Abstract
In Central America, the traditional temazcales or wood-fired steam baths, commonly used by many Native American populations, are often heated by wood fires with little ventilation, and this use results in high wood smoke exposure. Urinary mutagenicity has been previously employed as a non-invasive biomarker of human exposure to combustion emissions. This study examined the urinary mutagenicity in 19 indigenous Mayan families from the highlands of Guatemala who regularly use temazcales (N = 32), as well as control (unexposed) individuals from the same population (N = 9). Urine samples collected before and after temazcal exposure were enzymatically deconjugated and extracted using solid-phase extraction. The creatinine-adjusted mutagenic potency of urine extracts was assessed using the plate-incorporation version of the Salmonella mutagenicity assay with strain YG1041 in the presence of exogenous metabolic activation. The post-exposure mutagenic potency of urine extracts were, on average, 1.7-fold higher than pre-exposure samples (P < 0.005) and also significantly more mutagenic than the control samples (P < 0.05). Exhaled carbon monoxide (CO) was ~10 times higher following temazcal use (P < 0.0001), and both CO level and time spent in temazcal were positively associated with urinary mutagenic potency (i.e. P < 0.0001 and P = 0.01, respectively). Thus, the wood smoke exposure associated with temazcal use contributes to increased excretion of conjugated mutagenic metabolites. Moreover, urinary mutagenic potency is correlated with other metrics of exposure (i.e. exhaled CO, duration of exposure). Since urinary mutagenicity is a biomarker associated with genetic damage, temazcal use may therefore be expected to contribute to an increased risk of DNA damage and mutation, effects associated with the initiation of cancer.
Introduction
Wood smoke and wood smoke extract, which contain a complex mixture of substances including polycyclic aromatic hydrocarbons (PAHs), formaldehyde and respirable particulate matter (1), are mutagenic and genotoxic in bacterial and mammalian cells (2–9), carcinogenic in experimental animals (10,11) and, in epidemiologic studies, positively associated with increased human lung cancer incidence (12). Indoor air containing emissions from the combustion of biomass fuel (primarily wood) has been classified by the International Agency for Research on Cancer as a Group 2A carcinogen (i.e. probably carcinogenic to humans) (1). Exposure to the particulate portion of combustion emissions has also been associated with adverse health effects such as pulmonary injury, upper respiratory tract infection, asthma, chronic obstructive pulmonary disease, low birth-weight and infant mortality (for a review, see Naeher et al. (13)).
Existing monitoring methods for wood smoke exposure assessment include personal exposure devices for particulate matter, monitoring of exhaled or blood carbon monoxide (CO) levels and monitoring of urinary concentrations of various components of wood smoke such as PAHs (or their metabolites), potassium, methoxyphenols and levoglucosan (for a review see Simpson and Naeher) (14). Urinary methoxyphenol concentration was assessed in individuals from the same study population as that examined in the current work, comparing levels between individuals who use open fires for cooking versus vented wood stoves (i.e. planchas) (15). Urinary methoxyphenol levels were significantly increased in the individuals using open fires, relative to those that used planchas, indicating that this biomarker is effective for quantifying exposures to inhaled wood smoke. However, biomarkers such as urinary methoxyphenols and levoglucosan require a priori knowledge of components in wood smoke, as well as reliable information on metabolism and urinary excretion. Although promising, since levoglucosan is solely produced by the combustion of cellulose, which is a major component of biomass combustion emissions (16,17), urinary levoglucosan levels are also affected by dietary consumption of smoked or grilled meats and caramelized sugars (18). Additionally, from a genetic toxicology perspective, individual chemical biomarkers cannot provide an integrated appraisal of total exposure to agents capable of causing genetic damage, an effect related to the initiation of tumour formation. It has recently been recognized that new methods for biological monitoring of wood smoke exposure are required in order to fill knowledge gaps relating to determination of (i) safe exposure levels and (ii) regulatory options for control of biomass combustion emissions (13,14,19). An ideal biomarker would be specific to wood smoke and/or biomass combustion emissions, be non-invasive, be able to suitably account for variations in exposure magnitude and differences in individual susceptibility and not require a priori knowledge of wood smoke composition (should that information not be available).
Urinary excretion of mutagens, as detected using the Ames/Salmonella reverse mutation assay, has previously been shown to be a useful biomarker of human exposure to combustion emissions, including wood smoke (20–23) and polluted ambient air (24,25). Kato et al. (21) examined urinary mutagenicity levels in employees of a charcoal plant and demonstrated that urinary mutagenicity was associated with wood smoke exposure level (estimated based on task), as well as genetic damage (i.e. DNA adducts in urothelial cells). Assessment of urinary mutagenic activity is non-invasive, does not require a priori knowledge about the components of wood/biomass smoke, and can be effectively used to investigate interindividual variability. Although extrapolations from urinary mutagenicity to risk of adverse effect are tenuous, this biomarker has thus far been shown to be effective for quantitative assessment of occupational and/or environmental exposures to mutagenic combustion emissions.
A particular form of the traditional temazcal (i.e. wood-fired steam baths) used by Native American groups are routinely used by indigenous populations in Central America, including an estimated 1–2 million indigenous Mayan people in the western highlands of Guatemala (26). The temazcales used by these populations are typically small (~5.5 m3), minimally ventilated rooms (<0.5 air changes per hour) that are heated by burning a wood fire inside the structure to warm volcanic rocks (26) (Figure 1). Users enter the temazcal once the fire has reduced to smoke-emitting embers, which are traditionally left inside the structure during use. A recent survey of 97 households in the western highlands of Guatemala showed that >90% of respondents use the temazcal once or twice per week, and 4% use it as frequently as three times per week, typically for hygienic or ceremonial purposes (26). Only 4% of surveyed individuals indicated that they use the temazcal less than twice per month. A second study of the same population found that the average CO concentration in the temazcal during use was 661±62 ppm, with a range from <7 ppm to >1980 ppm (27). Average exhaled (alveolar) CO levels in adults following temazcal was 61 ppm, and ranged from 3 to 230 ppm (26). These environmental and biological concentrations are high enough to pose a risk of acute effects from CO poisoning.
Fig. 1.
The interior of a traditional temazcal used by the study population. From Thompson et al. (26), reproduced with permission.
This study evaluated urinary mutagenicity in adults and children of Mayan families from the same highland area in Guatemala as that examined in the aforementioned studies (26,27). The population of temazcal users constitutes a well-defined group of individuals exposed to emissions from wood combustion, and the study employed urinary mutagenicity to evaluate exposures to mutagenic combustion emissions associated with temazcal use.
Materials and methods
Study population identification and sample collection
Study subjects were 41 individuals from 20 Mam-speaking indigenous Mayan families from the western highlands of Guatemala. The study population included 17 children (ages 2–15), 15 adult females (ages 16–53) and 9 adult males (ages 18–69; Table I). The study included both exposed subjects, who regularly use traditional temazcales (N = 32), as well as control individuals from the same population who do not use temazcales (N = 9; Table I). All participants were healthy at the time of enrolment. Study subjects completed a questionnaire that captured information on demographics and exposure frequency. Usually, families use temazcales before going to bed at night. All study subjects were non-smokers. Urine samples were collected in the morning (i.e. first morning void) the day of temazcal use (i.e. pre-exposure sample), as well as in the morning the day following temazcal exposure (i.e. post-exposure sample). For each exposure, the amount of time spent in the temazcal was recorded by the head of the household. Control urine samples were collected from subjects in the same population. Urine volume was measured and recorded by field staff, and the samples were subsequently aliquoted, individually barcoded to ensure confidentiality and objective analyses and frozen at −80°C until analysis. All aspects of this study conform to standards and practices outlined in the protocol approved by the Research Ethics Board of Health Canada and the Committee for Protection of Human Subjects at the University of California, Berkeley. Informed consent and assent forms were signed by study participants prior to sample collection.
Table I.
Description of study participants
| Exposure condition | Total subjects | No. families | No. individuals (age range) | ||
|---|---|---|---|---|---|
| Adult females | Adult males | Childern (≤15 years) | |||
| Unexposed (control) | 9 | 3 | 3 | 1 | 5 |
| Exposed | 32 | 17 | 11 | 9 | 12 |
| Total | 41 | 20 | 14 (16–53) | 10 (18–69) | 17 (2–15) |
Exhaled CO analysis
Exhaled CO levels (expressed as ppm) were measured both pre- and post-exposure (i.e. immediately before and after temazcal use), as described in (27). Briefly, a MicroCO electrochemical breath monitor (MicroDirect, Lewiston, ME, USA) was employed to measure CO concentrations in exhaled breath. Study subjects first held their breath for 20 s, and then exhaled continuously and completely. Three replicate measurements were taken per individual, with 1–2min between attempts. The average of three successful attempts is reported. MicroCO breath monitors were calibrated using a laboratory standard each week during the study period.
