Abstract
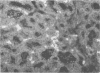
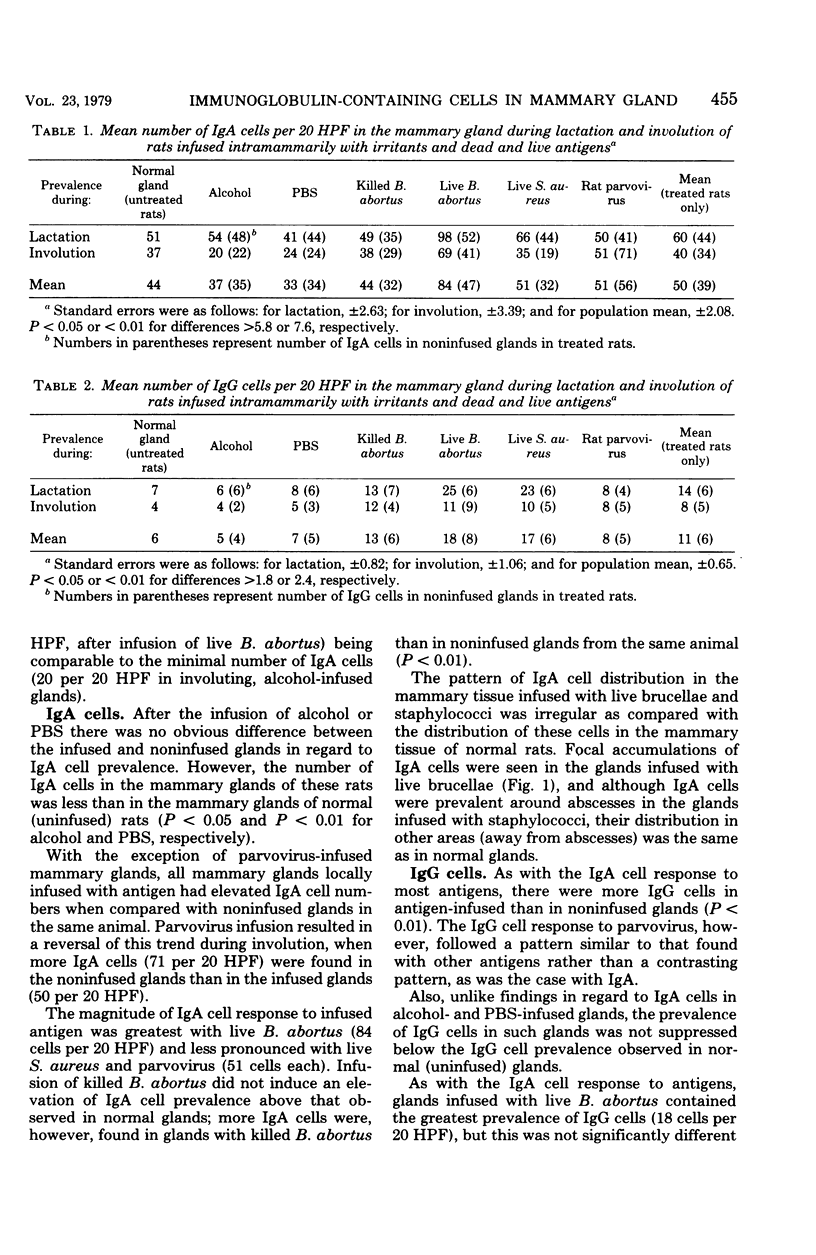
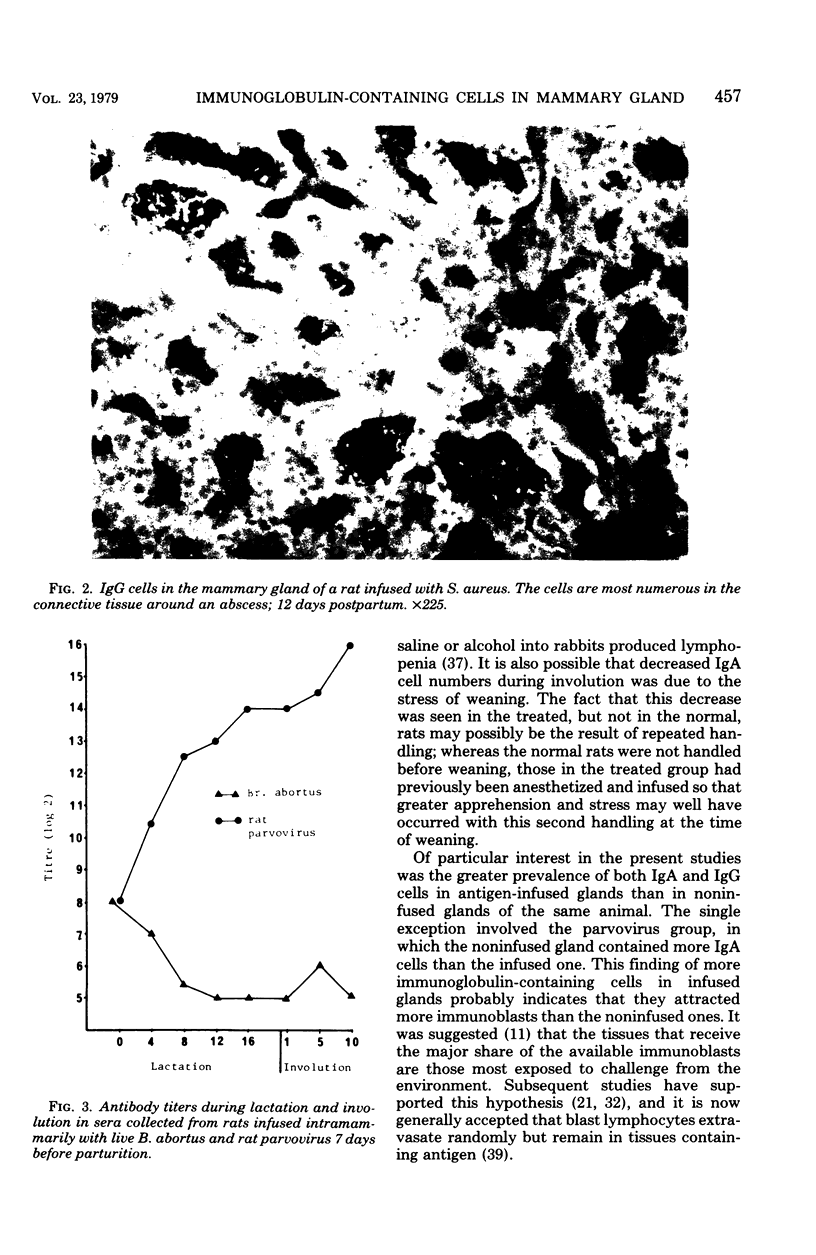
Two irritants, phosphate-buffered saline and alcohol, and antigens including killed Brucella abortus, live B. abortus, Staphylococcus aureus, and rat parvovirus were separately infused into rat mammary glands during pregnancy, and by using immunofluorescent techniques, the numbers of immunoglobulin-containing cells in glands during lactation and involution were determined. The study provided basic information on the local immune response of the mammary gland to antigens of various types. In all experiments, the number of immunoglobulin M (IgM) cells present was small and no trends were apparent. IgA cells were always more prevalent than IgG cells. Fewer IgA cells were in the glands of rats infused with phosphate-buffered saline and alcohol than in normal rats. IgA cell prevalence was greatest in response to infusion of live B. abortus. Responses to live S. aureus and parvovirus were less pronounced, and infusion of killed B. abortus did not induce an elevation in IgA cell prevalence. IgG cell prevalence was greatest in response to infusion of live B. abortus or S. aureus and was decreasingly less pronounced in response to killed B. abortus and rat parvovirus. With the exception of parvovirus infusion, in regard to IgA cells, all glands locally infused with antigen had elevated IgA and IgG cell numbers when compared with noninfused glands in the same animal.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Thorley C. M., Ward P. D., Collins M., Lucken R. N., Knight P. A. Natural and experimental staphylococcal mastitis in rabbits. J Comp Pathol. 1976 Oct;86(4):581–593. doi: 10.1016/0021-9975(76)90067-0. [DOI] [PubMed] [Google Scholar]
- Beh K. J., Lascelles A. K. The use of the antiglobulin test in the diagnosis of bovine brucellosis. Res Vet Sci. 1973 Mar;14(2):239–244. [PubMed] [Google Scholar]
- Bohl E. H., Gupta R. K., Olquin M. V., Saif L. J. Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect Immun. 1972 Sep;6(3):289–301. doi: 10.1128/iai.6.3.289-301.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl E. H., Saif L. J., Gupta R. K., Frederick G. T. Secretory antibodies in milk of swine against transmissible gastroenteritis virus. Adv Exp Med Biol. 1974;45(0):337–342. doi: 10.1007/978-1-4613-4550-3_40. [DOI] [PubMed] [Google Scholar]
- Burrin D. H., Keppie J., Smith H. The isolation of phagocytes and lymphocytes from bovine blood and the effect of their extracts on the growth of Brucella abortus. Br J Exp Pathol. 1966 Feb;47(1):70–75. [PMC free article] [PubMed] [Google Scholar]
