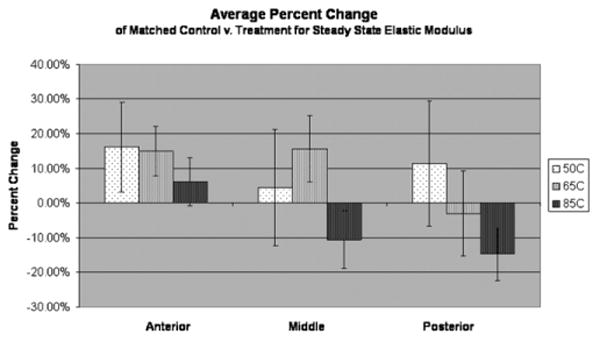
The percent change between each matched control and post-treatment value was calculated and averaged for each respective region and maximum temperature. Standard error bars are shown, which indicate high variability in percent change. At 85°C there is a slight trend of decreased elastic modulus at middle and posterior regions.
Table 1. Summary of Steady State Elastic Modulus Values With Dependent t-test P values (vs. Respective Controls).
| Temperature | Region | Average (MPa) | Standard error (MPa) | Ave. % change | Standard error (%) | n | P-value |
|---|---|---|---|---|---|---|---|
| Control | Anterior | 1.07 | 0.06 | — | — | 50 | — |
| Middle | 0.94 | 0.05 | — | — | 47 | — | |
| Posterior | 0.81 | 0.05 | — | — | 42 | — | |
| 50°C | Anterior | 1.04 | 0.10 | 16.1 | 12.9 | 16 | 0.59 |
| Middle | 0.83 | 0.09 | 4.5 | 16.8 | 14 | 0.38 | |
| Posterior | 0.71 | 0.09 | 11.4 | 17.9 | 13 | 0.56 | |
| 65°C | Anterior | 0.92 | 0.09 | 15.0 | 7.1 | 18 | 0.06 |
| Middle | 1.12 | 0.11 | 15.6 | 9.6 | 17 | 0.29 | |
| Posterior | 0.70 | 0.08 | −3.1 | 12.4 | 14 | 0.38 | |
| 85°C | Anterior | 1.08 | 0.08 | 6.2 | 6.9 | 16 | 0.49 |
| Middle | 0.81 | 0.07 | −10.6 | 8.4 | 16 | 0.09 | |
| Posterior | 0.79 | 0.07 | −14.8 | 7.6 | 15 | 0.06 |
Discussion
The original motivation for this study was to obtain information about steady state mechanical behavior of septal cartilage to be used in a finite element model (FEM) to approximate the solid mechanics of cartilage and the effects of laser cartilage reshaping (LCR). Over the past 15 years, LCR has slowly gained acceptance into the clinical realm with commercial systems and treatment centers becoming more available, providing treatments for the nasal septum and external ear. In order to optimize and improve LCR, it would be important to model the shape change process. Opto-thermal interactions in cartilage have been rigorously studied for well over three decades, and because of clinical impact of lasers these models are very sophisticated. However, there is no detailed model linking heat generation and peak temperature to shape change. Our group has developed theoretical models to show how changes in mechanical properties after simulated LCR impact residual stress distribution [18]. We do not have experimental data on how the steady state elastic modulus of cartilage changes after heating in areas that are not mechanically deformed. This has been a major limitation. The aim of this study was to determine these equilibrium moduli for use in FEM modeling of the optothermo-mechanical response of cartilage, at least in steady state. Of course, one important limitation of this study is that porcine cartilage was used in order to characterize steady state properties. Ultimately the results of this study do not directly relate to regions of heated cartilage that are shaped to hold a new form; however, it does show that the regions that are not mechanically deformed do return to the largely original pre-treatment elastic modulus. This is practical information that may be incorporated into finite element models as boundary conditions to predict changes in cartilage after laser reshaping. Although cartilage reshaping depends on applying thermal damage to an area with applied strain, there are still regions which receive heating but do not undergo bending, and it is useful to know that the post-treatment elastic modulus in these areas remains consistent.
Previous experiments using laser cartilage reshaping (LCR) have demonstrated that laser heating is an effective way to produce shape-change in irradiated cartilage samples [8–11]. It is reasonable to assume that the internal mechanisms of heat-induced shape-change also produce change in cartilage mechanical properties [19]. Although many experiments have been conducted on laser reshaping, detailed quantitative studies concerning the dependence of cartilage elastic modulus on heating parameters have not been performed. The difficulty of characterizing elastic modulus measurements arises from a non-uniform distribution of energy in the laser beam, and the resultant non-uniform pattern of local heating on the treated cartilage tissue. A Gaussian or similar beam profile combined with heat transfer effects produces a non-uniform heating pattern with the maximum temperature at the irradiation center (Fig. 3A and C) [17]. Furthermore, multiple sites of irradiation in adjacent locations on the cartilage sample produce further variations in the temperature distribution. The combination of these effects produces variations in thermo-mechanical changes within laser spot and across the treated tissue, which make accurate measurements of elastic modulus extremely complicated.
