Abstract
Background
High intensity focused ultrasound (HIFU) is an alterative ablative technique currently being investigated for local treatment of breast cancer and fibroadenomas. Current HIFU therapies require concurrent MRI monitoring. Biodegradable 500 nm perfluoropentane (PFP) filled iron-silica nanoshells have been synthesized as a sensitizing agent for HIFU therapies, which aid both mechanical and thermal ablation of tissues. In low duty cycle high-intensity applications, rapid tissue damage occurs from mechanical rather than thermal effects, which can be monitored closely by ultrasound obviating the need for concurrent MRI.
Materials and Methods
Iron-silica nanoshells were synthesized by a sol-gel method on polystyrene templates and calcined to yield hollow nanoshells. The nanoshells were filled with PFP and injected directly into excised human breast tumor, and intravenously (IV) into healthy rabbits and Py8119 tumor bearing nu/nu mice. HIFU was applied at 1.1 MHz and 3.5 MPa at a 2% duty cycle to achieve mechanical ablation.
Results
Ex vivo in excised rabbit livers, the time to visually observable damage with HIFU was 20 seconds without nanoshells and only 2 seconds with nanoshells administered IV prior to sacrifice. Nanoshells administered IV into nu/nu mice with xenograft tumors were activated in vivo by HIFU 24 hours after administration. In this xenograft model, applied HIFU resulted in a 13.6 ± 6.1 mm3 bubble cloud with the IV injected particles and no bubble cloud without particles.
Conclusions
Iron-silica nanoshells can reduce the power and time to perform HIFU ablative therapy and can be monitored by ultrasound during low duty cycle operation.
Keywords: Nanoparticles, HIFU, Silica, Breast Cancer, Histotripsy
Introduction
High intensity focused ultrasound (HIFU) is an ablative technique that is currently employed for treatment of uterine fibroids in the United States and is being investigated for other applications.(1–4) Less invasive techniques, which still provide good local control and prevent recurrence, are desirable for treating solid tumors. Silica nanoshells can accurately localize non-palpable tumors(5–7) and are being investigated for HIFU ablation of small breast cancers without an operation or incision. Previous researchers have investigated MRI guided HIFU of breast fibroadenomas.(8) However, MRI guided HIFU is associated with patient discomfort of lying face down for over an hour and is not associated with complete ablation.(9, 10) Ongoing clinical trials use thermal HIFU ablation for small breast cancers under MRI guidance.(11) With traditional HIFU therapy, some energy is converted to heat, which at the focal zone of the HIFU is sufficient to cause a thermal burn and induce coagulative necrosis of the tissue. For thermal ablation with HIFU, MRI is required to guide and monitor tissue temperature to minimize off target heating or over- or under-treatment.(12) Silica nanoshells could be utilized in a more convenient non-thermal technique of supine ablation under ultrasound guidance obviating the need for MRI monitoring while improving patient comfort and speed.
In addition to thermal damage, HIFU also causes mechanical damage by cavitation and can liquefy tissue; this is denoted as histotripsy.(13) Normally, for strictly mechanical damage to occur with HIFU, an intense pulsed ultrasound with a duty cycle below 4% with a time average intensity of 2 W/mm2 is required to minimize tissue heating.(14) Due to the minimal tissue heating, mechanical damage caused with HIFU can be monitored by ultrasound instead of MRI. Xu et al. demonstrated that during histotripsy in vitro, bubbles were generated that grew from less than 10 microns in size to over 100 microns.(15) Roberts et al. applied focused, pulsed high power, low frequency ultrasound for histrotripsy in rabbit kidneys.(16) With 25 MPa peak negative pressure output, it was found that 1000 or 10000 pulses resulted in formation of a 3 mm by 10 mm ellipsoidal cavity at the focal zone. Vlaisavljevich et al. demonstrated that histotripsy can be performed on much larger volumes in vivo in porcine livers through intervening tissue and ribs.(17) Although histotripsy without enhancement is a very promising technique, it utilizes very large pressures and insonation times.
Ultrasound contrast agents, such as microbubbles and nanoemulsions can enhance thermal and mechanical HIFU ablation.(18–22) Kaneko et al. performed HIFU on rabbit livers in vivo which had been dosed with Levovist® microbubbles and doubled the burn volume compared to saline control rabbits after 60 seconds of HIFU application.(23) Tran et al. performed cavitation in canine kidneys with and without (Optison®) microbubbles; continuous perfusion of microbubbles during insonation reduced the ultrasound intensity and exposure time needed.(24) For microbubbles, the rapid clearance from circulation due to their large sizes suggests that for practical use it would be necessary to administer large doses or have multiple/continuous dosing.(25) Nanoemulsions/nanodroplets are particularly attractive because they have a small size until acoustic excitation induces droplet vaporization, which greatly increases their size. In vitro, perfluoropentane core nanodroplets have the potential to substantially reduce the threshold for cavitation and histrotripsy.(26) The minimum pressure to generate cavitation bubbles in the presence of nanodroplets was ~3 MPa versus ~15.6 MPa without the nanodroplets.