- Butler J. E., Maxwell C. F., Pierce C. S., Hylton M. B., Asofsky R., Kiddy C. A. Studies on the relative synthesis and distribution of IgA and IgG1 in various tissues and body fluids of the cow. J Immunol. 1972 Jul;109(1):38–46. [PubMed] [Google Scholar]
- Chandler R. L., Anger H. S., Smith K. Observations on experimental mastitis in mice with reference to summer mastitis in cattle. J Comp Pathol. 1976 Apr;86(2):319–327. doi: 10.1016/0021-9975(76)90056-6. [DOI] [PubMed] [Google Scholar]
- Chandler R. L. Experimental bacterial mastitis in the mouse. J Med Microbiol. 1970 May;3(2):273–282. doi: 10.1099/00222615-3-2-273. [DOI] [PubMed] [Google Scholar]
- Corner A. H., Bannister G. L., Hill D. P. A histological study of the effects of enzootic abortion of ewes virus in the lactating bovine mammary gland. Can J Comp Med Vet Sci. 1968 Jan;32(1):372–381. [PMC free article] [PubMed] [Google Scholar]
- Corner A. H., Greig A. S., Hill D. P. A histological study of the effects of the herpesvirus of infectious bovine rhinotracheitis in the lactating bovine mammary gland. Can J Comp Med Vet Sci. 1967 Dec;31(12):320–330. [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Hall J. G. Effector mechanisms in immunity. Lancet. 1969 Jan 4;1(7584):25–28. doi: 10.1016/s0140-6736(69)90988-x. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Smith M. E. Homing of lymph-borne immunoblasts to the gut. Nature. 1970 Apr 18;226(5242):262–263. doi: 10.1038/226262a0. [DOI] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Transmission of rat virus in milk of rats. J Infect Dis. 1974 Jun;129(6):737–740. doi: 10.1093/infdis/129.6.737. [DOI] [PubMed] [Google Scholar]
- Lascelles A. K., Outteridge P. M., Mackenzie D. D. Local production of antibody by the lactating mammary gland following antigenic stimulation. Aust J Exp Biol Med Sci. 1966 Apr;44(2):169–180. doi: 10.1038/icb.1966.18. [DOI] [PubMed] [Google Scholar]
- Lee C. G., Ladds P. W., Watson D. L. Immunocyte populations in the mammary gland of the rat at different stages of pregnancy and lactation. Res Vet Sci. 1978 May;24(3):322–327. [PubMed] [Google Scholar]
- Lee C. S., Lascelles A. K. Antibody-producing cells in antigenically stimulated mammary glands and in the gastro-intestinal tract of sheep. Aust J Exp Biol Med Sci. 1970 Oct;48(5):525–535. doi: 10.1038/icb.1970.52. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Lascelles A. K. The histological changes in involuting mammary glands of ewes in relation to the local allergic response. Aust J Exp Biol Med Sci. 1969 Oct;47(5):613–623. doi: 10.1038/icb.1969.155. [DOI] [PubMed] [Google Scholar]
- McDowell G. H., Lascelles A. K. Local immunization of ewes with staphylococcal cell and cell-toxoid vaccines. Res Vet Sci. 1971 May;12(3):258–264. [PubMed] [Google Scholar]