As previously noted, measuring mechanical properties in the region of laser irradiated septal cartilage has many technical challenges; therefore, we opted for using RF. To produce a uniform distribution of thermo-mechanical change in the sample, we developed a heating method using an RF power source. The electric field created in a cartilage sample sandwiched between two plate electrodes is relatively constant (except at the sides of the sample where an edge effect is present), and produces uniform heating throughout most of the sample (Fig. 3B and D). Of note, though RF produces a more uniform temperature distribution, the heating rate is still very similar between the laser and RF (Fig. 3C and D). Varying the RF power level changes the heating rate, and can thus be used to create a look-up table of equilibrium mechanical properties as a function of heating rate and peak temperature. Using this information combined with knowledge of the heating rate within discrete regions of a laser spot, we could, in theory, estimate the mechanical changes in cartilage tissue produced by a laser after LCR.
The advantages of using RF include achieving similar heating rates as lasers and uniform bulk heating of large specimens, which is necessary for mechanical compression testing. However, the RF approach has intrinsic limitations, such as non-uniform heating at the edges of the sample and in the contact area, due to the edge effects resulting in small variations and less precise control of heating rates. Because of these factors, mechanical analysis was performed on punch biopsies taken from the center of each sample to reduce non-uniformity in temperature and potential thermally induced mechanical changes.
Unconfined compression was used to evaluate cartilage properties because it is a replicable and reliable method of determining steady state elastic modulus. Furthermore, compression was used in order to be able to compare data with another study that has been done to evaluate porcine elastic modulus [10]. The central punch biopsy avoided sampling tissue that could be altered by edge-effects, but this region of tissue was inadequate for reliable tension or cantilever testing with our equipment because of its small size.
One other limitation is that porcine septal cartilage is not a good surrogate for human septal cartilage. On gross physical inspection they are very different: porcine cartilage is much thicker, larger, and denser than human. The porcine septum has a much greater elastic modulus and may differ greatly in biochemical composition [13]. Regardless, porcine cartilage is the best available surrogate. Some of the problems encountered with other tissue models include: (1) fresh cadaver septal samples are difficult, if not impossible, to obtain; (2) tissue remnants from rhinoplasty or septoplasty are severely limited in size, number and uniformity; (3) rodent cartilage is generally too small; (4) bovine tissue may carry the remote risk of spongiform encephalitis and is anatomically less similar to human cartilage to a greater degree than porcine cartilage; and (5) tissue from other primate species is essentially impossible to obtain. Rabbit septal cartilage bears special consideration because it is significantly larger than rat or murine tissue and is available from consumer/commercial packing houses. However, the septum is very thin, not as broad, and subsequently much more difficult to heat uniformly with RF and to measure mechanical properties using compression testing.
Based on the high variability between matched treatment and baseline control samples, we have observed that RF heating, which achieves a temperature range and heating rate typical for LCR, unpredictably increases or decreases the steady state elastic modulus, but not to a statistically significant extent. Nonetheless, it is important to note that the finding that little post-treatment change in steady state elastic modulus was observed is still useful information for clinical applications such as rhinoplasty or otoplasty, because that suggests post-treatment cartilage will be still able to bear its steady-state anatomical loads.
It is known from previous work that during heating above the 65–75°C temperature range, and depending on the heating rate, transient softening occurs [1,2,4,9–11,17,18]. A change in mechanical properties may be proportional to higher temperatures, and may be dependent on initial pre-treatment elastic modulus. One possibility is that heating initiates at least two competing processes that are responsible for changes in elastic modulus: one results in increased elastic modulus which is more pronounced in cartilage with a high baseline elastic modulus, while the other results in decreased elastic modulus and is associated with a low baseline elastic modulus. For example, in anterior regions with comparatively higher baseline elastic modulus, there is presumably a higher proportion of cross-linking. When the anterior region is heated, perhaps the result is in an increase in cross-linking causing in an increase in elastic modulus. Conversely, in posterior regions with relatively lower baseline elastic modulus and lower proportion of cross-linking, the elastic modulus may be more dependent on the fluid component of its viscoelasticity. When the posterior region is heated, fluid may be more easily mobilized resulting in a decrease in post-treatment elastic modulus. This combined phenomenon could account for the lack of statistically significant change in mechanical properties at temperatures below the threshold of softening. Interestingly, a slight trend of softening was observed in middle and posterior sections at 85°C. This result potentially supports the previous hypothesis because at these higher temperatures there is permanent damage to cross-linked structures, which outweighs the affects of the competing processes, regardless of the nature of the initial composition of the cartilage.
