Abstract
Assessment of management-induced changes in soil quality is important to sustaining high crop yield. A large diversity of cultivated soils necessitate identification development of an appropriate soil quality index (SQI) based on relative soil properties and crop yield. Whereas numerous attempts have been made to estimate SQI for major soils across the World, there is no standard method established and thus, a strong need exists for developing a user-friendly and credible SQI through comparison of various available methods. Therefore, the objective of this article is to compare three widely used methods to estimate SQI using the data collected from 72 soil samples from three on-farm study sites in Ohio. Additionally, challenge lies in establishing a correlation between crop yield versus SQI calculated either depth wise or in combination of soil layers as standard methodology is not yet available and was not given much attention to date. Predominant soils of the study included one organic (Mc), and two mineral (CrB, Ko) soils. Three methods used to estimate SQI were: (i) simple additive SQI (SQI-1), (ii) weighted additive SQI (SQI-2), and (iii) statistically modeled SQI (SQI-3) based on principal component analysis (PCA). The SQI varied between treatments and soil types and ranged between 0–0.9 (1 being the maximum SQI). In general, SQIs did not significantly differ at depths under any method suggesting that soil quality did not significantly differ for different depths at the studied sites. Additionally, data indicate that SQI-3 was most strongly correlated with crop yield, the correlation coefficient ranged between 0.74–0.78. All three SQIs were significantly correlated (r = 0.92–0.97) to each other and with crop yield (r = 0.65–0.79). Separate analyses by crop variety revealed that correlation was low indicating that some key aspects of soil quality related to crop response are important requirements for estimating SQI.
Introduction
A wide range of agricultural soils represents diversely managed arable lands while the main goal to improve soil quality, crop yield, and reduce the ecological foot print. Soil quality is defined as the soil’s capacity to function within natural or managed ecosystem boundaries and to sustain plant productivity while reducing soil degradation [1]–[4]. As soil quality is a complex functional concept and cannot be measured directly in the field or laboratory [5] but can only be inferred from soil characteristics [6], a range of soil parameters or indicators has been identified to estimate soil quality. However, soil quality is often related to the management goal and practices as well to soil characteristics. Thus, a mathematical or statistical framework was put forward in early 1990s to estimate soil quality index (SQI) [1], [3], [4]. The SQI was assessed so that the management goals are not only focused on productivity per se, which may result in soil degradation [7], but also on environmental issues. Thus, an appropriate SQI may have three component goals: environmental quality, agronomic sustainability, and socio-economic viability [8].
Estimation of SQI is a complex process and difficult task [9], especially when linked with several functional goals. Yet a considerable progress has been made towards estimating SQI across a number of soil types and management practices [8]–[14]. Most studies indexed soil quality employing only one method, with a few exceptions [8], [15], [16]. As computation of SQI is difficult, a strong need exists for developing a user-friendly and credible SQI through comparison of various available methods. Thus, the objective of this study was to compare SQIs computed by three methods which are conceptually different from each other. The study is based on the hypothesis that SQIs computed from three methods have similar relationship with crop yields. Data are scarce on validation of SQI against crop yield as most studies focused on the environmental aspects of the soil as end point variable and that SQI of various soil layers has not been computed [13], [16]–[18]. Nevertheless, there is a challenge in validating SQI against crop yield as SQI computed only from surface soils (0–10 and/or 0–20 cm) may not evaluate realistic relationship between soil quality and crop yield because root system can extend to deeper layers [19]. Thus, the other objective of this study was to compute SQI from multiple depths of the soil to examine its relationship with crop yield.