Hydrolysis and extraction of urine samples
Coded urine samples (~50ml) were thawed and filtered to remove urothelial cells. The exact volume of each sample was recorded, and the samples enzymatically deconjugated in 0.2M (10% v/v) sodium acetate buffer (pH 5.0) (Sigma–Aldrich Canada, Oakville, Ontario, Canada), containing β-glucuronidase (6 units/ml urine; Sigma–Aldrich Canada) and sulphatase (2 units/ml urine; Sigma–Aldrich Canada) for 16h at 37°C. Deconjugated urinary metabolites were then concentrated using solid-phase extraction on C18 silica (Avantor Performance Materials, Center Valley, PA, USA) with methanol elution (EMD Chemicals, Billerica, MA, USA) as described previously (21,28). The resulting extracts were reconstituted in dimethylsulphoxide (DMSO; Sigma–Aldrich Canada) to produce urine extracts suitable for mutagenicity assessment. Extracts were stored at 4°C until use.
Mutagenicity assessment of urine extracts
The mutagenic activity of urine extracts was assessed using the standard plate-incorporation version of the Ames/Salmonella reverse mutation assay with Salmonella strain YG1041(obtained from Dr Takehiko Nohmi) and Aroclor-induced rat liver S9 (i.e. S9, Molecular Toxicology Inc., Boone, NC), as described in Mortelmans and Zeiger (29). These conditions were selected based on the results published by Kato et al. (21), which showed that the combination of strain YG1041, a metabolically enhanced version of the frameshift-detecting strain TA98, and Aroclor-induced S9 yielded the highest response for urine extracts from wood smoke-exposed individuals. An initial range-finding test showed that some of the post-exposure extracts were cytotoxic at 12-ml equivalents per plate (data not shown); therefore, the concentrations selected for mutagenicity testing were 0.3, 0.6, 1.2, 3.0 and 6.0 ml-equivalent urine per plate. All concentrations were tested in duplicate. A simultaneous positive control (i.e. 0.1 µg/plate 2-aminoanthracene, Molecular Toxicology Inc.) and negative solvent control (i.e. DMSO) were used on each day of mutagenicity testing. Following a 72-h incubation at 37°C, the frequency of mutant (i.e. revertant; rev) colonies was scored using a ProtoCol automated colony counter (Synbiosis Corporation, Exton, PA, USA). Mean positive control values were 1857.4±104.7 rev/plate, and mean negative control values were 58.5±3.2 rev/plate. Mutagenic potency was calculated as the slope of the linear portion of the concentration-response function as described below, and expressed as revertants/ml-equivalent urine.
Urine samples were adjusted for creatinine concentration in order to reduce the effects of interindividual variability in glomerular filtration rate (i.e. urine dilution). Urinary creatinine analysis was conducted using a colorimetric assay (Oxford Biomedical, Abingdon, UK) and levels employed to correct for urinary flow rate. Mutagenic potency values corrected for creatinine concentration were expressed as revertants/µmole urinary creatinine. When comparing creatinine-adjusted urinary mutagenic potency with other metrics (e.g. time in temazcal), particular attention was paid to the age of the subjects (e.g. adults versus children), since urinary creatinine levels have been shown to be dependent on both body mass and age. Consequently, comparisons of creatinine-adjusted values between adults and children must be interpreted with caution (30,31). Individual subject mutagenic potency values are available from the first author upon request.
Statistical analyses
Statistical analyses employed SAS v9.2 for Windows (SAS Institute, Cary, NC, USA) and Microsoft Excel. Mutagenic potency values were determined using ordinary least squares linear regression analysis. A paired (dependent) t-test was used to evaluate the treatment effect (i.e. pre- versus post-exposure) for unadjusted urinary mutagenic potency, creatinine-adjusted urinary mutagenic potency as well as exhaled CO (i.e. H0 that the mean difference between pre- and post-exposure is zero). The test assumes that the dependant variable (e.g. mutagenic potency) is continuous, that the subjects are matched, and the differences between the pre- and post-exposure measurements are independent and normally distributed. Normality of the difference values was confirmed using the Shapiro–Wilk test, the Kolmogorov–Smirnov test and visual inspection of a normal probability plot. Single-factor analysis of variance (ANOVA) was also employed to examine differences between the exposure groups (i.e. pre-exposure and post-exposure) and the control group for unadjusted urinary mutagenic potency, creatinine-adjusted urinary mutagenic potency and exhaled CO. Least squares linear regression was also employed to investigate empirical relationships between mutagenic potency and other variables that reflect the magnitude of the exposure (e.g. exhaled CO level, time spent in temazcal), as well as between exhaled CO level and time spent in temazcal. Analysis of the studentised deleted residuals (32) was employed to detect outliers from the empirical relationships between urinary mutagenicity and exposure time, and between urinary mutagenicity and exhaled CO. Empirical relationships between the various variables investigated (e.g. creatinine-adjusted urinary mutagenic potency, subject age, time spent in temazcal and exhaled CO) were investigated using ordinary least squared linear regression and analysis of covariance (ANCOVA). The analysis assumes that disturbance term µ i (also known as the residuals) are independent and normally distributed with mean of zero and constant variance of σ 2. The assumption of independence is easily met since each observation represents a different subject. The assumption of normality was examined using the Shapiro–Wilk test, the Kolmogorov–Smirnov test and visual inspection of a normal probability plot.
Results
Urinary mutagenic potency
All control and post-exposure urine extracts induced a concentration-dependent mutagenicity response in Salmonella YG1041, as did all but three of the pre-exposure extracts. Substantial cytotoxicity was observed in some samples at 3- and 6-ml equivalent concentrations, several samples also exhibited toxicity at 1.2-ml equivalent urine, and a few samples exhibited toxicity at 0.6-ml equivalent urine. One sample elicited toxicity at all concentrations above 0.3-ml equivalent urine. Consequently, a mutagenic potency could not be calculated for this sample and it was excluded from further analyses. Crude mutagenic potencies ranged from 0 (i.e. no significant response compared with solvent control) to 120 rev/ml-equivalent urine. Adjustment of urinary mutagenicity for variations in creatinine concentration permits control for variations in glomerular filtration rate across subjects and reduces interperson variability (31,33,34). Creatinine data were not available for two post-exposure samples, and therefore creatinine-adjusted mutagenic potencies could not be calculated for these samples. Creatinine-adjusted mutagenic potencies calculated for all samples ranged from 0 to 3.06 rev/µmol urinary creatinine.
