Abstract
Despite recent declines in the rates of cigarette smoking, smoking remains prevalent among individuals with lower income, less education, and those with mental illness or HIV. Exercise is promoted as an aid to smoking cessation; however, the evidence for this recommendation is equivocal. To date, the majority of studies have only examined aerobic exercise; there is a poor understanding of the mechanisms of action; and there is an under-representation of male smokers. The goal of this trial is to produce new data that will help to address each of these gaps. A total of 206 male and female smokers will receive a brief smoking cessation education session prior to being randomized into a 12-week Resistance Training (RT) or Wellness Contact Control group. Both groups will have the option of using nicotine replacement therapy (NRT), and both will meet on-site twice per week during the 12-week program (24 total sessions). Follow-up assessments will occur at the end of the 12-weeks (3-month), and at a 6-month and 12-month (post-randomization) visit. Participants will not receive any additional smoking cessation treatment during follow-up; however, the RT group will receive a 9-month membership to a fitness center to encourage continued resistance training as a way to maintain cessation, and attendance will be tracked. The primary outcome is salivary-cotinine-verified 7-Day Point Prevalence Abstinence (PPA) at the 3-month assessment, and at the 6 and 12-month follow-ups. Secondary outcomes include effects of resistance training on nicotine withdrawal symptoms, indicators of mental health, and markers of disease risk.
Introduction
Each year, tobacco use kills nearly 6 million people and costs more than half a trillion dollars worldwide (WHO, 2013). In the United States (US), the primary method of tobacco use is cigarette smoking, and approximately 19.9% of men and 15.2% of women currently smoke (Schiller, Ward, & Freeman, 2013). These rates are known to differ by other demographic variables such as race, education, and income level (Schoenborn, Adams, Peregoy, 2013; CDC, 2012) and they differ by current health status. For example, the rate of cigarette smoking is estimated to be highest among persons living with human immunodeficiency virus (HIV; 59%–85%; Marshall et al., 2011; Tesoriero, Gieryic, Carrascal, & Lavigne, 2010) and those with a diagnosed mental illness (36.1%; CDC, 2013).
In the US, cigarette smoking and exposure to secondhand smoke is estimated to cause 480,000 deaths annually, or about one out of every five deaths (USDHHS, 2014; CDC, 2008). Lung cancer claims the most lives, followed by ischemic heart disease, and chronic obstructive pulmonary disease (COPD; CDC, 2008). In total, more deaths are caused by tobacco use in the US than by all deaths from illegal drug and alcohol use, HIV/AIDS, motor vehicle injuries, murders, and suicides combined (USDHHS, 2004). Fortunately, quitting smoking results in a number of short and long-term benefits. For example, the risk of developing heart disease drops by 50% within one year after quitting, and the risk for a stroke can fall to about the same as a nonsmoker’s, 2–5 years after quitting (USDHHS, 2010). Other risks, such as cancer of the mouth or throat are cut in half five years after quitting, and the risk of dying from lung cancer drops by half 10 years after quitting (USDHHS, 2010). Smoking cessation can also improve mental health, as it is known to be associated with reduced depression, anxiety, and stress and improved mood and quality of life (Taylor, McNeill, Girling, Farley, Lindson-Hawley, & Aveyar, 2014).
Recent estimates indicate that the majority of US smokers would like to quit, with 45.8% having tried in the past year (Schoenborn, Adams, & Peregoy, 2013). Unfortunately, less than 5% of those who attempt to quit are able to maintain long-term abstinence (Rafful, García-Rodríguez, Wang, Secades-Villa, Martínez-Ortega, & Blanco, 2013), particularly greater than six months (Murthy & Subodh, 2010). There are several prescription and over-the-counter medications that have been shown to nearly double the success rate of smoking cessation when compared to a placebo (Herman & Sofuoglu, 2010); however, cost, access, and the perception of medication risk are well known barriers to use (Foulds et al., 2013). In addition, the weight gain associated with quitting can be problematic for both men and women, as current evidence indicates that post-cessation weight gain can range from 4–10 kilograms (Aubin, Farley, Lycett, Lahmek, & Aveyard, 2012). Notably, the increases in body weight following smoking cessation may be attributed to a lower metabolic rate and increased amount of body fat (Kleppinger, Litt, Kenny, & Oncken, 2010; Pistelli, Aquilini, & Carrozzi, 2009). Such changes can significantly diminish the positive health effects of smoking cessation via associated reductions in glucose metabolism (Yeh, Duncan, Schmidt, Wang, & Brancati, 2010), lung function (Chinn et al., 2005), and increases in the risk of developing type 2 diabetes (Luo et al., 2013) and hypertension (Gratziou, 2009).
The U.S. Department of Health and Human Services currently advocates the use of exercise as an aid to quitting (USDHHS, 2008), as do researchers, tobacco treatment specialists and former smokers (Haasova et al., 2014; Everson, Taylor, & Ussher, 2010). However, the overall evidence for using exercise as an aid is equivocal. Specifically, a 2012 Cochrane review determined that larger, adequately powered, sufficiently intense interventions with equal contact control conditions are needed (Ussher, Taylor, & Faulkner, 2012). The authors identified a number of limitations of previous research that included a lack of testing potential mechanisms of action (e.g., reduction in nicotine withdrawal symptoms), a female-only sample, and a strict focus on using aerobic exercise. As such, newer, rigorous research is needed to elucidate the role exercise can play in aiding smoking cessation, particularly for male smokers and anyone who would prefer to engage in various different physical activity modalities. This could potentially enhance compliance and ultimately prevent relapse.
In a prior pilot study, we explored the feasibility of resistance training (i.e., weight training) as an aid to smoking cessation (Ciccolo et al., 2011). Briefly, 12 male and 13 female smokers received a 20-minute smoking cessation counseling session and Nicotine Replacement Therapy (NRT). Participants were then randomized into a two-session per week, 12-week resistance training or contact control program (i.e., 24 total sessions). At the end of treatment, carbon-monoxide (CO)-verified 7-day point prevalence abstinence (PPA) rates were 46% for the resistance training group and 17% for contact control (OR 4.3, 95% CI=0.7–27.8). Additionally, participants in the resistance training group had more favorable changes in body weight (Cohen’s d = −0.7) and body fat (d = −0.8) when compared to the control.