Materials and Methods
Soil sampling and analyses
Soil samples from the field (on-farm) were collected from Logan county, Ohio that included an organic (Martisco Variant silt loam: Mc, organic parent material, >26% organic matter, 40°25′12.4″N, 83°40′55.9″W), and a mineral soil (Crosby silt loam: CrB: Fine, mixed, active, mesic Aeric Epiaqualfs, sand: 23%, silt: 35%, clay: 42%, 40°24′52.4″N, 83°39′21.9″W), and from Franklin county, Ohio that included a Kokomo silty clay loam (Ko: Fine, mixed, superactive, mesic Typic Argiaquolls, sand: 9%, silt: 62%, clay: 29%, 40°00′41.2″N, 83°12′23.1″W) in May, 2013. These locations were farmer-owned field sites and before collecting the soils from specific sites permissions of the farmers were obtained from the land-owners or farmers. Initial permission was granted through The Ohio State University’s extension managers and thereafter communication was established directly by the primary author. Additionally, no endangered or protected species were involved in the current study sites, and thus no such permission was required from any other regulatory agencies. The properties of the soils and other management details were presented elsewhere [20], [21] and briefly presented in the discussion section. Soil samples were obtained from 0–10, 10–20, 20–40, and 40–60 cm depths under no-tillage (NT) and conventional tillage (CT) practices for Mc and CrB soil series and under NT for one year cover crop (CC) and no-cover crop (NCC) for Ko series. The organic soil (Mc) has been under practice of CT corn (Zea mays) for 10 years which was compared with the adjacent grassland soil (termed as NT). The mineral soil (CrB) was under NT and recent introduction (one year) of CT corn practices. Both soils received fertilizer (N:P:K as 5.5∶26∶30) with the rates of 336 (30, 142 and 164 kg ha−1 of N, P, and K, respectively) and 280 (25, 118 and 137 kg ha−1 of N, P, and K, respectively) kg ha−1 for corn, for Mc and CrB soils, respectively. The other site (Ko) was under NT corn and soybean (Glycin max L.) annual rotation. A mixture of pea (Pisum sativum L.) and turnip (Brassica rapa L.) cover crops with the seeding rate of 140 kg ha−1 was seeded prior to growing soybean in 2013. Roundup-ready soybean was seeded on 20 April, 2013 and harvested on 30 September, 2013. A row spacing of 38 cm was used during planting for corn and soybean. Soil core (inside diameter: 5.3 cm, height: 5.9 cm) samples were collected manually using soil sampler before planting (7 May, 2013) from four depths (0–10, 10–20, 20–40, and 40–60) for determination of bulk density (BD) by dividing dry mass of soil in the core by core volume [22] and hydrologic properties. Undisturbed soil cores were used to determine the water retention at field capacity (0.033 MPa), while sieved samples (<2-mm size) were used to determine the permanent wilting point (1.5 MPa). The potential available water capacity (AWC) of the soil was calculated as the difference in volumetric water content at 0.033 and 1.5 MPa moisture potentials. The water content of a soil layer was calculated by multiplying thickness of the layer with the volumetric water capacity. A minimum of three field penetration resistance (PR) measurements for soil’s mechanical strength were made for each depth using a CP40II cone penetrometer [23]. Bulk samples were obtained to measure the aggregate size distributions and fraction of water stable aggregates (WSA) using the wet sieving method [24]. Five sieves of diameter sizes 4.75, 2, 1, 0.5, and 0.25 mm openings were placed into a Yoder apparatus. Air-dried soils (5–8 mm, 51 g) were slowly wetted by capillarity action by adjusting the water level in the container so that the base of the top sieve just touched the water. The sieve combination was oscillated mechanically in the water at 60 oscillations per minute for 30 min. Aggregates retained in the sieves were transferred to glass beakers and the weight of each of five fractions was measured after drying at 60°C overnight. The data were used to compute WSA, mean weight diameter (MWD), and geometric mean diameter (GMD) [25]. Soil water retention at matrix potentials of 0.033 and 1.5 MPa were measured using a pressure plate apparatus [26]. The pH and electrical conductivity (EC) of soil were determined in 1∶2 soil:water ratio slurry using a Thermo-scientific Orion Star Series pH/Conductivity Meter. Concentrations of soil organic C and total N were determined using an Elemental analyzer (Vario Max, Elementar Americas, Inc., Germany) by the dry combustion method (900°C) after grinding subsamples to 0.25 mm. Total C and N stocks were calculated by multiplying the respective elemental concentrations by BD and the thickness of the soil layer [27].
