Abstract
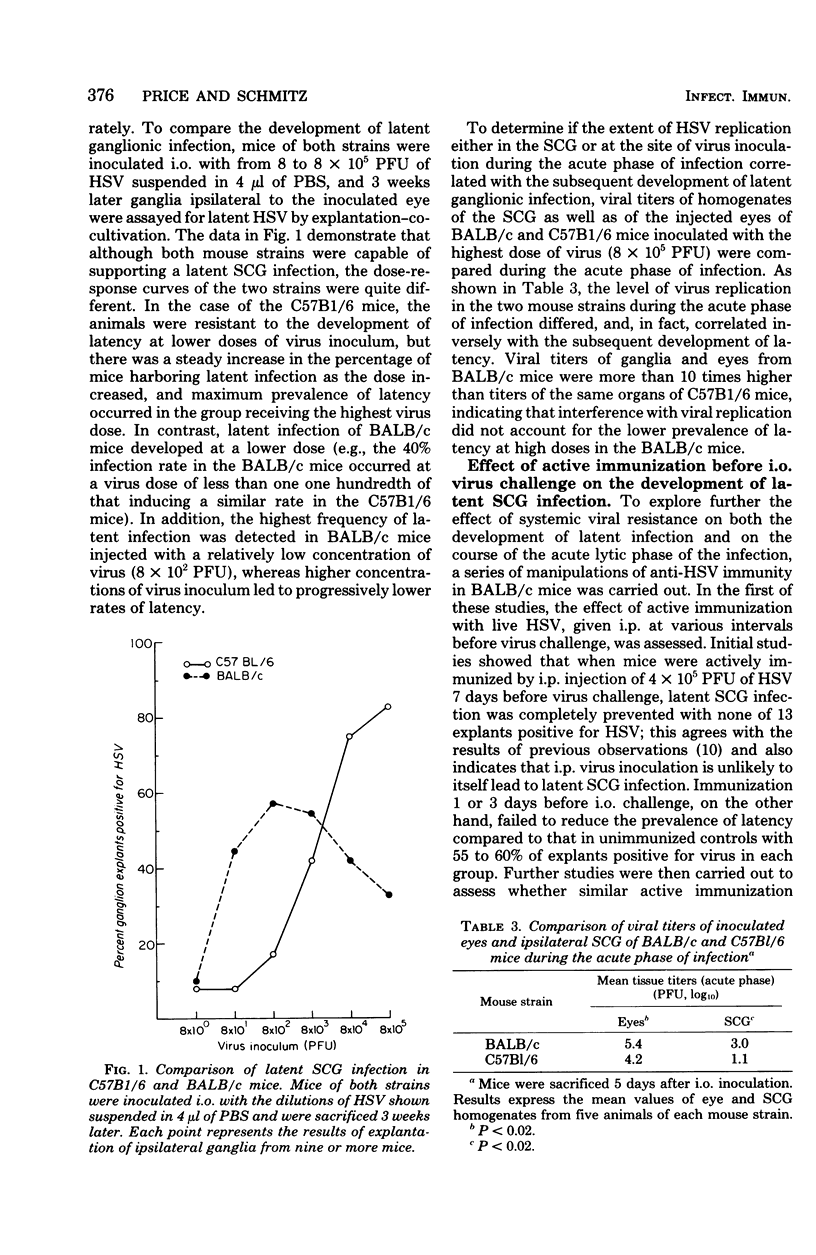
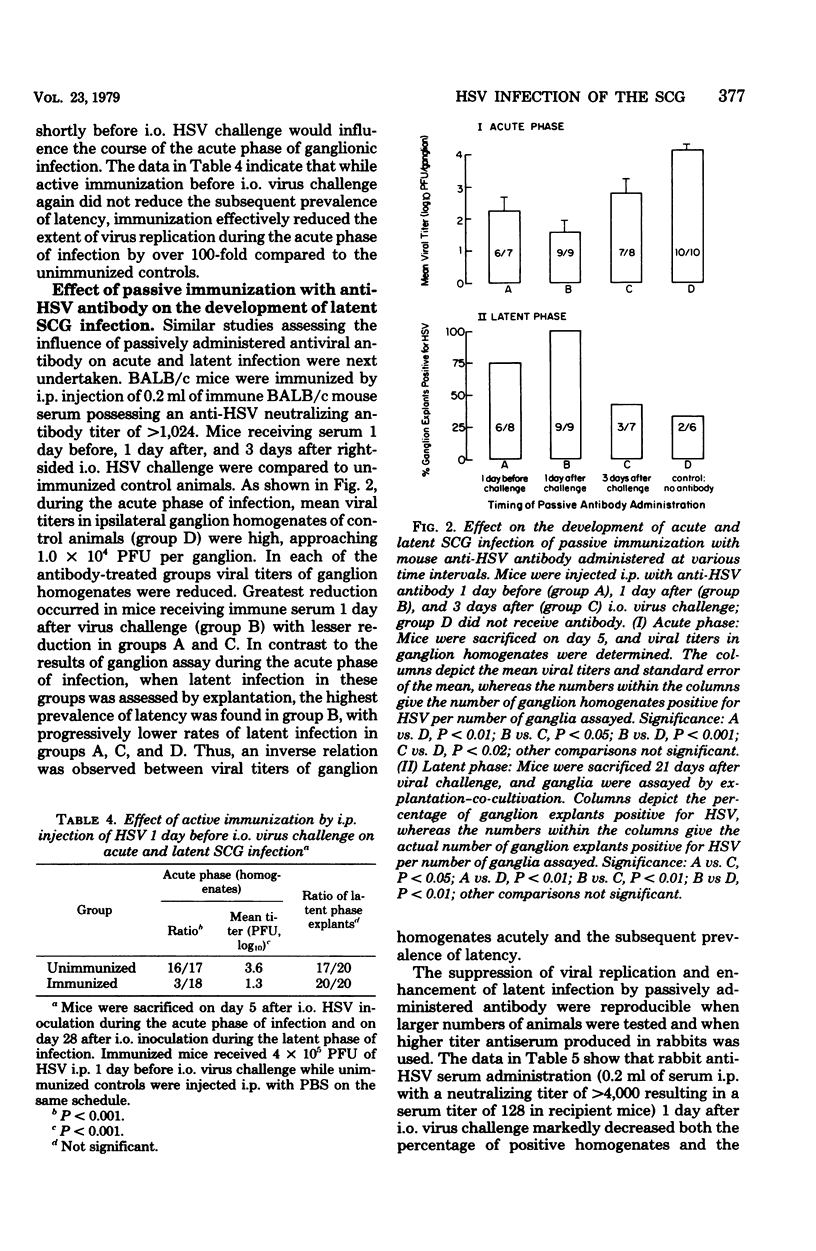
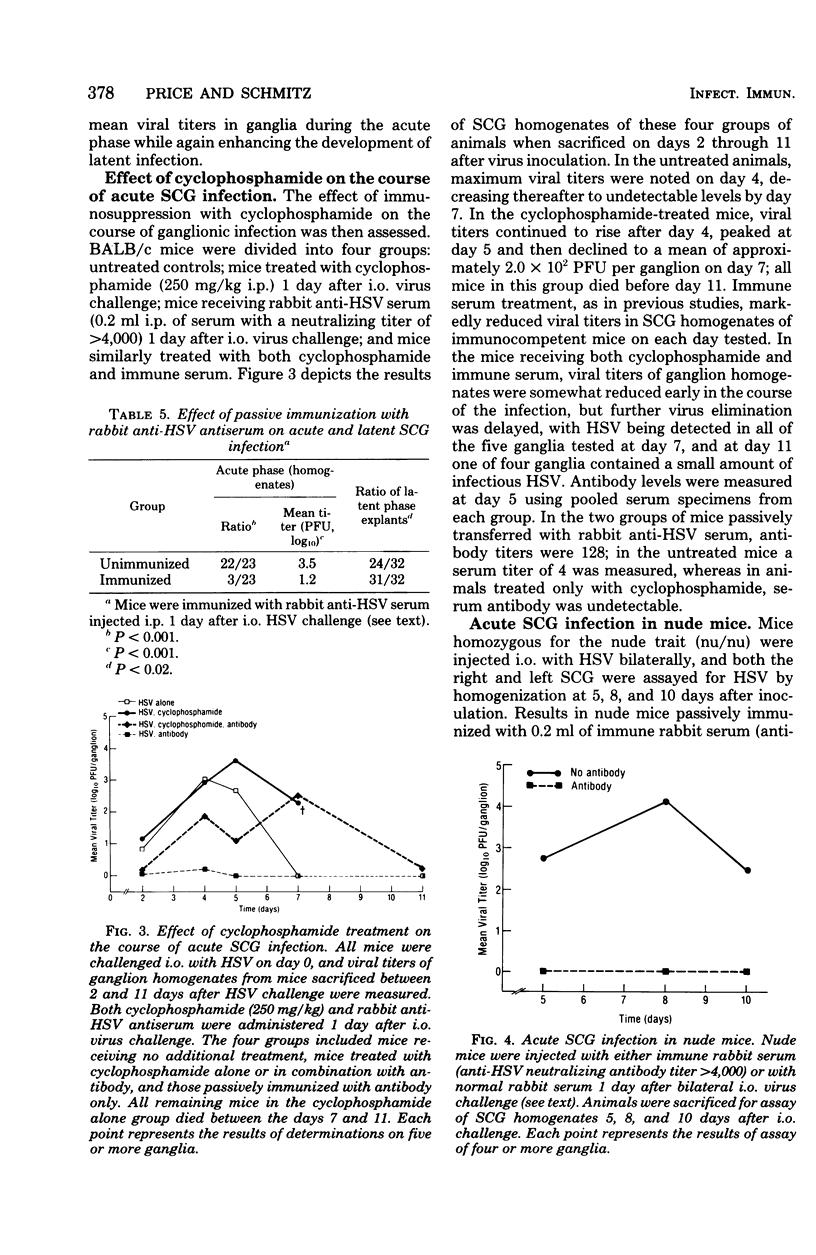
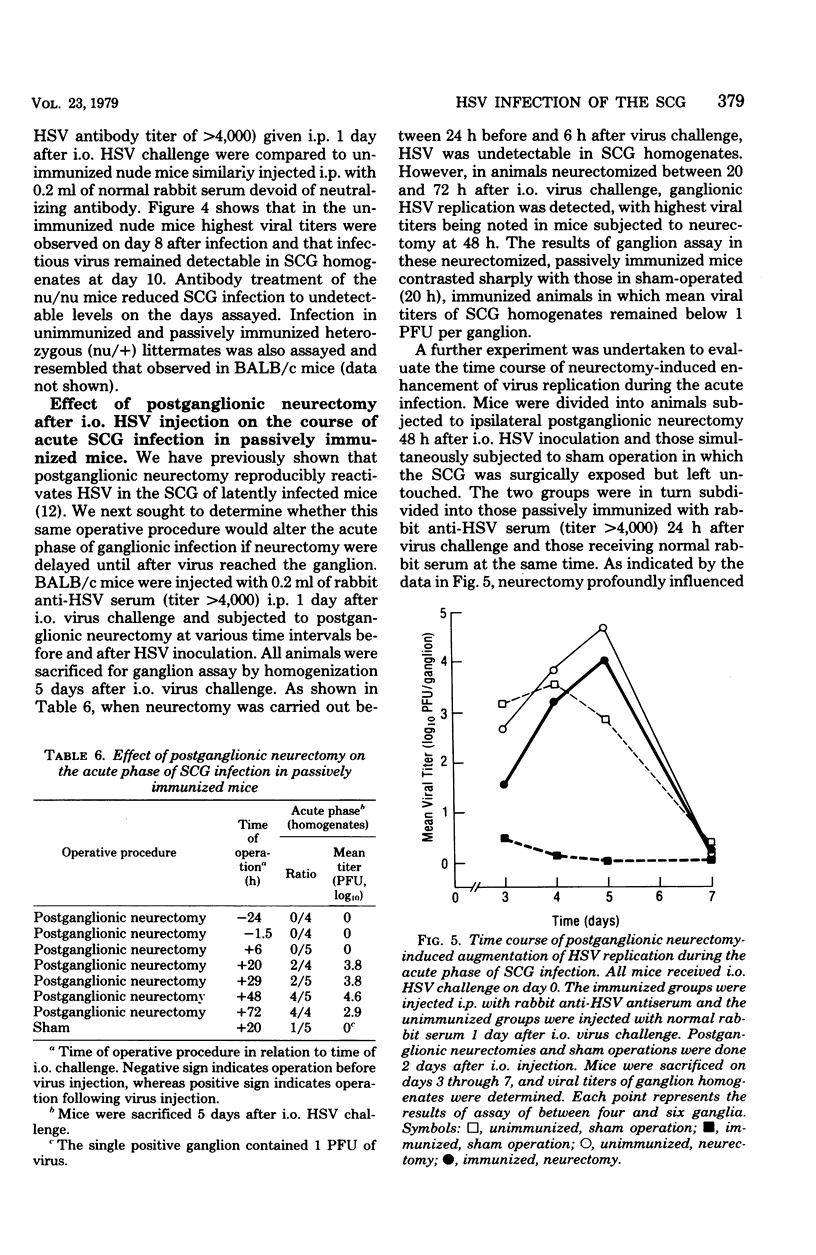
The character of acute and latent herpes simplex virus (HSV) infection of the superior cervical ganglion (SCG) in mice depended on the route by which the virus reached the ganglion, the level of systemic host resistance, and the integrity of postganglionic nerves. Prevention of ganglionic infection by postganglionic neurectomy carried out before intraocular (i.o.) virus challenge established the importance of the neural route in the development of SCG infection. However, hematogenous virus dissemination also led to SCG infection although with reduced frequency compared to that with i.o. inoculation. Enhanced host systemic antiviral resistance had two divergent effects on ganglionic infection depending on the dose and timing of virus inoculation. Thus, both acute and latent ganglionic infections were concomitantly reduced when resistant C57B1/6 mice were challenged with low doses of virus or when