The above pilot results warrant additional research, as the data suggest that resistance training could (1) be a potential aid for smoking cessation and (2) provide smokers with a method to reduce other health risks when trying to quit. More specifically, the potential mechanisms supporting resistance training as an aid for smoking cessation are that it could beneficially affect some of the most well known predictors of relapse, such as negative affect (Leventhal et al., 2013) and sleep disturbance (Hamidovic & de Wit, 2009), as well as barriers to quitting, such as weight gain (Pistelli, Aquilini, & Carrozzi, 2009). For example, studies have shown that resistance training can reduce many of the same negative affective states frequently reported during nicotine withdrawal, such as tension, anxiety, and depression (Arent, Landers, Matt, & Etnier, 2005; Singh, Stavrinos, Scarbek, Galambos, Liber, & Fiatarone-Singh, 2005). Other research has shown that there is a significant association between resistance training and increased quality of sleep (Yang, Ho, Chen & Chien, 2012); and the effects of resistance training on metabolism and body composition have been rigorously tested and are well established (Strasser, Siebert, & Schobersberger, 2010). In addition to these potential positive effects, the health benefits gained from resistance training could also be particularly helpful for smokers, as resistance training has been shown to improve lung function (Singh, Jani, John, Singh, & Joseley, 2011), blood lipids (Kelley & Kelley, 2009), and blood glucose control (Strasser, Siebert, & Schobersberger, 2010). All of these factors are known indicators of disease risk that are also significantly associated with smoking (USDHHS, 2014).
As such, the purpose of this paper is to outline the rationale and design of Strength To Quit, a large, laboratory-based, randomized controlled trial (RCT) testing the use of resistance training as an aid to smoking cessation. Strength To Quit was specifically designed to examine the following three questions: (a) What is the efficacy of resistance training as an aid to smoking cessation for male and female smokers? (b) What are the psychological and physiological mechanisms of the effects of resistance training on smoking cessation? and (c) Does resistance training attenuate detrimental changes in markers of disease risk associated with quitting (e.g., body fat gain)?
Study Methods
Study Design
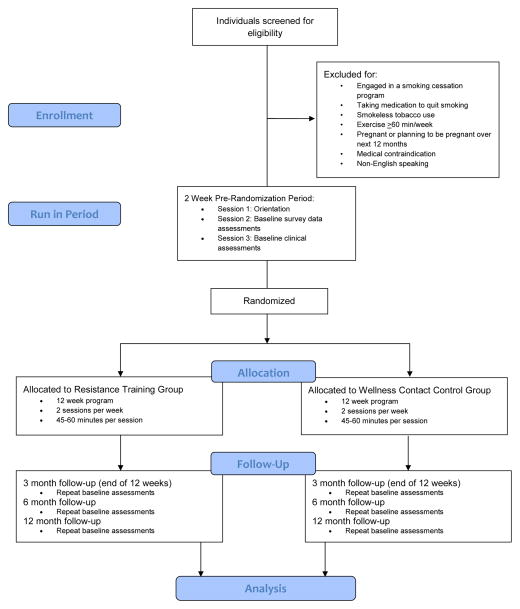
Strength To Quit uses a 2-group design in which 206 participants will be randomized into 12-week Resistance Training (n=103) or Wellness Contact Control (n=103) conditions. A stratified block randomization procedure is used to achieve balance between the two groups on gender and intention to use the nicotine patch. All participants receive a brief smoking cessation education session prior to being randomized. Both groups meet on-site twice per week during the 12-week program (24 total sessions), and follow-up assessments occur at the end of the 12-weeks (3-month) and at a 6-month and 12-month (post-randomization) follow-up (See Figure 1). The primary outcome is salivary-cotinine-verified 7-Day PPA measured at each of the follow-up assessments.
Figure 1.
Study Design.
Participants and Eligibility
Eligible participants are male and female smokers, age >18, who have a desire to quit smoking and have regularly smoked five or more cigarettes a day for at least the past 12 months. Potential participants are excluded if they currently engage in any combination of aerobic exercise or resistance training for >60 minutes/week or have over the past 3 months, currently use smokeless tobacco, are taking medication to quit smoking, or are participating in an ongoing smoking cessation treatment. Women who are pregnant or planning to become pregnant are excluded. Anyone with cardiovascular or pulmonary disease (e.g., coronary artery disease, emphysema), and anyone with orthopedic limitations that would prevent their ability to complete a full-body resistance training program are also excluded. Lastly, participants must be able to speak and read English, and have no plans to move out of the area within the next 12 months.
Procedures
Recruitment
Advertisements for this study are posted in newspapers, at subway and bus stops, on the Internet (e.g., Craigslist), on bulletin boards in the local area (i.e., flyers), and on the radio. The bulk of advertisements are placed in a free metro newspaper that is circulated throughout the New York City subway system, as it has a large readership that includes significant numbers of racial and ethnic minorities.
Pre-Randomization Sessions
Individuals who appear to be eligible at the phone screen are asked to attend three sessions prior to randomization. The first session is an orientation to provide additional information about the study and to obtain informed consent. The second and third sessions are used to collect survey data and conduct clinical assessments (See Figure 1). After completing the three pre-randomization sessions participants are eligible to be randomized and begin the 12-week program.
Baseline Smoking Education and Randomization
At the first session of the 12-week program, all participants receive a brief (20-minute) smoking cessation education session based on the Centers for Disease Control and Prevention’s (CDC) “You Can Quit Smoking” consumer guide consisting of a manualized, five-step program (i.e., get ready, get support, learn new skills and behaviors, get medication and use it correctly, and be prepared for difficult situations). Participants are asked to set their quit day one week from this education session. Immediately following the smoking education (i.e., at the same session), participants are randomized into either the Resistance Training or Wellness Contact Control condition. Specifically, participants open a sequentially numbered envelope, which reveals the group allocation. A blinded, off-site biostatistician previously determined the randomization sequence.