Silica nanoparticles and nanoshells have been shown useful as ultrasound contrast agents(5–7) and as sensitizing agents for HIFU therapy.(27–29) While the in vivo lifetime of ultrasound contrast agents is only minutes for microbubbles or several hours for nanodroplets, silica nanoshells have been shown to retain the loaded perfluorocarbon in vivo over the course of 10 days and remain in xenograft tumors after intravenous administration over the course of several days from a single dose of nanoshells.(5–7) In contrast to both microbubbles and nanodroplets silica nanoshells have an almost infinite shelf life and facile surface functionalization. Previous studies employed perfluorohexane liquid filled silica nanoparticles and very short insonation times at high ultrasound power to cause thermal damage. The present study uses biodegradable 500 nm perfluoropentane filled Fe-SiO2 nanoshells for applications in histotripsy, as well as thermal ablation at relatively modest peak negative pressures. Furthermore, it is demonstrated ex vivo in human mastectomy tissue that the use of the Fe-SiO2 nanoshells may be used to ablate small and targeted tissue volumes as detailed by color Doppler imaging. Finally, it is shown that IV injection of the nanoshells, which accumulate in Py8119 tumors grown in mouse flanks, ablate tumors in vivo. To the best of our knowledge, this is the first report applying silica nanoparticle for a strictly histotripsy type therapy.
Materials and Methods
Materials
Tetramethyl orthosilicate (TMOS) was purchased from Sigma-Aldrich (St. Louis, MO); iron (III) ethoxide was obtained from Gelest Inc. (Moorisville, PA), 500 nm aminated polystyrene templates were purchased Polysciences (Warrington, PA); perfluoropentane was procured from Strem Chemicals (Newburyport, MA). All ultrasound imaging was performed using a Siemens Sequoia 512 (Mountainview, CA) with an Acuson 15L8 imaging transducer. HIFU was performed using a Sonic Concepts Inc. (Bothell, WA) H-102 single element transducer, driven by ac AG 1006 Amplifier/Generator (Rochester, NY). FGEN Soft Front Panel v2.6 software by National Instruments (Austin, TX) was used to generate the waveform and control the HIFU. Tissue sectioning and hematoxylin and eosin staining was performed by the UCSD Histology core.
Animals and Tissue
Female, 3 to 4 kg New Zealand White rabbits were housed individually in a UCSD approved vivarium and fed Harlan Teklad pelleted commercial feed. Prior to experimentation, each animal was anesthetized with 2% isoflurane gas through a nose cone. Particle injections took place through the ear vein of each animal under anesthesia. Animals were monitored in accordance with UCSD guidelines throughout all procedures. Following experimentation, each animal was euthanized with an IV injection of sodium pentobarbital through the ear vein. All procedures performed on rabbits were approved by UCSD IACUC board.
Female, 4 to 6 week old nude mice from UCSD’s in house breeding colony were housed in UCSD approved vivarium on a twelve hour light/dark cycle and fed Harlan Teklad rodent feed. All tumor cell injections took place under anesthesia induced by inhalation of 1–2% isoflurane. Each animal received two injections of 106 Py8119 cells in a single cell suspension in growth media, one over each hind limb. Animals were weighed and tumors were measured regularly in accordance with UCSD animal welfare policies until the tumors approached 1000mm3 in volume. Particle injections took place through the tail vein of each animal under anesthesia. Following the conclusion of treatments and scans, all animals were euthanized by CO2 inhalation. All procedures performed on mice were approved by UCSD IACUC board.
Human breast invasive ductal carcinoma and fibroadenoma tissues were received post operatively and in full cooperation and consent of UCSD IRB protocols. Tissues received were approximately 1 cubic centimeter in volume but varied in disease type and state. Particles were injected directly into tissue and after extraction of tissue sample.
Methods
Nanoparticle Synthesis and Perfluorocarbon filling process
Biodegradable 500 nm iron silica nanoshells were synthesized as described previously.(6, 30) A sol gel reaction was performed on a 500 nm aminated template using tetramethyl orthosilicate and iron (III) ethoxide in ethanol. After collection by centrifugation, the particles were calcined at 550 °C to remove the template and dehydrate the gel. After calcination, particles were stored dried until needed and, subsequently, filled with perfluoropentane gas or liquid. Gas filling was performed as previously described.(31) For perfluorocarbon liquid filling, nanoshells were first placed under vacuum in an amber vial with a self-sealing silicone top. After 30 minutes under vacuum, the vacuum line was removed, and the vial filled with perfluoropentane gas using a gas syringe filled with vaporized perfluoropentane in order to equalize the pressure in the container such that the inserted liquid PFP does not vaporize in the container. With a syringe, 25 ul PFP/mg of particles was added to the container, and the container was briefly bath sonicated to disperse the particles. After the particles were filled with PFP liquid or gas, the particles were resuspended in MilliQ water at a concentration of 4 mg/ml.
Ex Vivo HIFU of Rabbit Liver
2 mg (500 μl) of gas filled Fe-SiO2 nanoshells were administered to the rabbits intravenously via the marginal ear vein and allowed to circulate for 15 minutes. The rabbits were sacrificed and the livers were removed and partitioned for HIFU application. Liver partitions were placed in a water bath within the focal distance of the HIFU transducer, and HIFU was applied at 800 KHz with 100% duty cycle at 3 MPa for variable amounts of time. After HIFU, the livers were coarsely sectioned to reveal the damaged sections. A total of six rabbits were used, three rabbits received particle injections and three were used as a control group. Two insonations per time point were performed in each animal for a total of six insonations per time point. Effected volumes were measured by calipers.