- McDowell G., Grov A., Oeding P. Local immunization of guinea pig mammary gland with staphylococcal antigens. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(6):805–811. doi: 10.1111/j.1699-0463.1971.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Mitchell C. A. Production of antibodies in the mammary gland with especial reference to virus neutralizing antibody. Can J Comp Med Vet Sci. 1965 Oct;29(10):262–265. [PMC free article] [PubMed] [Google Scholar]
- Newby T. J., Bourne J. The nature of the local immune system of the bovine mammary gland. J Immunol. 1977 Feb;118(2):461–465. [PubMed] [Google Scholar]
- Norcross N. L., Stark D. M. Immunity to mastitis. A review. J Dairy Sci. 1970 Apr;53(4):387–393. doi: 10.3168/jds.S0022-0302(70)86217-8. [DOI] [PubMed] [Google Scholar]
- PUTT F. A. A modified Ziehl-Neelsen method for demonstration of leprosy bacilli and other acid-fast organisms. Am J Clin Pathol. 1951 Jan;21(1):92–95. [PubMed] [Google Scholar]
- Pasieka A. E., Guerin L. F., Mitchell C. A. Antibody production in goat milk serum after virus instillation of goat mammary gland. V. Biochemical isolation and further characterization of antibodies to various influenza and mumps viruses. Can J Microbiol. 1970 Dec;16(12):1153–1159. doi: 10.1139/m70-195. [DOI] [PubMed] [Google Scholar]
- Pasieka A. E., Guerin L. F., Mitchell C. A. Antibody production in milk serum after virus instillation of goat mammary gland. II. Biochemical isolation and purification of antibody to influenza virus. Can J Microbiol. 1967 Sep;13(9):1195–1201. doi: 10.1139/m67-164. [DOI] [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- STAVITSKY A. B. The influence of adrenal cortical extract on immunological phenomena in vivo. I. Antigen-antibody reactions as indicated by hypocomplementemia, granulocytopenia and reduction of circulating antibody. J Immunol. 1952 Jul;69(1):63–73. [PubMed] [Google Scholar]
- Saif L. J., Bohl E. H., Gupta R. K. Isolation of porcine immunoglobulins and determination of the immunoglobulin classes of transmissible gastroenteritis viral antibodies. Infect Immun. 1972 Oct;6(4):600–609. doi: 10.1128/iai.6.4.600-609.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Unanue E. R., Goralnick S. J., Schreiner G. F. Chemotaxis of rat lymphocytes. J Immunol. 1977 Aug;119(2):416–421. [PubMed] [Google Scholar]
- Wilson M. R. The influence of preparturient intramammary vaccination on bovine mamary secretions. Antibody activity and protective value against Escherichia coli enteric infections. Immunology. 1972 Dec;23(6):947–955. [PMC free article] [PubMed] [Google Scholar]