Provision of the Nicotine Patch
All participants are given the option of using nicotine replacement therapy during the trial. Use of the nicotine patch is in accordance with the current Clinical Practice Guidelines from the United States Department of Health and Human Services (Fiore et al., 2008). If a participant chooses to use nicotine patches, s/he is asked to use the patch at the beginning the first session of week 2 (quit day). Each week thereafter participants are given a two-week supply of the nicotine patches on an as-needed basis. Participants who smoke more than 10 cigarettes per day are instructed to use a 21 mg patch for six weeks (Weeks 2–7), tapered to a 14 mg patch for the next two weeks (Weeks 8–9), and a 7 mg patch for the final two weeks (Weeks 10–11). Participants who smoke between 5 and 10 cigarettes per day are instructed to begin with a 14 mg patch for six weeks (Weeks 2–7), which is tapered to a 7 mg patch for the next four weeks (Weeks 8–11). The 10 weeks of nicotine patch use and dose tapering protocol are consistent with clinical recommendations (Fiore et al., 2008). All participants are asked to discontinue patch use after 10 weeks (beginning of week 12) in order to obtain valid saliva cotinine assessment at the post-treatment assessment session.
Experimental Conditions
Resistance Training Program
Participants in this study arm attend two, 45–60-minute, progressive resistance training sessions per week for 12 weeks. The program manipulates the key resistance training program variables of volume (repetitions/sets) and intensity (% of 1-repetition maximum; 1-RM), while maintaining a format of a 5-minute (aerobic) warm-up, 35–50 minutes of resistance training and a 5-minute cool-down (stretching). Specifically, participants are guided through a 10-exercise, full-body routine by study staff. For the first 2–3 weeks, participants complete 1–2 sets of each exercise at an intensity that elicits muscular fatigue within 10–15 repetitions (approximately 65%–75% of 1-RM). From weeks 4–12, participants complete 2–3 sets per exercise, and the weight is systematically increased to elicit muscular fatigue within 8–10 repetitions (75%–80% of 1-RM). The rest interval between sets is held constant across the 12 weeks at 30 seconds-1.5 minutes. This program is consistent with the American College of Sports Medicine’s Position Stand on Progression Models in Resistance Training (Ratamess, et al., 2009). Similar programs are known to produce increases in both muscular strength and aerobic fitness (Tanaka & Swensen, 1998).
Wellness Contact Control Condition
Participants in this study arm are required to attend the same number of sessions, for the same amount of time as those in the Resistance Training group. Each session includes a handout on pertinent healthy lifestyle topics for adults (e.g., human anatomy, sex and relationships), an informational video, and a practical component/demonstration. None of the sessions include information on smoking, smoking cessation or exercise. Participants are asked to not change their current exercise behavior during the 12-week intervention.
Exercise Maintenance Program
At the end of the 12-week program, participants in the Resistance Training group receive a 9-month membership to a local fitness center, and they receive an individualized, progressive resistance training plan, which is an extension of the routine completed during the intervention. This is meant to provide the participants with the knowledge and guidance needed to facilitate continued training during the follow-up period. Study staff arrange initial appointments between research participants and the fitness center, but there is no further involvement from research staff once the initial appointment is arranged. For equity, participants in the Wellness Contact Control also receive a 9-month fitness center membership after their 12-month follow-up visit (i.e., after they have completed the trial). Card swipe data detailing the time and date of each visit to the fitness center are collected in the Resistance Training group at the 6 and 12-month follow-ups.
Incentives
Compliance with the resistance training and wellness control programs is crucial to addressing the primary aims of the study (i.e., testing the efficacy of resistance training), therefore, monetary incentives are given to the participants as compensation for their time, effort and attendance. In both groups, participants are paid $25 at week 4, $50 at week 8, and $75 at week 12, with a $50 bonus for completing >22 sessions. In addition, all participants receive $50 for attending each of the three follow-up assessments (3, 6, and 12 month), with a $50 bonus for completing all three of the follow-up assessments. Incentives are not dependent on smoking status.
Measures
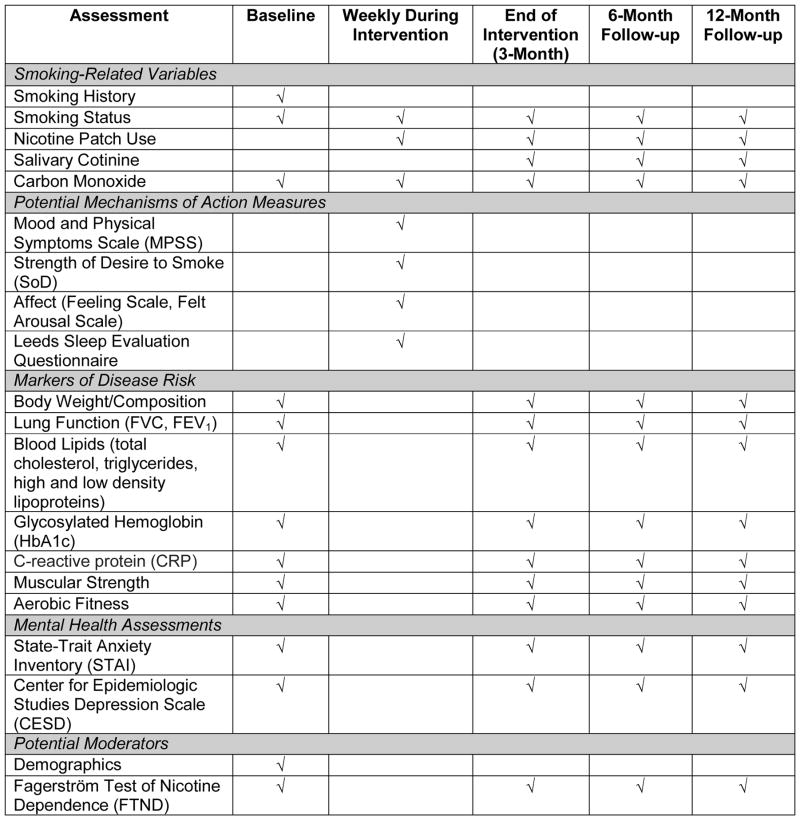
Participants complete a battery of assessments at baseline, weekly during the intervention, at the end of treatment (3-month), and at the 6 and 12-month follow-ups (see Figure 2).
Figure 2.
Schedule of Assessments.