Ex Vivo HIFU of Excised Breast Tumor
Patients underwent their standard procedures for resection of their benign or malignant tumor. A UCSD pathologist examined the tissue; after determining that removing this tissue would not interfere with their analysis, they removed a portion for experimentation. 200 μg (50 μl) of PFP liquid filled Fe-SiO2 nanoshells were injected directly into the provided tumor tissue. The tissue was placed inside of a plastic disposable transfer pipette bulb to aid handling. The bulb containing the tissue was placed in a water bath at the focus of the HIFU transducer. The HIFU transducer and the ultrasound imaging transducer were arranged orthogonal to one another in a water bath for simultaneous HIFU and US imaging. HIFU was applied for 1 minute at 1.1 MHz with a 2% duty cycle at 3.5 MPa. After HIFU, tissues were flash frozen and stored at −80 °C until they were submitted to histology for sectioning and H&E staining. Human tissue experiments were repeated six times with tissues from six different patients, but were not analyzed numerically due to large variations in tissue type and difficulty injecting consistent amounts of particles because of the variations of the tissue.
In Vivo HIFU of Py8119 Tumor Bearing Mice
800 μg (100 μl at 8 mg/ml) of PFP liquid filled Fe-SiO2 nanoshells were injected intravenously via the tail vein into Py8119 tumor bearing nu/nu mice (2 tumors/mouse), and the nanoshells were allowed to circulate for 24 hours prior to HIFU. The tumors were pressed against the HIFU transducer in a water bath for ease of focus. The HIFU transducer and the ultrasound imaging transducer were arranged orthogonal to one another in a water bath for simultaneous HIFU and US imaging. Three mice received particles, and an additional two mice served as a control group. In sum, six tumors were insonated with presence of particles and four tumors were insonated without particles. Raw ultrasound data was analyzed with Osirix Software and then numerical data was imported into Microsoft Excel. All data was analyzed with the analytical and statistical package available in Microsoft Excel. HIFU was applied for 1 minute at 1.1 MHz with a 2% duty cycle at 3.5 MPa. After HIFU, tissues were flash frozen and stored at −80 °C until they were submitted to histology for se ctioning and H&E staining.
Results and Discussion
PFP filled Fe-SiO2 nanoshells have been evaluated as a sensitizing agent for HIFU ablative therapies. New Zealand white rabbits received a 2 mg IV injection of PFP gas filled Fe-SiO2 nanoshells. After 15 minutes of particle circulation, the rabbits were sacrificed, and the livers were removed for HIFU application to overcome the attenuation and scattering of the rabbit skin and for more precise ablation of multiple distinct zones. It was found that at a given power of ultrasound energy, using a continuous 800KHz pure tone waveform with a peak negative pressure at 3 MPa, nanoshell enhancement could reduce the amount of time necessary to achieve a measurable response in tissue. As can be seen in Fig 1A, highly energetic ultrasound alone can cause thermal damage in the liver after 60 seconds of exposure at 100% duty cycle. However, an equally sized lesion, approximately 100 mm3 can be produced in 30 seconds at 100% duty cycle with nanoshell enhancement with the addition of mechanical damage (see Table 1 for time reduction and lesion volume data). Note within the ablated region, there is an area of mechanical damage and there is a zone of thermal ablation showing that the nanoshells enhance both processes because the ultrasound is strongly scattered by the nanoshells. HIFU causes thermal injury and coagulative necrosis which can be macroscopically viewed by tissue discoloration.(27, 28, 32) For these rabbit studies, the mechanical damage is differentiated from the strictly thermal injury by the presence of a large cavity within the thermally damaged region. The high degree of thermal damage as shown by macroscopic tissue necrosis in these rabbit studies is also attributed to the continuous use of the HIFU, which increases the deposition of thermal energy relative to pulsed HIFU. In the pulsed HIFU experiments in mice and human tissue below, the degree of thermal injury was assessed with H&E stained histology.
Figure 1. Ex vivo nanoshell enhanced ultrasonic ablation.
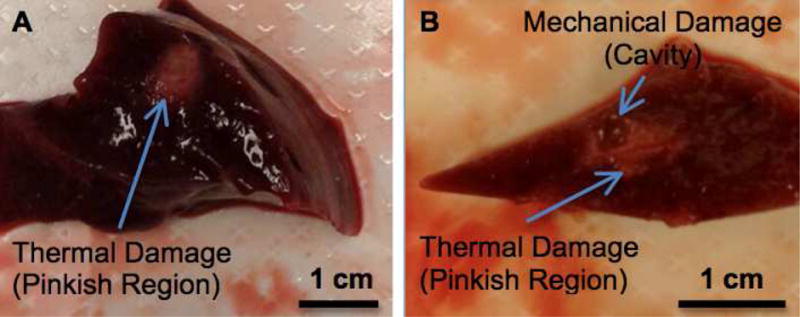
A) A thermal lesion is produced by highly energetic ultrasonic ablation without nanoshell enhancement after 60 seconds of exposure. B) Both mechanical and thermal damage are produced with nanoshell enhancement after only 30 seconds of ultrasonic ablation at an equivalent power. The thermal damage is the pink region compared to the dark red of the healthy liver tissue. The mechanical damage is seen as a physical cavity that was generated from nanoshell cavitation.