Soil quality index (SQI) calculations
All the SQI methods involved a set of 72 soil samples and a number of soil quality indicators as parameters. The 13 parameters used for developing SQIs were pH, EC, BD, WSA, GMD, MWD, PR, SOC concentration, N concentration, C-Stock, N-Stock, AWC, and soil water content. As some of these parameters were synthesized and redundant, only nine parameters were chosen omitting MWD, C and N concentrations, and water content from the SQI-1 and SQI-2 calculations to avoid redundancy. However, SQI-3 was not a primarily additive approach and redundancy of the parameters was eliminated through the processes of elimination as prescribed before. Thus, all 13 parameters were initially included in SQI-3 model and only four non-redundant soil parameters with maximum variations in the dataset were finally retained in the model as described in the following section. The average and standard deviation values of these parameters grouped by soil types are presented in Table 1. Under the proposed framework an ideal soil would have SQI value of 1 for the highest quality soil and 0 for the severely degraded soil [1]–[4].
Table 1. Descriptive statistics of all the soil indicators collected from four depths under three soil types used to estimate SQI.
| Ko | ||||||||
| 0–10 cm | 10–20 cm | 20–40 cm | 40–60 cm | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| pH | 6.0 | 0.5 | 6.2 | 0.6 | 6.4 | 0.6 | 6.4 | 0.6 |
| EC (µs cm−1) | 190.6 | 151.1 | 165.6 | 84.2 | 151.7 | 71.8 | 175.9 | 92.2 |
| BD (Mg m−3) | 1.4 | 0.2 | 1.5 | 0.1 | 1.6 | 0.1 | 1.6 | 0.1 |
| WSA % | 73.1 | 14.8 | 79.5 | 12.0 | 73.3 | 22.5 | 84.7 | 5.6 |
| GMD (mm) | 1.4 | 0.4 | 1.6 | 0.4 | 1.4 | 0.3 | 1.5 | 0.3 |
| MWD (mm) | 2.6 | 1.7 | 3.1 | 1.8 | 2.3 | 1.7 | 3.4 | 1.1 |
| PR (Mpa) | 2.2 | 0.3 | 2.4 | 0.2 | 2.3 | 0.2 | 2.2 | 0.4 |
| N (%) | 0.2 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 |
| SOC (%) | 2.4 | 0.2 | 2.1 | 0.2 | 2.0 | 0.2 | 2.0 | 0.3 |
| N-Stock (Mg/ha) | 3.5 | 0.5 | 3.0 | 0.3 | 6.4 | 0.6 | 6.4 | 1.1 |
| C-Stock (Mg/ha) | 33.7 | 3.7 | 30.6 | 2.8 | 62.5 | 6.8 | 63.7 | 7.2 |
| AWC (%) | 38.5 | 4.1 | 36.7 | 2.1 | 35.8 | 6.4 | 39.9 | 5.8 |
| Water content (cm) | 3.8 | 0.4 | 3.7 | 0.2 | 7.2 | 1.3 | 7.9 | 1.0 |
| CrB | ||||||||
| 0–10 cm | 10–20 cm | 20–40 cm | 40–60 cm | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| pH | 7.4 | 0.2 | 7.4 | 0.2 | 7.4 | 0.2 | 7.4 | 0.1 |
| EC (µs cm−1) | 250.3 | 44.6 | 217.8 | 26.8 | 212.3 | 31.8 | 223.5 | 45.0 |