Primary Outcome
The primary outcome is salivary-cotinine-verified 7-Day PPA at the 3-month assessment, and at the 6 and 12-month follow-ups. In addition, smoking status is assessed via self-report and measured objectively with a handheld breath carbon monoxide monitor at each of the two weekly sessions during the 12-week program, and at the 6 and 12-month follow-ups. Specifically, participants are asked whether or not “even a puff” was taken within the last 7 days, 24 hours, and the date of the last puff. This allows for an examination of the number of smoking cessation outcomes, including: (a) initial abstinence (24-hour abstinence); (b) time to first lapse (first smoking event following initial abstinence); (c) time to relapse (smoking at least 5 cigarettes/day for 3 consecutive days); (c) prolonged abstinence (reaching post-treatment without relapse following a two-week grace period after quit day); and (d) 7-day PPA. These outcome measures will be determined by self-report and confirmed by objective smoking assessments.
Covariate: Nicotine Replacement
Nicotine patch use is assessed at every session. Participants are asked if they used a nicotine patch since their last visit, how many, and if they have had any side effects. They are also asked if they have used any other supplemental nicotine (e.g., gum, lozenge), smoking cessation medications (e.g., varenicline, bupropion), or an electronic cigarette.
Secondary Outcomes
Secondary outcomes are divided into two major categories: (1) potential mechanisms of action; and (2) markers of disease risk associated (See Figure 2).
Potential Mechanisms of Action Measures
The potential mechanisms of action are measured at each session during the 12 weeks.
Withdrawal Symptoms
Nicotine withdrawal symptoms are regularly reported as the major barrier to successful smoking cessation (Piasecki, 2006). To be consistent with other research in this area, the Mood and Physical Symptoms Scale (MPSS; West & Hajek, 2004) is used to measure nicotine withdrawal symptoms. The MPSS is a 7-item survey that shows good sensitivity to changes in craving and withdrawal symptoms over 24 hours abstinence, and compares well with other scales of nicotine withdrawal (West, Ussher, Evans, & Rashid, 2006).
Cravings
Cigarette cravings and urges to smoke are reported by nearly all smokers following abstinence (Ussher, Beard, Abikoye, Hajek, & West, 2013). The Strength of Desire to Smoke (SoD), a single item measure (How strong are your smoking urges just now?), is used to assess cigarette craving. The SoD has been used in a number of previous studies testing the effects of aerobic exercise on cigarette craving with adequate reliability, validity and internal consistency (Taylor, Ussher, & Faulkner, 2007).
Affect
Negative shift in affective valence is often experienced during nicotine withdrawal and is a consistent, independent predictor of relapse (Leventhal et al., 2013). There is currently a substantial amount of evidence detailing the acute benefits of resistance training on various mood states and well-being (Bibeau, Moore, Mitchell, Vargas-Tonsing, Bartholomew, 2010; Arent, Landers, Matt, & Etnier, 2005). As such, affective valence is acutely assessed; this is by using the single-item Feeling Scale. The scale ranges from −5 (very bad) to +5 (very good) with 0 (neutral) at the mid-point. The Feeling Scale has been used in research testing the effects of aerobic exercise on nicotine withdrawal with adequate reliability, validity and internal consistency (Taylor, Ussher, & Faulkner, 2007).
Sleep
Sleep is a primary symptom of nicotine withdrawal (Hamidovic & de Wit, 2009), and can interfere with the ability to quit smoking or maintain long-term abstinence (Riemerth, Kunze, & Groman, 2009). Chronic and acute resistance training has been shown to result in improved quality of sleep in individuals with and without sleep problems (Yang, Ho, Chen & Chien, 2012; Viana et al., 2012). Thus, participants’ quality of sleep on the previous night is measured; this is by using the Leeds Sleep Evaluation Questionnaire (LSEQ), which has shown adequate consistency, reliability and validity in other research (Tarrasch, Laudon, & Zisapel, 2003).
Markers of Disease Risk
The markers of disease risk are measured by staff who are blind to the participant’s condition. All assessments are taken at baseline and each follow-up.
Body Weight and Composition
Unlike the majority of studies on exercise and smoking cessation, both body weight and composition are monitored in this study. These assessments will identify any changes in fat mass that occur over the 12-month trial. Body weight is measured using a calibrated electronic scale (Cosmed, Inc., Concord, CA, USA). Body composition is determined using whole-body air displacement plethysmography (Bod Pod, Cosmed, Inc., Concord, CA, USA) with estimated thoracic gas volume according to the manufacturer’s guidelines. Participants wear minimal clothing for each of these tests. The validity of air displacement plethysmography for male and female adults has been established (Baracos et al., 2012).
Lung Function
Smokers are at high-risk for developing impaired lung function (Nagelmann, Tonnov, Laks, Sepper, & Prikk, 2011). Resistance training can improve lung functioning (Singh, Jani, John, Singh, & Joseley, 2011) and is therefore monitored in this study. Lung function is assessed using a portable spirometer (Spirobank G Spirometer, Medical International Research, Inc., Waukesha, WI, USA). Forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) is determined from three trials, and the values for FEV1 and FVC are selected that fulfill the reproducibility and acceptability criteria in accordance with the American Thoracic Society criteria (Miller et al., 2005). Absolute values and percentages of predicted values of the lung function parameters will be used for analysis.
Blood Sample
Several studies have reported smokers to be at increased risk for developing insulin resistance (Luo et al., 2013) and dyslipidemia (Gepner, Piper, Johnson, Fiore, Baker, & Stein, 2011). Resistance training has been shown to result in statistically significant reductions in glycosylated hemoglobin (HbA1c), a marker of blood glucose control (Strasser, Siebert, & Schobersberger, 2010), and it may improve blood lipids (e.g., total cholesterol, triglycerides; Kelley & Kelley, 2009). As such, participants provide a sample of blood that is analyzed for HbA1c, total cholesterol, triglycerides, and high and low density lipoproteins. In addition, C-reactive protein (CRP) is measured, as it has been shown to be chronically elevated in smokers (Dietrich, Garcia, de Pablo, Schulze, & Hoffmann, 2007), and long-term elevations are consistently associated with an increased risk of coronary heart disease and stroke (Kaptoge et al., 2010). While research has shown that engaging in a resistance training program can lead to significant reductions in CRP concentrations (Donges, Duffield, & Drinkwater, 2010), we are unaware of any published study that has investigated the relationships among CRP, resistance training and smoking.