Table 1.
HIFU Application at 100% duty cycle on Rabbits Treated with and without Nanoshells
| Time (Seconds) | Nanoshell Enhanced Ablated Volume (mm3) | Standard Deviation | Control Ablated Volume (mm3) | Standard Deviation |
|---|---|---|---|---|
| 2 | 1.3 | 1.9 | 0.0 | 0.0 |
| 5 | 12.5 | 10.9 | 0.0 | 0.0 |
| 10 | 24.0 | 17.0 | 0.0 | 0.0 |
| 20 | 27.7 | 13.8 | 0.7 | 1.5 |
| 30 | 107.1 | 74.9 | 17.2 | 10.7 |
| 60 | 171.3 | 128.5 | 94.9 | 47.1 |
Nanoshell enhanced ablation can dramatically reduce the amount of time required for HIFU especially for HIFU which contain a thermal component. When HIFU alone was focused on a rabbit liver using a continuous 800KHz pure tone waveform with a peak negative pressure at 3 MPa at 100% duty cycle, no measurable response could be observed visually with less than a 20 second exposure. However, after giving a rabbit a dose of particles at 0.5 mg/kg and allowing for a 15 minute circulation time, a measureable response could be detected with as little as 2 seconds of HIFU exposure at 100% duty cycle. Table 1 shows the average volumes of the affected areas in the rabbit livers at times where HIFU alone would have no effect. As shown in Table 1, in only 2 seconds a response can be observed. The large standard deviations are attributed to a short incubation/circulation time resulting in an uneven distribution of particles. Alternatively, it is possible that the large standard deviations are due to regions which have a selectively higher accumulation of particles which when insonated result in a larger ablated area. These issues can be potentially overcome by changing the concentration of the delivered dose or increasing the circulation time. For the studies described below, the duty cycle was reduced to 2% in order to avoid thermal damage and to allow for more precise ultrasound imaging as thermal injury is not always readily visible under B-mode imaging. The lower duty cycle is also expected to increase the relative enhancement with silica nanoshells while remaining innocuous to off target tissues. Furthermore to potentially gain an increased effect from the pulsed HIFU which is less energetic than continuous HIFU, the particles in the following experiments utilized PFP-liquid filled particles. The advantage of using the PFP-liquid filled particles is that when the liquid is converted to gas under HIFU, the volume occupied by the PFP increases substantially and generates multiple gas bubbles from a single liquid droplet which can cavitate and cause mechanical damage. The increased number of bubbles from the conversion from liquid to gas also allows for very facile monitoring during ultrasound even in non-contrast specific imaging modalities.
To demonstrate that nanoshells could potentially be used for histotripsy HIFU therapy in humans, PFP liquid filled 500 nm nanoshells were injected intratumorally into excised human mastectomy tissue and HIFU was performed at 2% duty cycle. It has been previously shown that 500 nm silica nanoshells remain sequestered to the injection site during intratumoral or intramuscular injections and do not extravasate.(6, 7) It was hypothesized that due to the low functional porosity of tissue (approx. 10 nm) that fluids and small molecules can easily diffuse from the injection site but the nanoshells remain stationary. As shown in Fig 2A, the nanoshells are clearly visible under color Doppler ultrasound imaging. The long tail from the Doppler image seen in Fig 2A is due to shadowing from the high reflectivity of the particles. Once the HIFU is activated (Fig 2C), the cavitation and bubble generation can be observed at the site where the color Doppler signal originated. Comparing the images before (Fig. 2B) and after HIFU was applied (Fig. 2D), a cavity is clearly formed within the tumor. This effect is seen in the change in color of the injection site between Fig 2B and 2D, as can be seen in it goes from white to black after insonation. This change in color is indicative of a change of local echogenicity which was due to the formation of a liquid pocket. It is hypothesized that the relatively small volume that was ablated was due to the relatively small volume occupied by the directly injected nanoshells as well as the small focal zone of the transducer (cross sectional area ≈ 1.44 mm2). However the small volume overlap between the focal zone of the HIFU and the volume occupied by the nanoshells allows for very precise ablation and may be advantageous in delicate therapies to avoid damaging sensitive or off target tissues.
Figure 2. Ex vivo HIFU of excised mastectomy tissue.