less resistant BALB/c mice were actively immunized 1 week before virus challenge. On the other hand, when resistant mice were challenged with high doses of virus or when either active or passive (antibody) immunization was delayed long enough to assure viral access to the ganglion, intraganglionic viral replication during the acute phase of infection was reduced, but the prevalence of subsequent latent infection was either unaffected or actually enhanced. Postganglionic neurectomy, performed after virus had reached the ganglion, altered the course of SCG infection in a direction opposite that of immunization, augmenting the acute phase of viral replication while reducing latency. In athymic nude mice and mice immunosuppressed with cyclophosphamide, intraganglionic viral replication was prolonged. These results emphasize that host factors both extrinsic and intrinsic to the SCG modify the course of ganglionic infection.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R. Herpes simplex virus infection of nervous tissue in animals and man. Prog Med Virol. 1975;20:1–26. [PubMed] [Google Scholar]
- Baringer J. R. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med. 1974 Oct 17;291(16):828–830. doi: 10.1056/NEJM197410172911606. [DOI] [PubMed] [Google Scholar]
- Bastian F. O., Rabson A. S., Yee C. L., Tralka T. S. Herpesvirus hominis: isolation from human trigeminal ganglion. Science. 1972 Oct 20;178(4058):306–307. doi: 10.1126/science.178.4058.306. [DOI] [PubMed] [Google Scholar]
- Burns W., Billups L. C., Notkins A. L. Thymus dependence of viral antigens. Nature. 1975 Aug 21;256(5519):654–656. doi: 10.1038/256654a0. [DOI] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. I. VIRUS PATHWAYS TO THE NERVOUS SYSTEM OF SUCKLING MICE DEMONSTRATED BY FLUORESCENT ANTIBODY STAINING. J Exp Med. 1964 Feb 1;119:343–356. doi: 10.1084/jem.119.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensson K., Olsson Y. Diffusion pathways and retrograde axonal transport of protein tracers in peripheral nerves. Prog Neurobiol. 1973;1(2):87–109. [PubMed] [Google Scholar]
- Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975 Nov 13;258(5531):152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- Price R. W., Katz B. J., Notkins A. L. Latent infection of the peripheral ANS with herpes simplex virus. Nature. 1975 Oct 23;257(5528):686–688. doi: 10.1038/257686a0. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Maintenance of latent herpetic infection: an apparent role for anti-viral IgG. J Immunol. 1974 Dec;113(6):1685–1693. [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Hayashi K., Katz B. J., Notkins A. L. Effect of immunization on acute and latent infections of vaginouterine tissue with herpes simplex virus types 1 and 2. J Infect Dis. 1977 May;135(5):744–752. doi: 10.1093/infdis/135.5.744. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Notkins A. L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974 Jun 14;184(4142):1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Brown S. M., Wroblewska Z., Gilden D., Koprowski H., Subak-Sharpe J. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med. 1978 May 11;298(19):1068–1069. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]