Physical Fitness
Muscular strength (Ruiz et al., 2008) and aerobic capacity (Barry, Baruth, Beets, Durstine, Liu, & Blair, 2014) are well-known markers of disease risk and are predictors of mortality. The resistance training program used in this study is expected to impact both muscular strength and aerobic capacity (Tanaka & Swensen, 1998), thus, each is assessed. Muscular strength is measured using upper (chest press) and lower (leg extension) body exercises using the American College of Sports Medicine’s (ACSM) protocol for 1-RM testing (ACSM, 2014). Aerobic capacity is assessed six-minute treadmill walk test (6MWT; Laskin et al., 2007). Both measures are regularly used to determine physical fitness in diseased and healthy populations, and both have acceptable validity and reliability (ACSM, 2013; Laskin et al., 2007).
Mental Health Assessments
Cigarette smokers are significantly more likely than non-smokers to report having a mental illness (SAMHSA, 2013), and smokers with a mental illness may be more likely to make a quit attempt (Morris, Burns, Waxmonsky, & Levinson, 2014). As such, the following assessments are taken at baseline and each follow-up.
Anxiety
Smokers are well known to have elevated levels of anxiety (Moylan, Jacka, Pasco, & Berk, 2012) and regular resistance training has been shown to reduce anxiety in a variety of populations (Herring, Jacob, Suveg, Dishman, & O’Connor, 2012; Hale & Raglin 2002). Trait anxiety is measured using the trait component of the State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). This 20-item scale measures items (e.g., I worry too much over something that really doesn’t matter) on a 4-point scale from “Almost Never” to “Almost Always.” The scale has shown adequate consistency, reliability and validity in a wealth of research (Spielberger, 1989).
Depression
Similar to anxiety, smokers are also known to have elevated levels of depression (Tjora, Hetland, Aarø, Wold, Wiium, & Overland, 2014) and reductions in depression have been reported after resistance training in clinical and non-clinical samples (Cooney et al., 2013; Singh, Stavrinos, Scarbek, Galambos, Liber, & Fiatarone-Singh, 2005). The 10-item Center for Epidemiologic Studies Depression Scale (CES-D) is used to measure depression symptoms over the past week (Björgvinsson, Kertz, Bigda-Peyton, McCoy, & Aderka, 2013). Items are rated on a 4-point scale from 0 (less than one day) to 4 (5–7 days). The scale has been validated (Björgvinsson, Kertz, Bigda-Peyton, McCoy, & Aderka, 2013) and used in other smoking cessation trials (e.g., Hennrikus et al., 2010).
Potential Moderators
Each of the following measures will be collected at baseline and tested as a potential moderator of the primary outcome.
Demographics
This questionnaire asks for information on the participant’s age, gender, race, ethnicity, relationship status, family size, occupation, total household income and level of education.
Nicotine Dependence
The Fagerström Test for Nicotine Dependence (FTND) is used to measure nicotine dependence. The FTND is a 6-item measure with excellent reliability and validity in smoking research. It is considered the standard measure of nicotine dependence (Heatherton et al., 1991).
Manipulation Check
The resistance training completed on-site as part of the 12-week program is directly observed by research staff. Compliance to the program will be calculated as the number of sessions attended over the 12-weeks. Additional training outside of the on-site sessions is discouraged for the Resistance Training group, as is any exercise for the Wellness Control group. To monitor this throughout the trial, the past week Modifiable Activity Questionnaire (Pettee Gabriel, McClain, Schmid, Storti, Ainsworth, 2011) is administered at weeks 4, 8, 12, and at each follow-up assessment. In addition, for participants in the Resistance Training group, frequency of attendance at the fitness center during the follow-up period is monitored via their card swipe data; and any changes in fitness will be identified with the physical fitness tests.
ANALYSIS
Sample Size and Power Considerations
The sample size is designed to have 80% power for testing the null hypothesis that the intention to treat effect is zero, versus the two-sided alternative. In the analysis and sample size calculations, all dropouts will be coded as treatment failures (smokers). This approach has empirical justification in studies of smoking cessation (Lichtenstein & Glasgow, 1992). Sample size estimates for this trial were based on 6-month quit rates of 38% in the Resistance Training group versus 17% in the Contact Control (Ciccolo et al., 2011). While it is possible that effects may be stronger or weaker at the 12-month follow up (compared to those currently estimated from the 6-month follow-up in the pilot study), we do not anticipate significant differences from 6-month quit rates. Thus, using a multiplicity-adjusted two-tailed alpha level of 0.05/3 (1 outcome x 3 time points), a sample size of 103 participants per arm will be needed to have at least 80% power to detect between-group differences in objectively verified 7-day PPA at each of the follow-up times, for a total sample size of 206.
Planned Analysis for Primary Outcomes
Using a longitudinal regression model implemented with Generalized Estimating Equations (GEEs; Zeger & Liang, 1986), with robust standard errors, the effect of treatment assignment on 7-day PPA will be tested. Specifically, the probability of being quit will be regressed on treatment assigned to, as well as any baseline variables that are not equally distributed across the two conditions. Furthermore, we will explore the effect of intention to use NRT and self-reported use on the estimated treatment effect by including each as a covariate in the model. Similar analyses will be conducted to examine the effects of treatment on prolonged abstinence.
Planned Analysis for Secondary Outcomes
The treatment effects of resistance training on potential mechanisms of action will be tested using a series of longitudinal regression models implemented with GEEs (Zeger & Liang, 1986). The weekly measure of the mechanism (e.g., nicotine withdrawal symptoms) will be regressed on the treatment assignment, while controlling for baseline values of the mechanism, quit status at the time the mechanism was assessed, and potential confounders, as determined by preliminary analyses.
To examine potential mechanisms as predictors of smoking cessation outcomes, 7 day PPA, time to first lapse, and time to the start of relapse during the 12-week program will be considered as smoking endpoints. This will allow for a focus on quitting outcomes and on data that is reported before a participant returns to smoking. Time to the start of relapse requires first identifying a relapse, as defined by 5 cigarettes per day on 3 consecutive days, and then using the first cigarette smoked during the 3-day relapse process as the start of relapse. When the outcome of interest is time to a defined event (i.e., first lapse, start of relapse), survival analysis (Hosmer & Lemeshow, 1999) will be used to model the risk of lapsing (relapsing) over time. Survival analysis makes use of the full data set to inform an estimate of the risk of lapse/relapse. That is, each participant contributes two outcome variables to the model: T*i, time to first lapse/relapse and Ci, censoring time. For participants who lapse/relapse before the end of treatment, T*i< Ci; for those who do not lapse/relapse before end of treatment or who discontinue the study protocol before lapsing/relapsing, T*i> Ci. Thus, the model uses Ti = min { T*i, Ci} as the response for each participant. Using a Cox model (Cox, 1972; Therneau & Grambsch, 2000), we can write the hazard function (which can be thought of as the number of lapses/relapses per patient-day of follow-up time) as a function of a baseline hazard rate λ0(t) and covariates X(t). Note that the set X(t) can include both time-variant and time-invariant covariates. Lastly, when the outcome of interest is 7-day PPA, logistic regression models will be used to regress the logit of the probability of 7-day PPA on withdrawal symptoms over time (for example), when controlling for treatment assignment and potential confounders of the association under consideration.