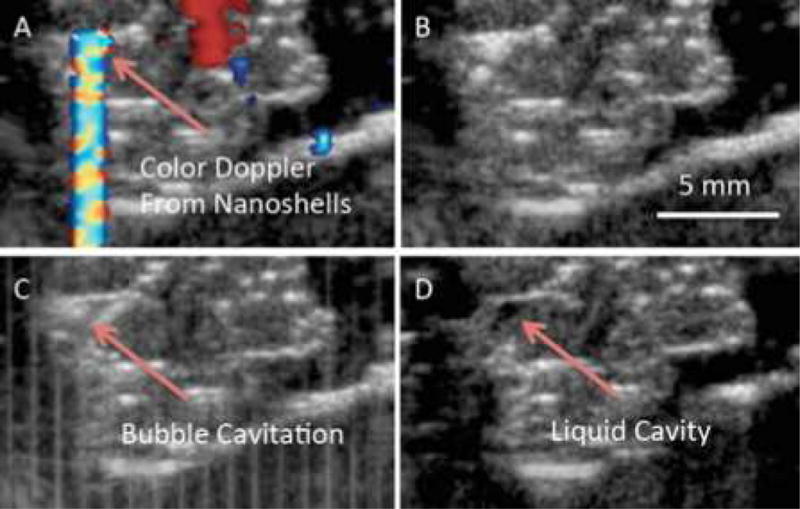
50 ul at 4 mg/ml of PFC liquid filled 500 nm nanoshells were injected intratumorally ex vivo. A) Color Doppler ultrasound displays the location of the nanoshells allowing for better targeting of the HIFU transducer. B) B-mode image of tissue prior to HIFU. C) HIFU was applied for 1 min at 1.1 MHz and 3.5 MPa with a 2% duty cycle. Bubble cavitation/formation is readily observed. D) After HIFU a pocket (black spot) filled is created which is filled with the liquefied tissue.
It was previously demonstrated with 500 nm nanoshells that it is possible to detect Py8119 epithelial breast cancer tumors in nu/nu by gamma scinitigraphy.(6) Since nanoshells can accumulate in this tumor model, nanoshells were administered intravenously into the same Py8119 breast tumor bearing nu/nu mice. PFP liquid filled nanoshells were allowed to circulate and accumulate in the tumors for 24 hours before HIFU administration. HIFU was applied for 1 minute at 3.5 MPa and 1.1 MHz with a 2% duty cycle. As HIFU was applied, the nanoshells were fractured and the liquid perfluoropentane within the nanoshells underwent acoustic droplet vaporization (Fig 3B) and began to cavitate. This cavitation liquefied the tissue within the focal zone (Fig 3C) while tissues outside the focal zone remained unaffected. The affected region in Fig 3C is black, identical to the region in Fig 2D despite the different injection types. The average size of the bubble cloud generated in response to insonation was found to be 13.6 ± 6.1 mm3 with the particles and no bubble cloud was observed without particles. Control mouse tumor insonation without particles can be seen in supplemental Figure 1. Figure 3 demonstrates that perfluoropentane filled 500 nm Fe-SiO2 nanoshells can be used as HIFU sensitizing agents, specifically for enhancing mechanical cavitation and liquefaction of tissue in vivo. Previous results in an identical mouse xenograft model showed that only ~2.5% of the injected dose, approximately 20 μg of nanoshells, actually reach the tumors.(6) Despite the low concentration of nanoshells within the tumors it was sufficient to generate a nanoshell dependent response when exposed to HIFU. This indicates that very few nanoshells are necessary to reduce the cavitation threshold and cause mechanical ablation.
Figure 3. Nanoshell enhanced HIFU in vivo in Py8119 tumor bearing nu/nu mice.
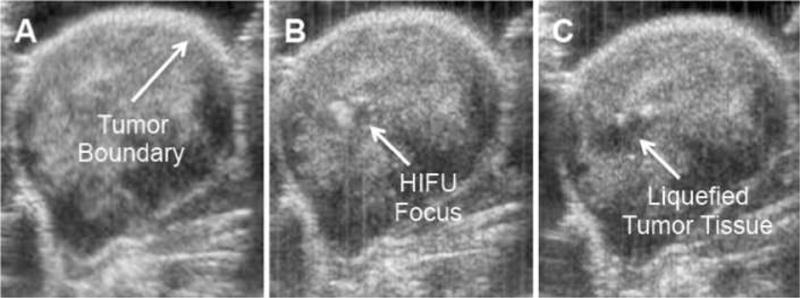
800 ug of 500 nm liquid PFP filled nanoshells were administered IV. HIFU was applied 24 hours after administration for 1 min at 3.5 MPa and 1.1 MHz with a 2% Duty Cycle. A) Before HIFU B) During HIFU, bubble movement/generation was noticed at the focal zone. C) Post HIFU. Blackened area at HIFU focus was liquefied tissue.
In both the mouse model and human mastectomy tissue, nanoshell enhanced ablation resulted in the generation of a physical cavity. It should be noted that during gross examination, when the tumor was cut along the ablated region, the dark area was liquefied. In both human breast tissue and mouse tumor tissue (Fig 4), the application of high intensity ultrasound in the presence of nanoshells results in mechanical destruction and liquefaction of tissue. Furthermore, due to the low duty cycle of the ultrasound, which results in a low time-averaged intensity, no coagulative necrosis or thermal damage is observed in the surrounding tissue in H&E histology. Previous histology reported from histotripsy showed that the region ablated was a homogenated field of cellular debris.(14, 17, 33) It is hypothesized that the difference in the present study is due to a difference in handling the tissue after insonation. Previous reports fixed the tissue in formalin solution, which may have preserved the ablated region for examination, whereas flash freezing the sample resulted in the ablated region being lost during the tissue sectioning process.