Lastly, this study shows that without applied shape change, the elastic modulus does not change significantly. Ideally, the next step will be to characterize the changes in elastic modulus in cartilage that has undergone shape change. However, this is a formidable task as shape change is generally affected over a very small region of interest that necessitates the use of specialized hardware, such as micro-indentation, to measure.
Conclusion
Thermally mediated shape change in facial cartilage is of interest because it can be performed via minimally invasive techniques that avoid the limitations and risks of traditional surgery. Although shape change and retention for different heating modalities have been fairly well characterized, little is known about the mechanical properties of facial cartilages after fast RF or laser heating. We determined that the difference of steady state elastic moduli between control and treated porcine septal samples were not statistically significant after uniform heating with RF to peak temperatures of 50 ± 5, 65 ± 5, and 85 ± 5°C. This is notable because 65°C is approximately the threshold for stress relaxation and 85°C is well above the threshold. Lack of steady state softening does have reassuring implications for thermally mediated cartilage reshaping modalities, because regions that are heated but not deformed will on-average return to pre-treatment elastic modulus. We also confirmed that porcine nasal septa have decreasing elastic modulus along the anterior-posterior axis.
Fig. 5.
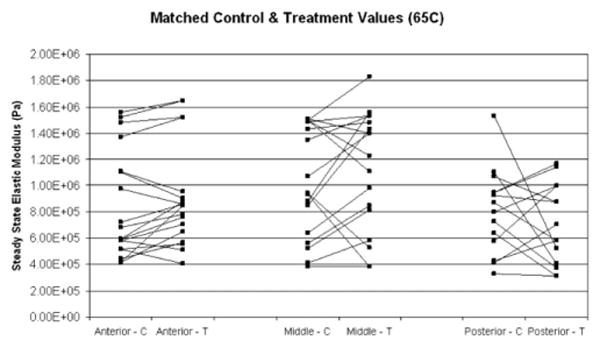
Matched control (C) and post-treatment (T) steady state elastic modulus values for samples treated at 65°C, for anterior, middle, and posterior regions of porcine septum.
Acknowledgments
This work was supported by the National Institute of Health (P41RR001192-28, P41RR001192-29, and R03 DC005572) and the U.S. Air Force Office of Scientific Research, Medical Free-Electron Laser Program (FA9550-04-1-0101 and FA9550-08-1-0384). The U.S. Government is authorized to reproduce and distribute reprints for Governmental purposes notwithstanding any copyright notation. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the Air Force Research Laboratory or the U.S. Government.
Contract grant sponsor: National Institute of Health; Contract grant numbers: P41RR001192-28; P41RR001192-29; R03 DC005572; Contract grant sponsor: U.S. Air Force Office of Scientific Research, Medical Free-Electron Laser Program; Contract grant numbers: FA9550-04-1-0101. FA9550-08-1-0384.
Footnotes
Conflict of Interest Disclosures: The authors certify that they have no affiliation with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the manuscript (e.g., employment, consultancies, stock ownership, honoraria) except as follows: Grant support from Lockheed Martin Corporation, equity with Praxis Biosciences LLC and consulting with Aerin Medical.
Allison Zemek is currently a medical student at Stanford University School of Medicine, Stanford, CA 94305.
Preliminary results were presented at the ASLMS annual conference in Grapevine, Texas on April 13, 2007.