The effects of treatment on markers of disease risk will be tested at each time point (the 3, 6, and 12-month follow-ups) using a repeated measures regression model implemented with GEEs with robust standard errors. These adjusted standard errors will account for repeated measurements. Appropriate link functions will be used (e.g., identity link for continuous outcomes). If needed, a normalizing transformation to the continuous response measures (such as taking the logarithm of lung function) before proceeding with the analysis will be completed. Models will also control for baseline variables that are not equally distributed across treatment conditions. Lastly, the association between smoking outcomes and changes in markers of disease risk (i.e., from baseline to follow-up, 3, 6, and 12 months) will be examined using a longitudinal regression model implemented with GEEs with a logit link (which will estimate, for example, the effect of changes from baseline to time t in markers on the logit of 7-day PPA at time t, controlling for potential confounders).
Finally, using a similar set of analyses to those planned for the primary outcome, we will examine potential moderators of the treatment effect on smoking outcomes (7 day PPA and prolonged abstinence). Specifically, models will include the main effect of the potential moderator (gender for example), the main effect of treatment and the interaction between the two.
LIMITATIONS
There are some limitations with the Strength To Quit trial that need to be acknowledged. First, the large incentives used to enhance compliance reduce the likelihood that the results from this study would easily translate into the community setting. However, because both conditions receive the same incentives and the incentives are not contingent on quitting, generalizability will be limited only to the extent that incentives moderate the effects of resistance training on smoking outcomes (i.e., the extent to which the differences between treatment conditions would be different in the absence of incentives). Moreover, the purpose of the Strength To Quit trial is to test the efficacy of resistance training as an aid for smoking cessation. If initial efficacy can be established, further research will be warranted, particularly studies that offer smokers a choice of different exercise programs that have been shown to be effective for aiding smoking cessation. Second, the mechanisms of action that are hypothesized to support sustained smoking abstinence during the follow-up period via continued resistance training (i.e., consistent reductions in negative affect, sleep disturbance, weight gain) are not monitored. As such, future studies should be designed in a way that would allow this data to be collected (e.g., using ecological momentary assessment) beyond the intervention period.
DISCUSSION
To our knowledge, this is the first large-scale randomized controlled trial to test the use of resistance training as an aid to smoking cessation. This trial was designed to produce new data that will address some of the limitations of previous work on exercise and smoking cessation. For example, the vast majority of studies have used an aerobic-only exercise program, with the use of a female-only sample (Ussher, Taylor, & Faulkner, 2012). Furthermore, the hypothesized mechanisms of action (e.g., reduced negative affect, withdrawal symptoms) supporting a reduction in smoking with exercise have largely been studied outside the context of making a quit attempt (Roberts, Maddison, Simpson, Bullen, & Prapavessis, 2012; Taylor, Ussher, & Faulkner, 2007). As such, the results of this study will add to the literature base and inform current public health initiatives that promote the use of exercise as a method to achieve smoking cessation. Lastly, data from this study will reveal any effects resistance training may have on markers of disease risk in male and female smokers. While the beneficial health effects of resistance training are well known and accepted, the various effects resistance training may have on male and female smokers when trying to quit has not been rigorously examined.
Highlights.
Tobacco use remains the leading worldwide preventable cause of death
Smoking rates remain high among certain groups (e.g., those with mental illness)
The research on the efficacy of exercise to enhance smoking cessation is equivocal
The effects of resistance training in smokers have not been adequately tested
Acknowledgments
The project described in this manuscript is supported by a grant from the National Heart, Lung, and Blood Institute (R01 HL117345 to J.T.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David M. Williams, Email: david_m_williams@brown.edu.
Shira I. Dunsiger, Email: sdunsiger@lifespan.org.
James W. Whitworth, Email: jww2127@tc.columbia.edu.
Aston K. McCullough, Email: akm2169@tc.columbia.edu.
Beth B. Bock, Email: BBock@lifespan.org.
Bess H. Marcus, Email: bmarcus@ucsd.edu.
Merle Myerson, Email: myersonm@optonline.net.