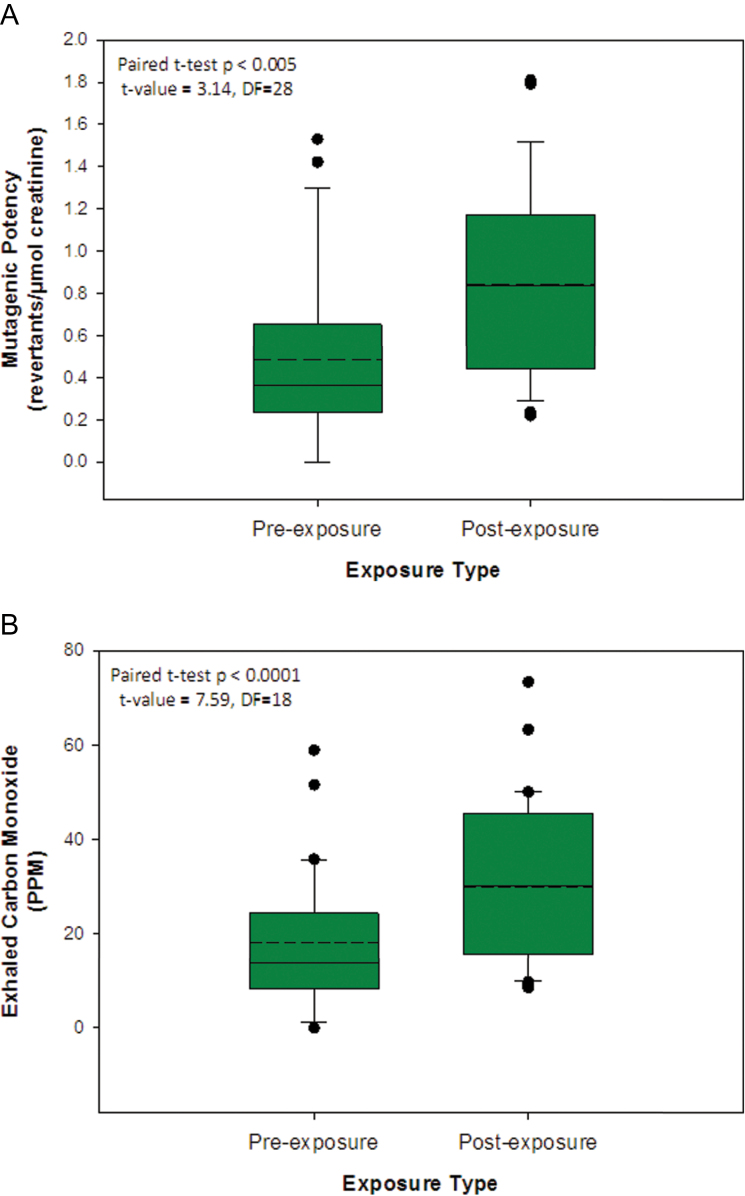
A paired t-test was conducted on creatinine-adjusted mutagenic potencies to compare pre- and post-exposure urinary mutagenicity in the same individual. This type of analysis requires a pre- and post-exposure value for each individual, and, as a result, one individual was removed from the analysis since the post-exposure mutagenic potency could not be calculated due to toxicity. Two additional individuals were removed from the analysis since there was no creatinine information for the post-exposure samples (see above). The results revealed a significant 1.74-fold increase (P < 0.005) between the urinary mutagenicity of the pre- and post-exposure samples (Figure 2A). Crude (i.e. unadjusted) urinary mutagenic potencies were also analysed in this manner, and the results also revealed a significant difference between the pre-exposure and post-exposure samples (1.66-fold post-exposure increase, P < 0.007). As noted, one individual was removed from the analysis because of sample toxicity. Scrutiny of the distribution of the difference values (i.e. between pre- and post-exposure) did not reveal any significant deviations from normality (i.e. P > 0.1). Nevertheless, for completeness, the data were also analysed using the non-parametric paired sample Wilcoxon-signed rank test. The results revealed a significant treatment effects for both the unadjusted (crude) mutagenic potency values (P < 0.005) as well as the creatinine-adjusted potency values (P < 0.003). All additional analyses were only conducted on creatinine-adjusted values.
Fig. 2.
(A) The distribution of creatinine-adjusted urinary mutagenic potency values for individuals examined the morning prior to temazcal use (i.e. pre-exposure) and the morning following temazcal use (i.e. post-exposure). Matched pre- and post-exposure data were available for 29 individuals. Post-exposure data were not available for individuals #431 and #512 (lack of creatinine data), as well as #1010 (toxicity). The box represents the interquartile range, the dashed lines denote mean values, the solid lines denote medians and the whiskers denote the 10th and 90th percentiles. (B) The distribution of exhaled CO concentration for individuals examined immediately prior to temazcal use (i.e. pre-exposure) and immediately following temazcal use (i.e. post-exposure). Matched pre- and post-exposure data were available for 19 individuals. There were data missing for either the pre- or post-exposure CO measurements (or both) for the following subject IDs: 113, 220, 222, 223, 312, 321, 431, 713, 1025, 430, 814 and 960. The box represents the interquartile range, the dashed lines denote mean values, the solid lines denote medians and the whiskers denote the 10th and 90th percentiles.
In order to compare the exposure groups with the control group, one-way ANOVA was conducted on creatinine-adjusted data (i.e. pre-exposure versus control and post-exposure versus control). This analysis revealed a significant difference (P < 0.05) between the control and post-exposure group (Table II). The mean urinary mutagenic potency for the post-exposure group was, on average, 1.7-fold greater than the control (unexposed) group (Table II). There was no statistically significant difference between the mutagenic potency of the unexposed controls and the pre-exposure samples (Table II). In addition, there was no significant difference between the post-exposure urinary mutagenic potency of adults versus children (Table III).
Table II.
Summary of creatinine-adjusted urinary mutagenic potency and exhaled CO levels for unexposed and exposed subjects (both pre- and post-exposure)
| Exposure condition | Urinary mutagenic potency (revertants/µmol creatinine) | Exhaled carbon moxidea (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Range | Mean (±SE) | Fold change (wrt child) | P-value | N | Range | Mean (±SE) | Fold change (wrt child) | P-value | |
| Unexposed (control) | 9 | 0.17–1.13 | 0.51 (0.11) | – | – | 2 | 4.0–5.5 | 4.8 (0.8) | – | – |
| Pre-exposure | 32 | 0–1.53 | 0.49 (0.07) | 1.0 | NS | 23 | 1.5–8.0 | 4.3 (0.3) | 0.9 | NS |
| Post-exposure | 29b | 0.22–1.81 | 0.84 (0.08) | 1.7 | <0.05 | 20 | 17.7–166.0 | 55.9 (8.1) | 11.8 | NS |
SE, standard error; NS, not significant; wrt, with respect to. A one-way ANOVA was employed to compare the pre-exposure and post-exposure groups with control individuals.
aCarbon monoxide data were not available for all individuals.
bThree samples were removed from this group. Subject ID #431 (M) and 512 (F) lacked creatinine data. Subject ID #1010 (F) was removed due to toxicity.
Table III.
Summary of exposure duration, post-exposure exhaled CO levels and post-exposure creatinine-adjusted urinary mutagenic potency for adults and children
| Study subject | Duration of exposurea (min) | Exhaled carbon monoxideb (post-exposure; ppm) | Urinary mutagenic potencyc (post-exposure; rev/µmol creatinine) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (±SE) | Range | Fold change (wrt child) | N | Mean (±SE) | Range | Fold change (wrt child) | N | Mean (±SE) | Range | Fold change (wrt child) | ||
| Age group | Child (≤15 years) | 8 | 29.5 (2.8) | 17.0–37.0 | – | 6 | 61.1 (14.1) | 30.0–111.0 | – | 12 | 0.92 (0.15) | 0.33–1.81 | – |
| Adult | 15 | 40.6 (6.5) | 11.0–98.0 | 1.4 | 14 | 53.6 (10.1) | 17.7–166.0 | 0.9 | 17 | 0.79 (0.10) | 0.22–1.52 | 0.9 | |
SE, standard error; NS, not significant; wrt, with respect to. Exposure duration and exhaled CO data were not collected for all study subjects, and analyses were conducted using all available data. Creatinine values were missing for two individuals in the post-exposure group, and one post-exposure urinary mutagenic potency value was excluded due to toxicity.
aExposure duration data were not available for four children (Subject ID #s 712, 713, 1012 and 1025), three adult females (#s 710, 920 and 1010) and two adult males (#s 721 and 911).
bCO data were not available for all individuals.
cThree post-exposure urinary mutagenic potency data were not available. Subject ID #431 (M) & 512 (F) lacked creatinine data. Subject ID #1010 (F) was removed due to toxicity.
Duration of temazcal exposure
The self-reported duration of temazcal use varied quite widely from only 11min (child) to 98min (adult; Table III). The mean duration time for temazcal use across all subjects was 37±4min, with the adults using the temazcal for almost 50% longer than the children (1.4-fold; Table III).
Exhaled CO levels
Pre- and post-exposure CO measurements were available for 19 individuals and these data were also analysed using a paired t-test. The results indicate that the use of temazcales is associated with significant modification of exhaled CO level. More specifically, there was a statistically significant increase in exhaled CO (ppm) following temazcal use relative to pre-exposure measurement (P < 0.0001; Figure 2B).
Pre-exposure exhaled CO values ranged from 1.5 ppm to 8.0 ppm (mean = 4.3 ppm), and post-exposure values ranged from 17.7 ppm to 166.0 ppm (mean = 55.9 ppm; Table II). One-way ANOVAs were conducted in order to compare the exposure measurements (i.e. pre- and post-exposure) with the control group; however, there was no significant difference in either case. The lack of significant difference between the control and post-exposure group is likely a consequence of the limited control data and lack of statistical power (i.e. only two control individuals with CO measurements, P = 0.065). Nevertheless, the mean exhaled CO for the post-exposure group was 11.8-fold greater than the mean control, whereas the pre-exposure group had roughly the same exhaled CO as the control group (Table II). Exhaled CO levels following temazcal use (i.e. post-exposure) were not significantly different between adults and children (Table III).