| BD (Mg m−3) | 1.5 | 0.2 | 1.5 | 0.1 | 1.5 | 0.3 | 1.5 | 0.3 |
| WSA % | 82.4 | 4.4 | 76.4 | 9.7 | 76.5 | 9.5 | 76.5 | 17.7 |
| GMD (mm) | 1.2 | 0.1 | 1.1 | 0.2 | 1.1 | 0.2 | 1.2 | 0.3 |
| MWD (mm) | 2.0 | 0.5 | 1.7 | 0.8 | 1.5 | 0.7 | 2.0 | 1.1 |
| PR (Mpa) | 2.4 | 0.2 | 2.5 | 0.5 | 2.6 | 0.2 | 2.9 | 0.7 |
| N (%) | 0.2 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 |
| SOC (%) | 2.4 | 0.3 | 2.1 | 0.4 | 1.8 | 0.4 | 1.8 | 0.5 |
| N-Stock (Mg/ha) | 3.2 | 0.2 | 3.1 | 0.3 | 5.2 | 1.6 | 5.1 | 2.2 |
| C-Stock (Mg/ha) | 34.3 | 3.2 | 32.2 | 4.1 | 53.8 | 16.8 | 54.6 | 24.4 |
| AWC (%) | 17.8 | 9.0 | 10.1 | 6.8 | 10.7 | 8.2 | 9.0 | 3.2 |
| Water content (cm) | 1.8 | 0.9 | 1.0 | 0.7 | 2.1 | 1.6 | 1.8 | 0.6 |
| Mc | ||||||||
| 0–10 cm | 10–20 cm | 20–40 cm | 40–60 cm | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| pH | 7.4 | 0.1 | 7.4 | 0.1 | 7.4 | 0.1 | 7.5 | 0.1 |
| EC (µs cm−1) | 616.3 | 40.1 | 570.0 | 92.8 | 633.2 | 126.4 | 599.2 | 129.2 |
| BD (Mg m−3) | 0.6 | 0.0 | 0.7 | 0.0 | 0.6 | 0.1 | 0.6 | 0.1 |
| WSA % | 91.2 | 0.5 | 91.0 | 2.5 | 89.7 | 2.5 | 82.5 | 8.7 |
| GMD (mm) | 2.1 | 0.0 | 2.2 | 0.0 | 2.2 | 0.0 | 2.2 | 0.1 |
| MWD (mm) | 5.5 | 0.1 | 5.5 | 0.2 | 5.7 | 0.4 | 5.0 | 0.5 |
| PR (Mpa) | 1.3 | 0.1 | 1.5 | 0.3 | 1.6 | 0.3 | 1.9 | 0.1 |
| N (%) | 1.0 | 0.1 | 0.9 | 0.1 | 0.9 | 0.1 | 1.0 | 0.1 |
| SOC (%) | 15.1 | 1.1 | 15.0 | 0.8 | 14.9 | 0.9 | 15.9 | 0.6 |
| N-Stock (Mg/ha) | 6.1 | 0.8 | 6.3 | 0.7 | 11.2 | 1.7 | 12.6 | 1.9 |
| C-Stock (Mg/ha) | 95.7 | 11.7 | 102.8 | 8.8 | 186.6 | 36.2 | 196.6 | 28.0 |
| AWC (%) | 248.3 | 151.3 | 84.8 | 42.4 | 48.2 | 13.8 | 36.1 | 22.1 |
| Water content(cm) | 24.8 | 15.1 | 8.5 | 4.2 | 9.6 | 2.8 | 7.2 | 4.4 |
Abbreviations: SD: standard deviation, EC: electrical conductivity, BD: bulk density, WSA: water stable aggregates, GMD: geometrical mean diameter, MWD: mean weight diameter, PR: penetration resistance, N: nitrogen, SOC: soil organic carbon, AWC: available water capacity.
Simple additive SQI (SQI-1)
Simple additive SQI was estimated following the method outlined by Amacher et al. [28]. In this method, soil parameters were given threshold values based primarily on the literature review and expert opinion of the authors. The threshold levels, interpretations, and associated unitless soil index score values are listed in Table 2. The individual index values were then summed up to obtain a total SQI:
| (1) |
Table 2. Soil indicators, threshold values, interpretations and scores.