References
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9. Philadelphia, PA: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- Arent SM, Landers DM, Matt KS, Etnier JL. Dose-response and mechanistic issues in the resistance training and affect relationship. Journal of Sport & Exercise Psychology. 2005;27:92–110. [Google Scholar]
- Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracos V, Caserotti P, Earthman CP, Fields D, Gallagher D, Hall KD, et al. Advances in the science and application of body composition measurement. Journal of Parenteral and Enteral Nutrition. 2012;36:96–107. doi: 10.1177/0148607111417448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs. fatness on all-cause mortality: a meta-analysis. Progress in Cardiovascular Diseases. 2014;56:382–390. doi: 10.1016/j.pcad.2013.09.002. Review. [DOI] [PubMed] [Google Scholar]
- Bibeau WS, Moore JB, Mitchell NG, Vargas-Tonsing T, Bartholomew JB. Effects of acute resistance training of different intensities and rest periods on anxiety and affect. Journal of Strength and Conditioning Research. 2010;24:2184–2191. doi: 10.1519/JSC.0b013e3181ae794b. [DOI] [PubMed] [Google Scholar]
- Björgvinsson T, Kertz SJ, Bigda-Peyton JS, McCoy KL, Aderka IM. Psychometric properties of the CES-D-10 in a psychiatric sample. Assessment. 2013;20:429–436. doi: 10.1177/1073191113481998. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Current cigarette smoking among adults - United States, 2011. Morbidity and Mortality Weekly Report. 2012;61:889–894. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses—United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged ≥18 years with mental illness - United States, 2009–2011. Morbidity and Mortality Weekly Report. 2013;62:81–87. [PMC free article] [PubMed] [Google Scholar]
- Chinn S, Jarvis D, Melotti R, Luczynska C, Ackermann-Liebrich U, Antó JM, et al. Smoking cessation, lung function, and weight gain: a follow-up study. Lancet. 2005;365:1629–1635. doi: 10.1016/S0140-6736(05)66511-7. [DOI] [PubMed] [Google Scholar]
- Ciccolo JT, Dunsiger SI, Williams DM, Bartholomew JB, Jennings EG, Ussher MH, et al. Resistance training as an aid to standard smoking cessation treatment: A pilot study. Nicotine & Tobacco Research. 2011;13:756–760. doi: 10.1093/ntr/ntr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, et al. Exercise for depression. Cochrane Database Systematic Reviews. 2013;9:CD004366. doi: 10.1002/14651858.CD004366.pub6. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR. Regression Models and Life Tables (with Discussion) Journal of the Royal Statistical Society: Series B. 1972;34:187–220. [Google Scholar]
- Dietrich T, Garcia RI, de Pablo P, Schulze PC, Hoffmann K. The effects of cigarette smoking on C-reactive protein concentrations in men and women and its modification by exogenous oral hormones in women. European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14:694–700. doi: 10.1097/HJR.0b013e328270b913. [DOI] [PubMed] [Google Scholar]
- Donges CE, Duffield R, Drinkwater EJ. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Medicine & Science in Sports and Exercise. 2010;42:304–313. doi: 10.1249/MSS.0b013e3181b117ca. [DOI] [PubMed] [Google Scholar]
- Everson ES, Taylor AH, Ussher M. Determinants of physical activity promotion by smoking cessation advisors as an aid for quitting: Support for the Transtheoretical Model. Patient Education Counseling. 2010;78:53–56. doi: 10.1016/j.pec.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen RC, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. US Department of Health & Human Services, Public Health Service; Rockville, MD: 2008. [Google Scholar]
- Foulds J, Russ C, Yu CR, Zou KH, Galaznik A, Franzon M, et al. Effect of varenicline on individual nicotine withdrawal symptoms: a combined analysis of eight randomized, placebo-controlled trials. Nicotine and Tobacco Research. 2013;15:1849–1857. doi: 10.1093/ntr/ntt066. [DOI] [PubMed] [Google Scholar]
- Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. American Heart Journal. 2011;161:145–151. doi: 10.1016/j.ahj.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratziou C. Respiratory, cardiovascular and other physiological consequences of smoking cessation. Current Medical Research Opinion. 2009;25:535–545. doi: 10.1185/03007990802707642. [DOI] [PubMed] [Google Scholar]
- Hale BS, Raglin JS. State anxiety responses to acute resistance training and step aerobic exercise across eight weeks of training. Journal of Sports Medicine and Physical Fitness. 2002;42:108–112. [PubMed] [Google Scholar]
- Hamidovic A, de Wit H. Sleep deprivation increases cigarette smoking. Pharmacol Biochem Behavior. 2009;93:263–269. doi: 10.1016/j.pbb.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T, Lozlowski L, Frecker T, Fagerstrom K. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, de Wit H. Sleep deprivation increases cigarette smoking. Pharmacology Biochemistry and Behavior. 2009;93:263–269. doi: 10.1016/j.pbb.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennrikus D, Joseph AM, Lando HA, Duval S, Ukestad L, Kodl M, Hirsch AT. Effectiveness of a smoking cessation program for peripheral artery disease patients: a randomized controlled trial. Journal of the American College of Cardiology. 2010;56:2105–2112. doi: 10.1016/j.jacc.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Herman A, Sofuoglu M. Comparison of available treatments for tobacco addiction. Current Psychiatry Reports. 2010;12:433–440. doi: 10.1007/s11920-010-0134-6. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring MP, Jacob ML, Suveg C, Dishman RK, O’Connor PJ. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychotherapy and Psychosomatics. 2012;81:21–28. doi: 10.1159/000327898. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. John Wiley & Sons, Inc; New York, NY: 1999. [Google Scholar]
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: another look at a meta-analysis using prediction intervals. Preventive Medicine. 2009;49:473–475. doi: 10.1016/j.ypmed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Kleppinger A, Litt MD, Kenny AM, Oncken CA. Effects of Smoking Cessation on Body Composition in Postmenopausal Women. Journal of Womens Health. 2010;19:1651–1657. doi: 10.1089/jwh.2009.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin JJ, Bundy S, Marron H, Moore H, Swanson M, Blair M, Humphrey R. Using a treadmill for the 6-minute walk test: reliability and validity. Journal of Cardiopulmonary Rehabilitation and Prevention. 2007;27:407–410. doi: 10.1097/01.HCR.0000300270.45881.d0. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Greenberg JB, Trujillo MA, Ameringer KJ, Lisha NE, Pang RD, et al. Positive and negative affect as predictors of urge to smoke: temporal factors and meditational pathways. Psychology of Addictive Behaviors. 2013;27:262–267. doi: 10.1037/a0031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein E, Glasgow RE. Smoking cessation: what have we learned over the past decade? Journal of Consulting and Clinical Psychology. 1992;60:518–527. doi: 10.1037//0022-006x.60.4.518. [DOI] [PubMed] [Google Scholar]