Relationships between exposure metrics
Analysis of the studentised deleted residuals from the mutagenic potency versus CO relationship revealed statistically significant outliers at P < 0.05, and these observations were removed from subsequent analyses (i.e. both the pre- and post- observations for individual #812, the pre-observation for #911, and the post-observation for #611). Urinary mutagenic potency was found to be empirically related to CO level (P < 0.0001, r 2 = 0.53), with increased CO associated with increases in urinary mutagenicity (Figure 3). This relationship was improved when only the data from the adults were included (P < 0.0001, r 2 = 0.57; supplementary Figure 1, available at Mutagenesis Online). Additionally, post-exposure urinary mutagenicity was found to be positively associated with the amount of time that each individual spent in the temazcal (P = 0.01, r 2 = 0.19; Figure 4), and again the relationship was improved when only the data from the adults were included (P < 0.005, r 2 = 0.44; supplementary Figure 2, available at Mutagenesis Online). Exhaled CO levels were also found to be positively associated with exposure duration (i.e. time spent in the temazcal; P < 0.005, r 2 = 0.57; Figure 5), and this relationship was also improved when only adult data were included (P < 0.0005, r 2 = 0.82; supplementary Figure 3, available at Mutagenesis Online).
Fig. 3.
The relationship between urinary mutagenicity, expressed as creatinine-adjusted potency, and exhaled CO concentration for control, pre-exposure and post-exposure samples. Thirty-nine observations had both creatinine-adjusted mutagenic potency and exhaled CO data. Four observations were determined to be statistical outliers and excluded from the analysis (i.e. both the pre- and post- observations for individual #812, the pre- observation for #911 and the post-observation for #611). Coefficient (i.e. slope) of the CO effect = 0.0071±0.0011. M = adult male, F = adult female, C = child. RMS = root mean square error.
Fig. 4.
The relationship between urinary mutagenicity, expressed as creatinine-adjusted potency and time spent in temazcal (i.e. exposure duration) for control and post-exposure samples. Thirty individuals had both creatinine-adjusted mutagenic potency and exposure duration data. Coefficient (i.e. slope) of the time effect = 0.0082±0.0032. M = adult male, F = adult female, C = child. RMS = root mean square error.
Fig. 5.
The relationship between exhaled CO, expressed as ppm and time spent in temazcal (i.e. exposure duration) for control and post-exposure samples. Fifteen individuals had both exhaled CO and exposure duration data. Coefficient (i.e. slope) of the time effect = 1.45±0.35. M = adult male, F = adult female, C = child. RMS = root mean square error.
ANCOVA examining the effects of both subject classification (i.e. adults versus children ≤15 years old) and time in temazcal revealed significant effects of both subject classification and time, with homogeneous slopes (i.e. no interaction between subject classification and time in temazcal). The results revealed a significant model with r 2 = 0.36 (N = 30) and F ratio = 7.67 (P < 0.005). The subject classification effect was significant at P < 0.05 (F = 7.14) with adult creatinine-adjusted mutagenic potency values, on average, 0.4 units lower than children. The time effect was significant at P < 0.005 (F = 12.2) with coefficient (i.e. slope) = 0.010±0.003. This coefficient is not significantly different from the slopes of the relationships shown in Figure 4 and supplementary Figure 2, available at Mutagenesis Online. Scrutiny of the distribution of the residuals for each of the aforementioned regression or ANCOVA models did not reveal any serious deviations from normality. Nevertheless, for the regression of post-exposure creatinine-adjusted mutagenic potency versus time, analysis of the residuals revealed the H0 of normality could be rejected at P = 0.06. Additional analyses using log10 transformed mutagenic potency revealed similar results (i.e. time effect with P<0.02) and residuals that showed no evidence of deviation from normality (Shapiro–Wilk P = 0.55).
Discussion
This study examined urinary mutagenicity in individuals exposed to wood combustion emissions during the use of traditional temazcales; moreover, empirical relationships between urinary mutagenic activity and other measures of wood smoke exposure (e.g. exhaled CO and time spent in the temazcal).
Urinary mutagenic potency
All urine extracts, with the exception of three pre-exposure samples, induced a significant mutagenic response in Salmonella strain YG1041 with S9 metabolic activation, and this is consistent with the aforementioned study by Kato et al. (21). YG1041, which overexpresses the Salmonella classical nitroreductase and O-acteytltransferase, is known to display increased sensitivity to nitroarenes and aromatic amines (35). Therefore, since extracts of urine from wood smoke exposed individuals have been previously shown to elicit a stronger response on YG1041, relative to its parent strain TA98, it seems likely that the extracts of deconjugated urinary metabolites contain aromatic amines as well as metabolites of homocyclic, unsubstituted PAHs. Indeed, studies have shown that the urine of individuals exposed to PAH-containing combustion emissions (i.e. wood smoke and cigarette smoke) contain metabolites of aromatic amines such as o- and p-toluidine, and 2-aminonapthalene, as well as metabolites of unsubstituted PAHs including 2-napthol and 1-hydroxypyrene (20,21,24,36,37). Furthermore, enhanced responses in the presence of S9 in this and other studies (20,21,23,24) are consistent with the excretion of PAHs and PAH metabolites that require metabolic activation to elicit a positive response in the Ames/Salmonella reverse mutation assay (38).
Toxicity was observed in some samples at concentrations of 6.0- and 12.0-ml equivalents of urine (per plate) and for one sample at a concentration of 3.0-ml equivalent urine. This latter sample was a post-exposure sample from an adult female with an exhaled CO value of 60 ppm, which is the highest exhaled CO value recorded for post-exposure adult women in this study. Toxicity of urine extracts was also observed in the Kato et al. (21) study of wood smoke exposed charcoal production workers, and the observation of cytotoxicity is consistent with studies that have noted the acute cytotoxicity of wood smoke condensates (39). Toxicity may also be unrelated to wood smoke exposure.
The range of urinary mutagenicity values in the current study was low relative to values presented in the Kato et al. (21) study. The mean mutagenic potency value recorded for adults in the current study (i.e. 0.79 rev/µmol creatinine) is ~5.5-fold lower than the mean value for the high exposure group (i.e. 4.5 rev/µmol creatinine), and ~3-fold lower than the mean value for the low exposure group in the Kato et al. study (21). This difference may not be surprising since the individuals in the Kato et al. (21) study were chronically exposed to wood smoke in an occupational setting, and the samples were collected after 3 days of exposure (i.e. third day of the work week). In contrast, the current study examined individuals exposed to high levels of wood smoke for brief periods of time (i.e. mean exposure time of 37min) with urine samples collected the morning following the exposure. Additionally, the individuals in the Kato et al. (21) study were exposed during occupational activities that would be expected to increase respiration rate and exposure level, in comparison with the individuals in our study who were exposed while seated in the temazcal.
There were only two studies that examined urinary mutagenicity in smokers using the same strain of bacteria (i.e. YG1041), and in both cases the mean value obtained following wood smoke exposure in our study was much lower than those obtained for the smokers. The Kato et al. (21) study examined urinary mutagenicity in smokers who worked at the charcoal plant and reported a mean of 4.23 rev/µmol creatinine. A second study examined urinary mutagenicity in smokers who worked in the rubber manufacturing industry and reported a mean of 4.43 rev/µmol creatinine (i.e. 39148 rev/g creatinine) (40). However, both of these studies examined urinary mutagenicity in smokers who were also occupationally exposed, and therefore it is difficult to compare the levels of urinary mutagenicity in our study, to those that would be obtained strictly from smoking.