Figure 4. Histological evaluation of tissues after nanoshell administration and insonation.
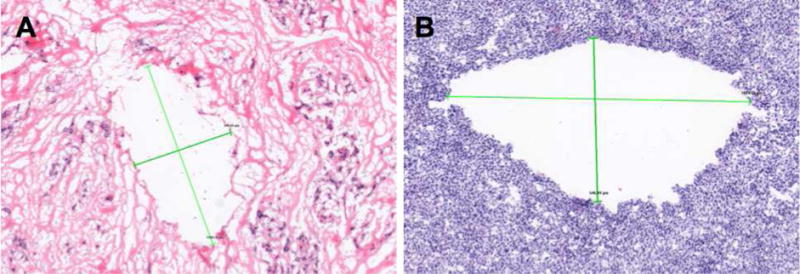
A) H&E stained image of human mastectomy tissue after HIFU at 4× magnification. Crosshairs measure a cavity found in the tissue at ~1600 × 800 μm. B) H&E stained image of Py8119 tumor tissue after HIFU at 10× magnification. Crosshairs measure a cavity found in the tissue at ~1000 × 550 μm.
Conclusions
Liquid PFP filled Fe-SiO2 nanoshells reduce the time and intensity needed to achieve visibly measurable damage in tissues in both standard and purely mechanical HIFU. With an insonation at 800 KHz and 3 MPa in healthy rabbit livers, the nanoshells could reduce the time to visually observed damage from 20 seconds to 2 seconds, while simultaneously accentuating thermal and mechanical damage. Upon gross examination of tissues, the mechanical damage resulted in a cavity filled with liquefied tissue. By reducing the duty cycle of the HIFU to 2% while employing nanoshells, the thermal damage was eliminated and only mechanical ablation occurred. After injecting ex vivo human breast tumor tissue with 50 μl of nanoshells, the mechanical ablation was limited to the volume overlap between the focal zone of the transducer and volume occupied by the nanoshells with precise spatial control of ablation. In vivo liquid PFP filled nanoshells intravenously injected into Py8119 breast tumor bearing mice could be activated by HIFU at 1.1 MHz and 3.5 MPa to enhance mechanical ablation 24 hours after administration. Under these conditions a 13.6 ± 6.1 mm3 bubble cloud response could be measured with the IV injected particles and no bubble cloud was observed without particles. Examination of breast and mouse tissues revealed similarly sized cavities from mechanical ablation of tissue. With intravenous administration in the in vivo model, a larger zone could be ablated by continuously moving the HIFU transducer throughout insonation. The Fe-SiO2 nanoshells have a much greater in vivo stability and tumor retention time as well as nearly infinite shelf life compared to other sensitizing agents. HIFU based therapies offer the promise for local control with low morbidity for treating solid tumors such as breast cancer.
Supplementary Material
HIFU was applied for 1 min at 3.5 MPa and 1.1 MHz with a 2% Duty Cycle. A) Before HIFU B) During HIFU C) Post HIFU. No bubble generation is observed.
Acknowledgments
This study has been funded by the Samsung Advanced Institute of Technology grant number 20125011 and by the R21-CA151140 IMAT grant from the National Institutes of Health. This study utilized the Small Animal Imaging Resource supported by the In vivo Cancer and Molecular Imaging Center (ICMIC) P50-CA128346. AL is supported by an R25 CA153915 training grant as well as 1F31CA174276 - 01A1 awarded by the National Institutes of Health. ZW was supported by a Career Development Award through the ICMIC and UCSD Academic Senate Grant RK131H. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. LGE was supported by NCI grant K22CA118182. The authors wish to thank Siemens Medical Solutions USA, Inc. for providing the Sequoia US system as equipment loans to UCSD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Alexander Liberman – Writing the Article/Analysis and Interpretation
Zhe Wu – Conception and design/Analysis and Interpretation
Christopher V. Barback – Data collection
Robert D. Viveros – Data Collection
James Wang – Critical Revision
Lesley G. Ellies – Conception and Design
Robert F. Mattrey – Conception and Design/Critical Revision
William C. Trogler – Conception and Design/Critical Revision
Andrew C. Kummel – Conception and design/Analysis and Interpretation/Obtaining Funding
Sarah L. Blair – Conception and design/Analysis and Interpretation/Obtaining Funding
Contributor Information
Alexander Liberman, Materials Science and Engineering Program, University of California, San Diego.
Zhe Wu, Department of Radiology, University of California, San Diego.
Christopher V. Barback, Department of Radiology, University of California, San Diego.
Robert D. Viveros, Department of Nanoengineering, University of California, San Diego.
James Wang, Department of Nanoengineering, University of California, San Diego.
Lesley G. Ellies, Department of Pathology, University of California, San Diego.
Robert F. Mattrey, Department of Radiology, University of California, San Diego; Moores Cancer Center, University of California, San Diego.
William C. Trogler, Department of Chemistry and Biochemistry, University of California, San Diego.