- Luo J, Rossouw J, Tong E, Giovino GA, Lee CC, Chen C, et al. Smoking and diabetes: does the increased risk ever go away? American Journal of Epidemiology. 2013;178:937–945. doi: 10.1093/aje/kwt071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MM, Kirk GD, Caporaso NE, McCormack MC, Merlo CA, Hague JC, et al. Tobacco use and nicotine dependence among HIV-infected and uninfected injection drug users. Addictive Behaviors. 2011;36:61–67. doi: 10.1016/j.addbeh.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. European Respiratory Journal. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Morris CD, Burns EK, Waxmonsky JA, Levinson AH. Smoking cessation behaviors among persons with psychiatric diagnoses: Results from a population-level state survey. Drug & Alcohol Dependence. 2014;136:63–68. doi: 10.1016/j.drugalcdep.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Moylan S, Jacka FN, Pasco JA, Berk M. Cigarette smoking, nicotine dependence and anxiety disorders: a systematic review of population-based, epidemiological studies. BMC Medicine. 2012;10:123. doi: 10.1186/1741-7015-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy P, Subodh BN. Current developments in behavioral interventions for tobacco cessation. Current Opinion in Psychiatry. 2010;23:151–156. doi: 10.1097/YCO.0b013e328336653f. Review. [DOI] [PubMed] [Google Scholar]
- Nagelmann A, Tonnov Ä, Laks T, Sepper R, Prikk K. Lung dysfunction of chronic smokers with no signs of COPD. Journal of Chronic Obstructive Pulmonary Disease. 2011;8:189–195. doi: 10.3109/15412555.2011.565090. [DOI] [PubMed] [Google Scholar]
- Pettee Gabriel K, McClain JJ, Schmid KK, Storti KL, Ainsworth BE. Reliability and convergent valididty of the past-week modifiable activity questionnaire. Public Health and Nutrition. 2011;14:435–442. doi: 10.1017/S1368980010002612. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Pistelli F, Aquilini F, Carrozzi L. Weight gain after smoking cessation. Monaldi Archives Chest Disease. 2009;71:81–87. doi: 10.4081/monaldi.2009.367. Review. [DOI] [PubMed] [Google Scholar]
- Rafful C, García-Rodríguez O, Wang S, Secades-Villa R, Martínez-Ortega JM, Blanco C, et al. Predictors of quit attempts and successful quit attempts in a nationally representative sample of smokers. Addict Behaviors. 2013;38:1920–1923. doi: 10.1016/j.addbeh.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratamess NA, Alvar BA, Evetoch TK, Housh TJ, Kinler WB, Kraemer WJ, et al. Progression models in resistance training for healthy adults. Special communication. Medicine & Science in Sports & Exercise. 2009;41:687–708. [Google Scholar]
- Riemerth A, Kunze U, Groman E. Nocturnal sleep-disturbing nicotine craving and accomplishment with a smoking cessation program. Wiener Medizinische Wochenschrift. 2009;159:47–52. doi: 10.1007/s10354-008-0640-x. [DOI] [PubMed] [Google Scholar]
- Roberts V, Maddison R, Simpson C, Bullen C, Prapavessis H. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review update and meta-analysis. Psychopharmacology. 2012;222:1–15. doi: 10.1007/s00213-012-2731-z. [DOI] [PubMed] [Google Scholar]
- Ruiz JR, Sui X, Lobelo F, Morrow JR, Jr, Jackson AW, Sjöström M, Blair SN. Association between muscular strength and mortality in men: prospective cohort study. British Medical Journal. 2008;337:a439. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller JS, Ward BW, Freeman G. Early release of selected estimates based on data from the January–June 2013 National Health Interview Survey. National Center for Health Statistics; 2013. Retrieved from: http://www.cdc.gov/nchs/nhis.htm. [Google Scholar]
- Schoenborn CA, Adams PF, Peregoy JA. Health behaviors of adults: United States, 2008–2010. National Center for Health Statistics. Vital Health Statistics. 2013;10:257. [PubMed] [Google Scholar]
- Singh NA, Stavrinos TM, Scarbek Y, Galambos G, Liber C, Fiatarone Singh MA. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2005;60:768–776. doi: 10.1093/gerona/60.6.768. [DOI] [PubMed] [Google Scholar]
- Singh VP, Jani H, John V, Singh P, Joseley T. Effects of upper body resistance training on pulmonary functions in sedentary male smokers. Lung India. 2011;28:169–173. doi: 10.4103/0970-2113.83971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory: Bibliography. 2. Palo Alto, CA: Consulting Psychologists Press; 1989. [Google Scholar]
- Strasser B, Siebert U, Schobersberger W. Resistance training in the treatment of the metabolic syndrome: a systematic review and meta-analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Medicine. 2010;40:397–415. doi: 10.2165/11531380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality. The NSDUH Report: Revised Estimates of Mental Illness from the National Survey on Drug Use and Health. Rockville, MD: 2013. [PubMed] [Google Scholar]
- Tanaka H, Swensen T. Impact of resistance training on endurance performance. A new form of cross-training? Sports Medicine. 1998;25:191–200. doi: 10.2165/00007256-199825030-00005. Review. [DOI] [PubMed] [Google Scholar]
- Tarrasch R, Laudon M, Zisapel N. Cross-cultural validation of the Leeds sleep evaluation questionnaire (LSEQ) in insomnia patients. Human Psychopharmacology. 2003;18:603–610. doi: 10.1002/hup.534. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. Review. [DOI] [PubMed] [Google Scholar]
- Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ. 2014;348:1151. doi: 10.1136/bmj.g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behavior. 2010;14:824–835. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- Tjora T, Hetland J, Aarø LE, Wold B, Wiium N, Overland S. The association between smoking and depression from adolescence to adulthood. Addiction. 2014 doi: 10.1111/add.12522. Ahead of Print. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- U.S. Department of Health & Human Services. A report of the Surgeon General. Rockville, MD: Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- Ussher M, Beard E, Abikoye G, Hajek P, West R. Urge to smoke over 52 weeks of abstinence. Psychopharmacology. 2013;226:83–89. doi: 10.1007/s00213-012-2886-7. [DOI] [PubMed] [Google Scholar]
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Systematic Reviews. 2012;18:CD002295. doi: 10.1002/14651858.CD002295.pub4. [DOI] [PubMed] [Google Scholar]
- Viana VA, Esteves AM, Boscolo RA, Grassmann V, Santana MG, Tufik S, de Mello MT. The effects of a session of resistance training on sleep patterns in the elderly. European Journal of Applied Physiology. 2012;112:2403–2408. doi: 10.1007/s00421-011-2219-2. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology. 2004;177:195–199. doi: 10.1007/s00213-004-1923-6. [DOI] [PubMed] [Google Scholar]
- West R, Ussher M, Evans M, Rashid M. Assessing DSM-IV nicotine withdrawal symptoms: a comparison and evaluation of five different scales. Psychopharmacology. 2006;184:619–627. doi: 10.1007/s00213-005-0216-z. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic, 2013. Enforcing bans on tobacco advertising, promotion and sponsorship. 2013 Retrieved from http://www.who.int/tobacco/global_report/2013/en/
- Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. Journal of Physiotherapy. 2012;58:157–163. doi: 10.1016/S1836-9553(12)70106-6. Review. [DOI] [PubMed] [Google Scholar]
- Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Annals of Internal Medicine. 2010;152:10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]