The absence of a significant difference between the urinary mutagenicity of the unexposed control samples and the pre-exposure samples suggests that the time interval between successive temazcal exposures (usually one week) was sufficient to permit the levels of urinary mutagenicity to return to background. However, since temazcal use is not consistent among all study participants (26) and can vary from 1 to 3 times per week, it was not possible to precisely determine the elapsed time between each exposure. Nevertheless, we contend that the effect of temazcal use, and the effect of the ensuing wood smoke exposure on urinary mutagenicity, is not persistent, and without consecutive daily exposures the urinary mutagenicity levels return to background. This contention is consistent with experimental observations of PAH excretion kinetics. For example, a study of F-344 rats orally exposed to BaP noted that maximum recovery of BaP metabolites in urine occurred within 48h after administration (41), and 15–25% of metabolites were recovered between 8 and 16h following administration (41). In addition, studies of Sprague–Dawley rats exposed to pyrene demonstrated that 60% of the original pyrene dose was recovered in urine 24h after the last dose, and about 35% of the dose was recovered at 10h post-exposure (42). The latter study also examined the influence of mixtures of PAHs (i.e. BaP, naphthalene and pyrene) on the excretion of pyrene and found that simple PAH mixtures did not influence excretion kinetics (42). More complex PAH mixtures, such as those that would be present in an environmental or occupational setting (e.g., wood smoke), were not examined. Importantly, another study found that the fraction of the original dose recovered in urine as 1-hydroxypyrene (mainly as sulpho-conjugates) was not affected by exposure route or dose and that there was a linear dose-excretion relationship (43) Thus, although available toxicokinetic information confirms that the urine extracts examined in this study would be expected to contain a diverse array of deconjugated metabolites from the investigated exposure, the toxicokinetics of combustion by-products such as pyrene suggest that the delay between the acute (i.e. single brief event) wood smoke exposure and the urine collection employed here may not have been optimal for detection of the peak in urinary mutagenicity. Future studies should ideally examine the kinetics of urinary mutagen excretion following discrete temazcal exposures.
In this study, we were able to detect a significant difference in urinary mutagenicity levels between pre- and post-exposure samples, as well as between the control group and post-exposure samples, without controlling for other sources of combustion exposures (i.e. wood stoves). Many of the subjects in this study would use wood stoves at home for cooking, and this would be expected to contribute to the total wood smoke exposure (44). Thus, the results obtained here indicate that urinary mutagenicity is a sensitive biomarker of single acute exposure events, and the exposure metrics appears to be sufficiently robust such that a correction for confounding exposures was not required to reveal a statistically significant relationship between exposure status/duration and urinary mutagenic activity. Nevertheless, control for confounding exposures would most likely strengthen the empirical relationships investigated (e.g. Figure 4, supplementary Figure 2, available at Mutagenesis Online).
Previous studies examining urinary mutagenicity have largely compared groups of individuals who were categorised into exposure groups based on their lifestyle (e.g. smoking status and area of residence) or occupational settings (21–24,45–48). Industrial exposures were examined by comparing work week urine samples with weekend/home urine samples (49–52) or simply by comparing individuals from the control populations to individuals from the exposed populations (20,53–56). In our study, we examined the same individuals before and after a single brief exposure event. This type of paired exposure design is advantageous since it is not prone to variations in the exposure metric attributable to interindividual variability. Only four other studies employed this type of study design to successfully detect an increase in urinary mutagenicity following a single exposure event, with one examining the effects of fried pork ingestion (57), one study looking at non-smoker’s urinary mutagenicity after smoking cigarettes (58) and two studies examining the effects of passive smoking on urinary mutagenicity (59,60). To our knowledge, this study constitutes the first use of a paired exposure design to show a significant increase in urinary mutagenicity following a single brief exposure to a wood smoke-contaminated aerosol.
Relationships between exposure metrics
Levels of urinary mutagenicity in individuals who used traditional temazcales was found to be significantly related to both the duration of wood smoke exposure (i.e. time spent in temazcal) and the exhaled CO measured following temazcal use. The fact that the post-exposure urinary mutagenic potency was significantly correlated with the time spent in the temazcal demonstrates that urinary mutagenicity is significantly augmented by the exposure that occurs during the use of traditional temazcales. Although the level of urinary mutagenicity is clearly influenced by other, hitherto unidentified factors, the observed empirical relationship permits the contention that urinary mutagenicity is a useful biomarker of exposure to combustion emissions associated with the use of traditional temazcales, particularly for adults.
Levels of urinary mutagenicity were also found to be significantly associated with exhaled CO levels (i.e. r 2 = 0.53 for all individuals, and r 2 = 0.57 for adults only; Figure 3, supplementary Figure 1, available at Mutagenesis Online). CO has been previously used as a biomarker of wood smoke exposure since it is known to be directly associated with inhalation of wood smoke (13,61–67). In our study, exhaled CO levels were also shown to be significantly associated with the exposure duration (Figure 5, supplementary Figure 3, available at Mutagenesis Online). However, CO levels do not provide an indication of the magnitude of the exposure to mutagenic and/or carcinogenic compounds that are known to be present in wood smoke and, moreover, known to augment the risk of tumour formation. Significant association of urinary mutagenicity with exhaled CO increases confidence in the use of urinary mutagenicity as an effective biomarker of wood smoke exposure; moreover, as a biomarker that may be used to indicate an increased risk of adverse health effects associated with exposures to environmental mutagens.
Although the paired exposure design controls for interindividual variability that could be attributed to susceptibility factors (i.e. genetic polymorphisms), it cannot account for confounding exposures that take place before or after temazcal use, nor when comparing post-exposure values to other exposure metrics. Genetic polymorphisms relating to the metabolism of combustion by-products could potentially be a factor in the individuals identified as statistical outliers. The Kato et al. (21) study noted that the urinary excretion of 2-naphthol and 1-pyrenol, PAH metabolites that have been employed as biomarkers of wood smoke exposure, was significantly elevated in wood smoke exposed individuals with the GSTM1 null genotype. It was also previously determined that in Polish children living in a region with high levels of and environmentally polluted air, polymorphisms in EPHX1 significantly affected both the 1-OHP concentration in urine, as well as urinary mutagenicity (68). Additionally, they determined that the GSTM1 null genotype or the deletion of GSTT1 both resulted in significantly increased levels of PAH-DNA adducts in the carriers. The authors also found that higher levels of PAH-DNA adducts were associated with individuals with the Lys/Lys genotype of the DNA repair gene XPD, in comparison with heterozygotes for this gene (68). Confounding exposures (e.g. wood stove use) were not controlled in this study; however, it should be noted that nearly all individuals in the examined population would be expected to experience additional wood smoke exposures in and around the home.
Children versus adults
In the population examined, it is common for an adult to enter the temazcal first to prepare the fire. The rest of the family enters afterwards, and, as a result, the children generally spend less time in the temazcal relative to adults. In our study, we noted that adults spent almost 50% more time in the temazcales in comparison with children (Table III); however, post-exposure levels of exhaled CO and the post-exposure urinary mutagenicity levels in adults were approximately the same as those of children (Table III). Moreover, the ANCOVA analysis revealed that for a given time in the temazcal, the creatinine-adjusted urinary mutagenic potency for children was on average 0.4 units greater than those of adults (P < 0.05). These results suggest that children may be more sensitive to wood smoke exposure, at least in terms of exhaled CO and urinary mutagenic activity. This contention is consistent with information in the literature pertaining to differences in the susceptibility of children and adults to the effects of environmental pollutants. More specifically, differences that can be attributed to well-known physiological differences that relate to body size, metabolic rate, organ size and metabolic capacity. For example, children can be exposed to disproportionately higher levels of environmental contaminants in aerosols, relative to adults, due to an elevated respiration rate (69–71); moreover, children have a larger liver (expressed per unit body weight) in comparison with adults (70). In the context of this study, an increased respiration rate would be expected to contribute to an elevated wood smoke exposure for a given exposure duration, relative to adults, and an increased liver size would be expected to permit increased metabolism and urinary excretion of wood smoke combustion by-products. Additionally, it is interesting to note that from the age of 6 months to 12 years, children are significantly more effective at clearing CYP1A2 substrates (72). Approximately 15% of the P450 content in human liver is CYP1A2, which is essential for the conversion of aromatic amines to hydroxyarylamines (73–75). This may result in a higher rate of metabolism and urinary excretion of selected combustion by-products, which is consistent with the observed increase, relative to adults, in urinary mutagenic potency observed for a given exposure time. The aforementioned results are also in agreement with those presented in Lam et al. (27), where it was noted that although children used the temazcal for half as long as adults, there was no significant difference in post-exposure exhaled CO level.