Andrew C. Kummel, Department of Chemistry and Biochemistry, University of California, San Diego.
Sarah L. Blair, Department of Surgery, University of California, San Diego; Moores Cancer Center, University of California, San Diego.
References
- 1.Chapman A, ter Haar G. Thermal ablation of uterine fibroids using MR-guided focused ultrasound-a truly non-invasive treatment modality. Eur Radiol. 2007;17(10):2505–11. doi: 10.1007/s00330-007-0644-8. [DOI] [PubMed] [Google Scholar]
- 2.Illing RO, Kennedy JE, Wu F, Ter Haar GR, Protheroe AS, Friend PJ, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93(8):890–5. doi: 10.1038/sj.bjc.6602803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poissonnier L, Chapelon J-Y, Rouvière O, Curiel L, Bouvier R, Martin X, et al. Control of Prostate Cancer by Transrectal HIFU in 227 Patients. Eur Urol. 2007;51(2):381–7. doi: 10.1016/j.eururo.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Wu F, Wang Z-B, Cao Y-D, Zhu X-Q, Zhu H, Chen W-Z, et al. “Wide local ablation” of localized breast cancer using high intensity focused ultrasound. J Surg Oncol. 2007;96(2):130–6. doi: 10.1002/jso.20769. [DOI] [PubMed] [Google Scholar]
- 5.Ta CN, Liberman A, Paul Martinez H, Barback CV, Mattrey RF, Blair SL, et al. Integrated processing of contrast pulse sequencing ultrasound imaging for enhanced active contrast of hollow gas filled silica nanoshells and microshells. J Vac Sci Technol B. 2012;30(2):02C104–02C-6. doi: 10.1116/1.3694835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberman A, Wu Z, Barback CV, Viveros R, Blair SL, Ellies LG, et al. Color Doppler Ultrasound and Gamma Imaging of Intratumorally Injected 500 nm Iron–Silica Nanoshells. ACS Nano. 2013;7(7):6367–77. doi: 10.1021/nn402507d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberman A, Martinez HP, Ta CN, Barback CV, Mattrey RF, Kono Y, et al. Hollow silica and silica-boron nano/microparticles for contrast-enhanced ultrasound to detect small tumors. Biomaterials. 2012;33(20):5124–9. doi: 10.1016/j.biomaterials.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach J, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219(1):176–85. doi: 10.1148/radiology.219.1.r01ap02176. [DOI] [PubMed] [Google Scholar]
- 9.Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR Imaging–guided Focused US Ablation of Breast Cancer: Histopathologic Assessment of Effectiveness—Initial Experience. Radiology. 2003;227(3):849–55. doi: 10.1148/radiol.2281012163. [DOI] [PubMed] [Google Scholar]
- 10.Gianfelice D, Khiat A, Boulanger Y, Amara M, Belblidia A. Feasibility of Magnetic Resonance Imaging–guided Focused Ultrasound Surgery as an Adjunct to Tamoxifen Therapy in High-risk Surgical Patients with Breast Carcinoma. Journal of Vascular and Interventional Radiology. 2003;14(10):1275–82. doi: 10.1097/01.rvi.0000092900.73329.a2. [DOI] [PubMed] [Google Scholar]
- 11.Study of ExAblate Focused Ultrasound Ablation of Breast Cancer Clinicaltrials.gov: Clinicaltrials.gov. 2012 [cited 2014 2/24/2014]. Available from: http://clinicaltrials.gov/ct2/show/NCT01620359.
- 12.Jolesz FA, Hynynen K. Magnetic resonance image-guided focused ultrasound surgery. The Cancer Journal. 2002;8:S100–S12. [PubMed] [Google Scholar]
- 13.Xu Z, Raghavan M, Hall TL, Chang C-W, Mycek MA, Fowlkes JB, et al. High speed imaging of bubble clouds generated in pulsed ultrasound cavitational therapy-histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54(10):2091–101. doi: 10.1109/TUFFC.2007.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kieran K, Hall TL, Parsons JE, Wolf JS, Jr, Fowlkes JB, Cain CA, et al. Refining Histotripsy: Defining the Parameter Space for the Creation of Nonthermal Lesions With High Intensity, Pulsed Focused Ultrasound of the In Vitro Kidney. J Urol. 2007;178(2):672–6. doi: 10.1016/j.juro.2007.03.093. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z, Raghavan M, Hall TL, Mycek MA, Fowlkes JB, Cain CA. Evolution of bubble clouds induced by pulsed cavitational ultrasound therapy-histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55(5):1122–32. doi: 10.1109/TUFFC.2008.764. [DOI] [PubMed] [Google Scholar]
- 16.Roberts WW, Hall TL, Ives K, Wolf JS, Jr, Fowlkes JB, Cain CA. Pulsed Cavitational Ultrasound: A Noninvasive Technology for Controlled Tissue Ablation (Histotripsy) in the Rabbit Kidney. J Urol. 2006;175(2):734–8. doi: 10.1016/S0022-5347(05)00141-2. [DOI] [PubMed] [Google Scholar]