References
- 1.Helidonis E, Sobol EN, Kavvalos G, Bizakis J, Christodoulou P, Velegrakis G, Segas J, Bagratashvili V. Laser shaping of composite cartilage grafts. Am J Otolaryngol. 1993;14:410–412. doi: 10.1016/0196-0709(93)90115-n. [DOI] [PubMed] [Google Scholar]
- 2.Sobol E, Sviridov A, Omel'chenko A, Bagratashvili V, Kitai M, Harding SE, Jones N, Jumel K, Mertig M, Pompe W, Ovchinnikov Y, Shekhter A, Svistushkin V. Laser reshaping of cartilage. Biotechnol Genet Eng Rev. 2000;17:539–564. doi: 10.1080/02648725.2000.10648005. [DOI] [PubMed] [Google Scholar]
- 3.Keefe MW, Rasouli A, Telenkov SA, Karamzadeh AM, Milner TE, Crumley RL, Wong BJ. Radiofrequency cartilage reshaping: Efficacy, biophysical measurements, and tissue viability. Arch Facial Plast Surg. 2003;5(1):46–52. doi: 10.1001/archfaci.5.1.46. [DOI] [PubMed] [Google Scholar]
- 4.Wright R, Protsenko DE, Diaz S, Ho K, Wong BJ. Shape retention in porcine and rabbit nasal septal cartilage using saline bath immersion and Nd:YAG laser irradiation. Laser Surg Med. 2005;37:201–209. doi: 10.1002/lsm.20212. [DOI] [PubMed] [Google Scholar]
- 5.Chao MC, Kim HK, Crumley RL, Wong BJ. Bipolar radiofrequency plasma-mediated ablation of porcine nasal septal cartilage: A pilot investigation. Am J Rhinol. 2005;19(5):488–494. [PubMed] [Google Scholar]
- 6.Gray DS, Kimball JA, Wong BJ. Shape retention in porcine-septal cartilage following Nd:YAG Laser-Mediated Reshaping. Laser Surg Med. 2001;29:160–164. doi: 10.1002/lsm.1104. [DOI] [PubMed] [Google Scholar]
- 7.Karam AM, Protsenko DE, Li C, Wright R, Liaw LH, Milner TE, Wong BJ. Long-term viability and mechanical behavior following laser cartilage reshaping. Arch Facial Plast Surg. 2006;8(2):105–116. doi: 10.1001/archfaci.8.2.105. [DOI] [PubMed] [Google Scholar]
- 8.Gaon MD, Ho KH, Wong BJ. Measurement of the elastic modulus of porcine septal cartilage specimens following Nd:YAG laser treatment. Laser Med Sci. 2003;18:148–153. doi: 10.1007/s10103-003-0275-5. [DOI] [PubMed] [Google Scholar]
- 9.Wong BJ, Milner TE, Kim HK, Nelson JS, Sobol EN. Stress relaxation of porcine septal cartilage during Nd:YAG laser irradiation: Mechanical, optical and thermal properties. J Biomed Opt. 1998;3:409–414. doi: 10.1117/1.429896. [DOI] [PubMed] [Google Scholar]
- 10.Protsenko DE, Zemek A, Wong BJ. Temperature dependent change in equilibrium elastic modulus after thermally induced stress relaxation in porcine septal cartilage. Laser Surg Med. 2008;40(3):202–210. doi: 10.1002/lsm.20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chae YS, Aguilar G, Lavernia EJ, Wong BJ. Characterization of temperature dependent mechanical behavior of cartilage. Laser Surg Med. 2003;32:271–278. doi: 10.1002/lsm.10167. [DOI] [PubMed] [Google Scholar]
- 12.Diaz SH, Nelson JS, Wong BJ. Rate process analysis of thermal damage in cartilage. Phys Med Biol. 2003;48(1):19–29. doi: 10.1088/0031-9155/48/1/302. [DOI] [PubMed] [Google Scholar]
- 13.Paun SH, Trenite GJ. Revision rhinoplasty: An overview of deformities and techniques. Facial Plast Surge. 2008;24(3):271–287. doi: 10.1055/s-0028-1083082. [DOI] [PubMed] [Google Scholar]
- 14.Thomson C, Mendelsohn M. Reducing the incidence of revision rhinoplasty. J Otolaryngol. 2007;36(2):130–134. doi: 10.2310/7070.2007.0012. [DOI] [PubMed] [Google Scholar]
- 15.Wong BJ, Chao KK, Kim HK, Chu EA, Dao X, Gaon M, Sun CH, Nelson JS. The porcine and lagomorph septal cartilages: Models for tissue engineering and morphologic cartilage research. Am J Rhinol. 2001;15(2):109–116. doi: 10.2500/105065801781543790. [DOI] [PubMed] [Google Scholar]
- 16.Youn JI, Telenkov SA, Kim E, Bhavaraju NC, Wong BJ, Valvano JW, Milner TE. Opitcal and thermal properties of nasal septal cartilage. Laser Surg Med. 2000;27:119–128. doi: 10.1002/1096-9101(2000)27:2<119::aid-lsm3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Protsenko DE, Zemek A, Chae YS, Wong B. Analysis of Nd:YAG laser-mediated thermal damage in rabbit nasal septal cartilage. Lasers Surg Med. 2007;39(5):451–457. doi: 10.1002/lsm.20514. [DOI] [PubMed] [Google Scholar]
- 18.Protsenko DE, Zemek A, Wong BJF. Simulation of laser-induced thermo-mechanical changes in tissue using RF heating method. Prog Biomed Opt Imaging Proc SPIE. 2007;6440:27. [Google Scholar]
- 19.Bagratashvili VN, Sobol EN, Sviridov AP, Popov VK, Omelchenko AI, Howdle SM. Thermal and diffusion processes in laser-induced stress relaxation and reshaping of cartilage. J Biomech. 1997;30(8):813–817. doi: 10.1016/s0021-9290(97)00028-6. [DOI] [PubMed] [Google Scholar]