As mentioned above, the urinary creatinine correction may not be as appropriate for children as it is for adults and, moreover, for comparing results across age groups. Removing the children’s data from the correlation analyses resulted in improved coefficients of correlation for the creatinine-adjusted urinary mutagenic potency versus exhaled CO, and creatinine-adjusted urinary mutagenic potency versus time in temazcal (supplementary Figures 1 and 2, available at Mutagenesis Online). The data points for children show considerable scatter for the plot of creatinine-adjusted mutagenic potency versus time (Figure 4), even at zero time. It is well known that children excrete lower levels of creatinine than adults as a result of their lower lean muscle mass. In a large-scale study that analysed National Health and Nutrition Examination Survey data, the authors reported that children between the ages of 6–11 had urinary creatinine concentrations that were only 60% of those for ages 20–29 (i.e. 102.1mg/dl versus 161.8mg/dl) (31). Following creatinine adjustment, lower urinary creatinine concentrations in children would result in relatively higher creatinine-adjusted urinary mutagenic potency values, compared with adults. This may explain some of the scatter observed for the children’s data points in Figure 4, as there are a number of data points that fall above the regressed line. Additionally, the aforementioned physiological differences between adults and children may be contributing to the considerable scatter observed for the children’s data points. The children in our study range from 2–15 years of age. Not only do children vary in a number of physiological capacities from adults, but there is also considerable variability in these physiological parameters between different age groups of children as they develop. For example, for drug clearance data, children who are between the ages of 12–18 years do not statistically differ from adults; however, children from 2–12 years have significantly higher clearance rates than adults (72). Additionally, young children have a proportionally larger liver weigh-to-body weight ratio (in comparison with adults), but this discrepancy levels off after once the child reaches 6 years of age (70). The removal of the children’s data from the correlation of exhaled CO versus time in temazcal also resulted in an improved correlation coefficient. This may be a result of the physiological differences between adults and children, as discussed above. Finally, there were only 12 children in the exposure group (only eight with CO values) and five children in the control group (1 with CO values), so the small sample size may also be contributing to the scatter and is a limitation of this study. Future research with larger groups of children is required to better understand the effects of wood smoke exposure in children.
Conclusions
The results of this study demonstrate that urinary mutagenicity is a reliable and sensitive biomarker for assessment of single, acute exposures to wood smoke-derived combustion emissions in an enclosed space. Moreover, the metric can be readily employed in the absence of a priori information regarding chemical identity of the putative mutagens in the combustion emission (i.e. wood smoke). This study constitutes the first demonstration of the genotoxic hazards associated with temazcal use, and the first demonstration of a significant increase in urinary mutagenicity following a single acute wood smoke-contaminated aerosol exposure event.
Combustion emissions, including wood smoke, are known to be mutagenic in animals and humans (1), and an increase in urinary mutagenicity has previously been shown to be associated with genetic damage, such as DNA adducts in urothelial cells (28). Temazcal use may therefore result in an increased likelihood of genetic damage and mutations, which have been associated with initiation of tumour formation and carcinogenesis.
Due to poverty and the cultural significance of the temazcal, it may be difficult to immediately reduce wood smoke exposures associated with temazcal use. Working with this population to increase ventilation in the temazcales, heat the rocks outside the structure (as is common in other Native American populations) or remove the embers before entering, as well as reducing exposure time, would reduce the likelihood of harmful effects, particularly in children.
Supplementary data
Supplementary Figures 1–3 are available at Mutagenesis Online.
Funding
This work was supported by the US National Institute of Environmental Health Sciences (R01ES010178); Health Canada’s Regulatory Strategy for Biotechnology; and a travel grant from the UC Berkeley Center for Global Health to conduct field work.
Supplementary Material
Acknowledgements
We are grateful to Dr David DeMarini for his advice on the urine mutagenicity method implementation for this study; Eduardo Canuz for his assistance with air pollution and exposure measurements; Nishat Shaikh for her help with collection of urine samples; Dr Lisa Thompson for providing suggestions and comments during the design of the study, as well as providing the photograph of the interior of a temazcal; Dr Takehiko Nohmi (at the National Institute of Health Sciences, Japan) for providing the Ames YG1041 tester strain and Dr Jason O’Brien and Rebecca Maertens for their helpful comments while reviewing the manuscript. Finally, we are especially grateful to the subjects and field personnel for their participation.
Conflict of interest statement: None declared.
References
- 1. IARC. (2010). Household use of solid fuels and high-temperature frying. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 95 World Health Organization, Lyon, France, pp. 1–444 [PMC free article] [PubMed] [Google Scholar]
- 2. Hytönen S., Alfheim I., Sorsa M. (1983). Effect of emissions from residential wood stoves on SCE induction in CHO cells. Mutat. Res., 118, 69–75 [DOI] [PubMed] [Google Scholar]
- 3. Alfheim I., Ramdahl T. (1984). Contribution of wood combustion to indoor air pollution as measured by mutagenicity in Salmonella and polycyclic aromatic hydrocarbon concentration. Environ. Mutagen., 6, 121–130 [DOI] [PubMed] [Google Scholar]
- 4. Alfheim I., Becher G., Hongslo J. K., et al. (1984). Short-term bioassays of fractionated emission samples from wood combustion. Teratog. Carcinog. Mutagen., 4, 459–475 [DOI] [PubMed] [Google Scholar]
- 5. van Houdt J. J., Daenen C. M., Boleij J. S., Alink G. M. (1986). Contribution of wood stoves and fire places to mutagenic activity of airborne particulate matter inside homes. Mutat. Res., 171, 91–98 [DOI] [PubMed] [Google Scholar]
- 6. Lewis C. W., Baumgardner R. E., Stevens R. K., Claxton L. D., Lewtas J. (1988). Contribution of woodsmoke and motor vehicle emissions to ambient aerosol mutagenicity. Environ. Sci. Technol., 22, 968–971 [DOI] [PubMed] [Google Scholar]
- 7. Bell D. A., Kamens R. M. (1990). Evaluation of the mutagenicity of combustion particles from several common biomass fuels in the Ames/Salmonella microsome test. Mutat. Res., 245, 177–183 [DOI] [PubMed] [Google Scholar]
- 8. Asita A. O., Matsui M., Nohmi T., Matsuoka A., Hayashi M., Ishidate M., Jr, Sofuni T., Koyano M., Matsushita H. (1991). Mutagenicity of wood smoke condensates in the Salmonella/microsome assay. Mutat. Res., 264, 7–14 [DOI] [PubMed] [Google Scholar]
- 9. Kamens R. M., Rives G. D., Perry J. M., Bell D. A., Paylo R. F., Goodman R. G., Claxton L. D. (1984). Mutagenic changes in dilute wood smoke as it ages and reacts with ozone and nitrogen dioxide. An outdoor chamber study. Environ Sci Tech, 18, 523–530 [Google Scholar]
- 10. Liang C. K., Quan N. Y., Cao S. R., He X. Z., Ma F. (1988). Natural inhalation exposure to coal smoke and wood smoke induces lung cancer in mice and rats. Biomed. Environ. Sci., 1, 42–50 [PubMed] [Google Scholar]