- 17.Vlaisavljevich E, Kim Y, Allen S, Owens G, Pelletier S, Cain C, et al. Image-Guided Non-Invasive Ultrasound Liver Ablation Using Histotripsy: Feasibility Study in an In Vivo Porcine Model. Ultrasound Med Biol. 2013;39(8):1398–409. doi: 10.1016/j.ultrasmedbio.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu T, Wang G, Hu K, Ma P, Bai J, Wang Z. A microbubble agent improves the therapeutic efficiency of high intensity focused ultrasound: a rabbit kidney study. Urol Res. 2004;32(1):14–9. doi: 10.1007/s00240-003-0362-x. [DOI] [PubMed] [Google Scholar]
- 19.Tran B, Hall T, Fowlkes J, Cain C, editors. Ultrasonics Symposium, 2000 IEEE. IEEE; 2000. Non-invasive ultrasound surgery: role of microbubble enhanced cavitation. [DOI] [PubMed] [Google Scholar]
- 20.Kopechek J, Zhang P, Burgess M, Porter T. Synthesis of Phase-shift Nanoemulsions with Narrow Size Distributions for Acoustic Droplet Vaporization and Bubble-enhanced Ultrasound-mediated Ablation. Journal of visualized experiments: JoVE. 2012;(67) doi: 10.3791/4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo W, Zhou X, Ren X, Zheng M, Zhang J, He G. Enhancing Effects of SonoVue, a Microbubble Sonographic Contrast Agent, on High-Intensity Focused Ultrasound Ablation in Rabbit Livers In Vivo. J Ultrasound Med. 2007;26(4):469–76. doi: 10.7863/jum.2007.26.4.469. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Fabiilli ML, Haworth KJ, Padilla F, Swanson SD, Kripfgans OD, et al. Acoustic Droplet Vaporization for Enhancement of Thermal Ablation by High Intensity Focused Ultrasound. Acad Radiol. 2011;18(9):1123–32. doi: 10.1016/j.acra.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneko Y, Maruyama T, Takegami K, Watanabe T, Mitsui H, Hanajiri K, et al. Use of a microbubble agent to increase the effects of high intensity focused ultrasound on liver tissue. Eur Radiol. 2005;15(7):1415–20. doi: 10.1007/s00330-005-2663-7. [DOI] [PubMed] [Google Scholar]
- 24.Tran BC, Seo J, Hall TL, Fowlkes JB, Cain CA. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 2003;50(10):1296–304. doi: 10.1109/tuffc.2003.1244746. [DOI] [PubMed] [Google Scholar]
- 25.Klibanov AL. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv Drug Del Rev. 1999;37(1–3):139–57. doi: 10.1016/s0169-409x(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 26.Vlaisavljevich E, Durmaz YY, Maxwell A, ElSayed M, Xu Z. Nanodroplet-Mediated Histotripsy for Image-guided Targeted Ultrasound Cell Ablation. Theranostics. 2013;3(11):851–64. doi: 10.7150/thno.6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Chen H, Zheng Y, Ma M, Chen Y, Zhang K, et al. Au-nanoparticle coated mesoporous silica nanocapsule-based multifunctional platform for ultrasound mediated imaging, cytoclasis and tumor ablation. Biomaterials. 2013;34(8):2057–68. doi: 10.1016/j.biomaterials.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Chen H, Chen Y, Ma M, Zhang K, Li F, et al. Perfluorohexane-Encapsulated Mesoporous Silica Nanocapsules as Enhancement Agents for Highly Efficient High Intensity Focused Ultrasound (HIFU) Adv Mater. 2012;24(6):785–91. doi: 10.1002/adma.201104033. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Chen H, Sun Y, Zheng Y, Zeng D, Li F, et al. Multifunctional Mesoporous Composite Nanocapsules for Highly Efficient MRI-Guided High-Intensity Focused Ultrasound Cancer Surgery. Angew Chem. 2011;123(52):12713–7. doi: 10.1002/anie.201106180. [DOI] [PubMed] [Google Scholar]
- 30.Pohaku Mitchell KK, Liberman A, Kummel AC, Trogler WC. Iron(III)-Doped, Silica Nanoshells: A Biodegradable Form of Silica. J Am Chem Soc. 2012;134(34):13997–4003. doi: 10.1021/ja3036114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez HP, Kono Y, Blair SL, Sandoval S, Wang-Rodriguez J, Mattrey RF, et al. Hard Shell Gas-Filled Contrast Enhancement Particles for Colour Doppler Ultrasound Imaging of Tumors. Medchemcomm. 2010;1(4):266–70. doi: 10.1039/c0md00139b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Zhu H, Jin C, Zhou K, Li K, Su H, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19(2):437–45. doi: 10.1007/s00330-008-1137-0. [DOI] [PubMed] [Google Scholar]
- 33.Lake AM, Hall TL, Kieran K, Fowlkes JB, Cain CA, Roberts WW. Histotripsy: Minimally Invasive Technology for Prostatic Tissue Ablation in an In Vivo Canine Model. Urology. 2008;72(3):682–6. doi: 10.1016/j.urology.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HIFU was applied for 1 min at 3.5 MPa and 1.1 MHz with a 2% Duty Cycle. A) Before HIFU B) During HIFU C) Post HIFU. No bubble generation is observed.