- 11. Mumford J. L., Helmes C. T., Lee X. M., Seidenberg J., Nesnow S. (1990). Mouse skin tumorigenicity studies of indoor coal and wood combustion emissions from homes of residents in Xuan Wei, China with high lung cancer mortality. Carcinogenesis, 11, 397–403 [DOI] [PubMed] [Google Scholar]
- 12. Hernández-Garduño E., Brauer M., Pérez-Neria J., Vedal S. (2004). Wood smoke exposure and lung adenocarcinoma in non-smoking Mexican women. Int. J. Tuberc. Lung Dis., 8, 377–383 [PubMed] [Google Scholar]
- 13. Naeher L. P., Brauer M., Lipsett M., Zelikoff J. T., Simpson C. D., Koenig J. Q., Smith K. R. (2007). Woodsmoke health effects: a review. Inhal. Toxicol., 19, 67–106 [DOI] [PubMed] [Google Scholar]
- 14. Simpson C. D., Naeher L. P. (2010). Biological monitoring of wood-smoke exposure. Inhal. Toxicol., 22, 99–103 [DOI] [PubMed] [Google Scholar]
- 15. Clark M., Paulsen M., Smith K. R., Canuz E., Simpson C. D. (2007). Urinary methoxyphenol biomarkers and woodsmoke exposure: comparisons in rural Guatemala with personal CO and kitchen CO, levoglucosan, and PM2.5. Environ. Sci. Technol., 41, 3481–3487 [DOI] [PubMed] [Google Scholar]
- 16. Simoneit B. R. T., Schauer J. J., Nolte C. G., Oros D. R., Elias V. O., Fraser M. P., Rogge W. F., Cass G. R. (1999). Levoglucosan, a tracer for cellulose in biomass burning and atmospheric particles. Atmos. Environ, 33, 173–182 [Google Scholar]
- 17. Zdráhal Z., Oliveira J., Vermeylen R., Claeys M., Maenhaut W. (2002). Improved method for quantifying levoglucosan and related monosaccharide anhydrides in atmospheric aerosols and application to samples from urban and tropical locations. Environ. Sci. Technol., 36, 747–753 [DOI] [PubMed] [Google Scholar]
- 18. Bergauff M. A., Ward T. J., Noonan C. W., Migliaccio C. T., Simpson C. D., Evanoski A. R., Palmer C. P. (2010). Urinary levoglucosan as a biomarker of wood smoke: results of human exposure studies. J. Expo. Sci. Environ. Epidemiol., 20, 385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith K. R. (2002). Indoor air pollution in developing countries: recommendations for research. Indoor Air, 12, 198–207 [DOI] [PubMed] [Google Scholar]
- 20. Ferreira M., Jr, Buchet J. P., Burrion J. B., Moro J., Cupers L., Delavignette J. P., Jacques J., Lauwerys R. (1994). Determinants of urinary thioethers, D-glucaric acid and mutagenicity after exposure to polycyclic aromatic hydrocarbons assessed by air monitoring and measurement of 1-hydroxypyrene in urine: a cross-sectional study in workers of coke and graphite-electrode-producing plants. Int. Arch. Occup. Environ. Health, 65, 329–338 [DOI] [PubMed] [Google Scholar]
- 21. Kato M., Loomis D., Brooks L. M., Gattas G. F., Gomes L., Carvalho A. B., Rego M. A., DeMarini D. M. (2004). Urinary biomarkers in charcoal workers exposed to wood smoke in Bahia State, Brazil. Cancer Epidemiol. Biomarkers Prev., 13, 1005–1012 [PubMed] [Google Scholar]
- 22. Kado N. Y., Langley D., Eisenstadt E. (1983). A simple modification of the Salmonella liquid-incubation assay. Increased sensitivity for detecting mutagens in human urine. Mutat. Res., 121, 25–32 [DOI] [PubMed] [Google Scholar]
- 23. Yamasaki E., Ames B. N. (1977). Concentration of mutagens from urine by absorption with the nonpolar resin XAD-2: cigarette smokers have mutagenic urine. Proc. Natl. Acad. Sci. U. S. A., 74, 3555–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cerná M., Pastorková A., Myers S. R., Rössner P., Binková B. (1997). The use of a urine mutagenicity assay in the monitoring of environmental exposure to genotoxins. Mutat. Res., 391, 99–110 [DOI] [PubMed] [Google Scholar]
- 25. Mielzyńska D., Siwińska E., Kapka L., Szyfter K., Knudsen L. E., Merlo D. F. (2006). The influence of environmental exposure to complex mixtures including PAHs and lead on genotoxic effects in children living in Upper Silesia, Poland. Mutagenesis, 21, 295–304 [DOI] [PubMed] [Google Scholar]
- 26. Thompson L. M., Clark M., Cadman B., Canúz E., Smith K. R. (2011). Exposures to high levels of carbon monoxide from wood-fired temazcal (steam bath) use in highland Guatemala. Int. J. Occup. Environ. Health, 17, 103–112 [DOI] [PubMed] [Google Scholar]
- 27. Lam N., Nicas M., Ruiz-Mercado I., Thompson L. M., Romero C., Smith K. R. (2011). Non-invasive measurement of carbon monoxide burden in Guatemalan children and adults following wood-fired temazcal (sauna-bath) use. J. Environ. Monit., 13, 2172–2181 [DOI] [PubMed] [Google Scholar]
- 28. DeMarini D. M., Brooks L. R., Bhatnagar V. K., et al. (1997). Urinary mutagenicity as a biomarker in workers exposed to benzidine: correlation with urinary metabolites and urothelial DNA adducts. Carcinogenesis, 18, 981–988 [DOI] [PubMed] [Google Scholar]
- 29. Mortelmans K., Zeiger E. (2000). The Ames Salmonella/microsome mutagenicity assay. Mutat. Res., 455, 29–60 [DOI] [PubMed] [Google Scholar]
- 30. Boeniger M. F., Lowry L. K., Rosenberg J. (1993). Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am. Ind. Hyg. Assoc. J., 54, 615–627 [DOI] [PubMed] [Google Scholar]
- 31. Barr D. B., Wilder L. C., Caudill S. P., Gonzalez A. J., Needham L. L., Pirkle J. L. (2005). Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect., 113, 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neter J., Wasserman W., Kutner M. H. (1990). Applied Linear Statistical Models: Regression, Analysis of Variance, and Experimental Designs. 3rd edn. Richard D. Irwin, Inc., Homewood, IL [Google Scholar]
- 33. Cline R. E., Hill R. H., Jr, Phillips D. L., Needham L. L. (1989). Pentachlorophenol measurements in body fluids of people in log homes and workplaces. Arch. Environ. Contam. Toxicol., 18, 475–481 [DOI] [PubMed] [Google Scholar]
- 34. Rehberg P. B. (1926). Studies on Kidney Function: The Rate of Filtration and Reabsorption in the Human Kidney. Biochem. J., 20, 447–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hagiwara Y., Watanabe M., Oda Y., Sofuni T., Nohmi T. (1993). Specificity and sensitivity of Salmonella typhimurium YG1041 and YG1042 strains possessing elevated levels of both nitroreductase and acetyltransferase activity. Mutat. Res., 291, 171–180 [DOI] [PubMed] [Google Scholar]
- 36. Riedel K., Scherer G., Engl J., Hagedorn H. W., Tricker A. R. (2006). Determination of three carcinogenic aromatic amines in urine of smokers and nonsmokers. J. Anal. Toxicol., 30, 187–195 [DOI] [PubMed] [Google Scholar]
- 37. Riffelmann M., Müller G., Schmieding W., Popp W., Norpoth K. (1995). Biomonitoring of urinary aromatic amines and arylamine hemoglobin adducts in exposed workers and nonexposed control persons. Int. Arch. Occup. Environ. Health, 68, 36–43 [DOI] [PubMed] [Google Scholar]
- 38. Xue W., Warshawsky D. (2005). Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol. Appl. Pharmacol., 206, 73–93 [DOI] [PubMed] [Google Scholar]
- 39. Pimenta A. S., Bayona J. M., García M. T., Solanas A. M. (2000). Evaluation of acute toxicity and genotoxicity of liquid products from pyrolysis of Eucalyptus grandis wood. Arch. Environ. Contam. Toxicol., 38, 169–175 [DOI] [PubMed] [Google Scholar]
- 40. Vermeulen R., Wegh H., Bos R. P., Kromhout H. (2000). Weekly patterns in smoking habits and influence on urinary cotinine and mutagenicity levels: confounding effect of nonsmoking policies in the workplace. Cancer Epidemiol. Biomarkers Prev., 9, 1205–1209 [PubMed] [Google Scholar]
- 41. Ramesh A., Inyang F., Hood D. B., Archibong A. E., Knuckles M. E., Nyanda A. M. (2001). Metabolism, bioavailability, and toxicokinetics of benzo(alpha)pyrene in F-344 rats following oral administration. Exp. Toxicol. Pathol., 53, 275–290 [DOI] [PubMed] [Google Scholar]
- 42. Viau C., Bouchard M., Carrier G., Brunet R., Krishnan K. (1999). The toxicokinetics of pyrene and its metabolites in rats. Toxicol. Lett., 108, 201–207 [DOI] [PubMed] [Google Scholar]
- 43. Bouchard M., Viau C. (1998). Urinary and biliary excretion kinetics of 1-hydroxypyrene following intravenous and oral administration of pyrene in rats. Toxicology, 127, 69–84 [DOI] [PubMed] [Google Scholar]
- 44. Smith K. R., McCracken J. P., Thompson L., Edwards R., Shields K. N., Canuz E., Bruce N. (2010). Personal child and mother carbon monoxide exposures and kitchen levels: methods and results from a randomized trial of woodfired chimney cookstoves in Guatemala (RESPIRE). J. Expo. Sci. Environ. Epidemiol., 20, 406–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nikoyan A., De Méo M., Sari-Minodier I., Chaspoul F., Gallice P., Botta A. (2007). Evaluation of a battery of Salmonella typhimurium tester strains for biomonitoring of mutagenic polycyclic aromatic hydrocarbons, nitroarenes and aromatic amines. Mutat. Res., 626, 88–101 [DOI] [PubMed] [Google Scholar]
- 46. Scarlett-Kranz J. M., Babish J. G., Strickland D., Goodrich R. M., Lisk D. J. (1986). Urinary mutagens in municipal sewage workers and water treatment workers. Am. J. Epidemiol., 124, 884–893 [DOI] [PubMed] [Google Scholar]
- 47. Falck K. (1983). Biological monitoring of occupational exposure to mutagenic chemicals in the rubber industry. Use of the bacterial urinary mutagenicity assay. Scand. J. Work. Environ. Health, 9 (Suppl. 2), 39–42 [PubMed] [Google Scholar]
- 48. Nguyen T. V., Theiss J. C., Matney T. S. (1982). Exposure of pharmacy personnel to mutagenic antineoplastic drugs. Cancer Res., 42, 4792–4796 [PubMed] [Google Scholar]
- 49. Peters S., Talaska G., Jönsson B. A., Kromhout H., Vermeulen R. (2008). Polycyclic aromatic hydrocarbon exposure, urinary mutagenicity, and DNA adducts in rubber manufacturing workers. Cancer Epidemiol. Biomarkers Prev., 17, 1452–1459 [DOI] [PubMed] [Google Scholar]
- 50. Choi B. C., Connolly J. G., Zhou R. H. (1995). Application of urinary mutagen testing to detect workplace hazardous exposure and bladder cancer. Mutat. Res., 341, 207–216 [DOI] [PubMed] [Google Scholar]
- 51. Bos R. P., Kromhout H., Ikink H., de Haan W., Koppejan J., Theuws J. L. (1989). Mutagens in urine of non-smoking and smoking workers in an aircraft tyre retreading plant. Skin exposure as a causal factor? Mutat. Res., 223, 41–48 [DOI] [PubMed] [Google Scholar]
- 52. Ahlborg G., Jr, Einistö P., Sorsa M. (1988). Mutagenic activity and metabolites in the urine of workers exposed to trinitrotoluene (TNT). Br. J. Ind. Med., 45, 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pasquini R., Monarca S., Scassellati Sforzolini G., Savino A., Bauleo F. A., Angeli G. (1989). Urinary excretion of mutagens, thioethers and D-glucaric acid in workers exposed to bitumen fumes. Int. Arch. Occup. Environ. Health, 61, 335–340 [DOI] [PubMed] [Google Scholar]
- 54. Dolara P., Mazzoli S., Rosi D., Buiatti E., Baccetti S., Turchi A., Vannucci V. (1981). Exposure to carcinogenic chemicals and smoking increases urinary excretion of mutagens in humans. J. Toxicol. Environ. Health, 8, 95–103 [DOI] [PubMed] [Google Scholar]
- 55. Falck K., Sorsa M., Vainio H., Kilpikari I. (1980). Mutagenicity in urine of workers in rubber industry. Mutat. Res., 79, 45–52 [DOI] [PubMed] [Google Scholar]
- 56. Falck K., Gröhn P., Sorsa M., Vainio H., Heinonen E., Holsti L. R. (1979). Mutagenicity in urine of nurses handling cytostatic drugs. Lancet, 1, 1250–1251 [DOI] [PubMed] [Google Scholar]
- 57. Baker R., Arlauskas A., Bonin A., Angus D. (1982). Detection of mutagenic activity in human urine following fried pork or bacon meals. Cancer Lett., 16, 81–89 [DOI] [PubMed] [Google Scholar]
- 58. Mohtashamipur E., Norpoth K., Lieder F. (1987). Urinary excretion of mutagens in smokers of cigarettes with various tar and nicotine yields, black tobacco, and cigars. Cancer Lett., 34, 103–112 [DOI] [PubMed] [Google Scholar]
- 59. Bos R. P., Theuws J. L., Henderson P. T. (1983). Excretion of mutagens in human urine after passive smoking. Cancer Lett., 19, 85–90 [DOI] [PubMed] [Google Scholar]
- 60. Smith C. J., Bombick D. W., Ryan B. A., Morgan W. T., Doolittle D. J. (2000). Urinary mutagenicity in nonsmokers following exposure to fresh diluted sidestream cigarette smoke. Mutat. Res., 470, 53–70 [DOI] [PubMed] [Google Scholar]
- 61. Smith-Sivertsen T., Díaz E., Pope D., et al. (2009). Effect of reducing indoor air pollution on women’s respiratory symptoms and lung function: the RESPIRE Randomized Trial, Guatemala. Am. J. Epidemiol., 170, 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Joshi V., Venkataraman C., Ahuja D. R. (1989). Emissions from burning biofuels in metal cookstoves. Environ. Manage., 13, 763–772 [Google Scholar]
- 63. Naeher L. P., Smith K. R., Leaderer B. P., Mage D., Grajeda R. (2000). Indoor and outdoor PM2.5 and CO in high- and low-density Guatemalan villages. J. Expo. Anal. Environ. Epidemiol., 10, 544–551 [DOI] [PubMed] [Google Scholar]
- 64. Smith K. R., McCracken J. P., Weber M. W., et al. (2011). Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet, 378, 1717–1726 [DOI] [PubMed] [Google Scholar]
- 65. Fitzgerald C., Aguilar-Villalobos M., Eppler A. R., Dorner S. C., Rathbun S. L., Naeher L. P. (2012). Testing the effectiveness of two improved cookstove interventions in the Santiago de Chuco Province of Peru. Sci. Total Environ., 420, 54–64 [DOI] [PubMed] [Google Scholar]
- 66. Adetona O., Dunn K., Hall D. B., Achtemeier G., Stock A., Naeher L. P. (2011). Personal PM(2.5) exposure among wildland firefighters working at prescribed forest burns in Southeastern United States. J. Occup. Environ. Hyg., 8, 503–511 [DOI] [PubMed] [Google Scholar]
- 67. Northcross A., Chowdhury Z., McCracken J., Canuz E., Smith K. R. (2010). Estimating personal PM2.5 exposures using CO measurements in Guatemalan households cooking with wood fuel. J. Environ. Monit., 12, 873–878 [DOI] [PubMed] [Google Scholar]
- 68. Mielzynska-Svach D., Blaszczyk E., Butkiewicz D., Durzynska J., Rydzanicz M. (2013). Influence of genetic polymorphisms on biomarkers of exposure and effects in children living in Upper Silesia. Mutagenesis, 28, 591–599 [DOI] [PubMed] [Google Scholar]
- 69. Neri M., Bonassi S., Knudsen L. E., Sram R. J., Holland N., Ugolini D., Merlo D. F. (2006). Children’s exposure to environmental pollutants and biomarkers of genetic damage. I. Overview and critical issues. Mutat. Res., 612, 1–13 [DOI] [PubMed] [Google Scholar]
- 70. Ginsberg G., Hattis D., Miller R., Sonawane B. (2004). Pediatric pharmacokinetic data: implications for environmental risk assessment for children. Pediatrics, 113, 973–983 [PubMed] [Google Scholar]
- 71. Wild C. P., Kleinjans J. (2003). Children and increased susceptibility to environmental carcinogens: evidence or empathy? Cancer Epidemiol. Biomarkers Prev., 12, 1389–1394 [PubMed] [Google Scholar]
- 72. Ginsberg G., Hattis D., Sonawane B., Russ A., Banati P., Kozlak M., Smolenski S., Goble R. (2002). Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol. Sci., 66, 185–200 [DOI] [PubMed] [Google Scholar]
- 73. Turesky R. J., Le Marchand L. (2011). Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem. Res. Toxicol., 24, 1169–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guengerich F. P., Turvy C. G. (1991). Comparison of levels of several human microsomal cytochrome P-450 enzymes and epoxide hydrolase in normal and disease states using immunochemical analysis of surgical liver samples. J. Pharmacol. Exp. Ther., 256, 1189–1194 [PubMed] [Google Scholar]
- 75. Oda Y., Aryal P., Terashita T., Gillam E. M., Guengerich F. P., Shimada T. (2001). Metabolic activation of heterocyclic amines and other procarcinogens in Salmonella typhimurium umu tester strains expressing human cytochrome P4501A1, 1A2, 1B1, 2C9, 2D6, 2E1, and 3A4 and human NADPH-P450 reductase and bacterial O-acetyltransferase. Mutat. Res., 492, 81–90 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.