| Indicators | Range | Interpretation | Score | Reference |
| pH | 5.5–7.2 | Slightly acidic to neutral: Optimum for plant growth | 2 | [28] |
| >7.2<8.0 | Slightly to moderately alkaline: Preferred by some plants,possible P and some metal deficiencies | 1 | ||
| EC (us/cm) | <200 | Low salt level | 0 | [42] |
| 200–500 | Optimum salt level for plants | 1 | ||
| >500 | High salt level, adverse effect likely | 0 | ||
| BD (Mg/m3) | <1.0 | High organic soil, supports plant roots | 2 | [28], [45]–[47] |
| 1.0–1.5 | Adverse effects unlikely | 1 | ||
| >1.5 | Adverse effects likely | 0 | ||
| WSA (%) | <50 | Infiltration and soil erosion problems likely | 0 | [30], [42] |
| 50–70 | Moderate constraints | 1 | ||
| 70–90 | Good soil | 2 | ||
| >90 | Excellent soil | 3 | ||
| GMD (mm) | <1.0 | Infiltration and soil erosion problems likely | 0 | [30], [42] |
| 1–2 | Moderate limitations | 1 | ||
| >2 | No limitation | 2 | ||
| MWD (mm) | <1.0 | Infiltration and soil erosion problems likely | 0 | [30], [42] |
| 1–2 | Moderate limitations | 1 | ||
| 2–5 | Slight limitations | 2 | ||
| >5.0 | No limitation | 3 | ||
| PR (Mpa) | 1–2 | Adverse effect on plant root unlikely | 2 | [42], [48] |
| 2–3 | Moderate adverse effect on plant root | 1 | ||
| >3.0 | Severe adverse effect on plant roots | 0 | ||
| N (%) | 0.2–0.3 | Moderate limitation | 1 | [41], [42] |
| >0.3 | Slight to no limitation | 2 | ||
| SOC (%) | 2–3 | Moderate limitation | 1 | [41], [42] |
| >3.0 | Slight to no limitation | 2 | ||
| N-Stock (Mg/ha) | <5.0 | N deficient | 1 | Authors’ opinion |
| 5–10 | Moderate to optimum N level | 2 | ||
| >10.0 | N-rich soil | 3 | ||
| C-Stock (Mg/ha) | <50.0 | C deficient | 1 | Authors’ opinion |
| 50–100 | Moderate to optimum C level | 2 | ||
| >100 | C-rich soil | 3 | ||
| AWC (%) | <20 | Water-stress to plants | 0 | [31], [42] |
| 20–50 | Moderate water availability | 1 | ||
| >50 | Good water capacity for plants | 2 | ||
| Water content(cm) | <5.0 | Water-stress to plants | 0 | Authors’ opinion |
| 5–10 | Moderate water availability | 1 | ||
| >10 | Good water capacity for plants | 2 |
Abbreviations are same as Table 1.
The scaled SQI (SQI-1) of individual soil, was computed by Eq. 2:
| (2) |
whereas, SQIMin = Minimum value of SQI, and SQIMax = Maximum value of SQI from the total dataset.
Weighted additive SQI (SQI-2)
In this approach, each soil parameter was first assigned unitless score ranging from o to 1 by employing linear scoring functions [8]. Non-linear scoring functions were avoided because of their lower capacity of predicting the end point variable or crop yield [8]. Soil parameters were divided into groups based on three mathematical algorithm functions: (a) ‘more is better’ (e.g., WSA, GMD, C-Stock, N-Stock, and AWC) (b) ‘less is better’ (e.g., BD, PR), and (c) ‘optimum’ (e.g., pH and EC). ‘Optimum’ properties are those which have positive influence up to a certain level beyond which the influence could be considered detrimental [13]. For ‘more is better’ parameters, each observation was divided by the highest observed value of the entire dataset so that the highest observed value would have a score of 1; for ‘less is better’ parameters, the lowest observed value in the entire dataset was divided by each observation so that the lowest observed value received a score of 1; and ‘optimum’ parameters were scored up to a threshold value as ‘more is better’, and thereafter above the threshold values were scored as ‘less is better’ [8], [29]. For example, pHs up to 5.5–7.2 were scored as ‘more is better’, and pHs >7.2 were scored as ‘less is better’.
After normalizing soil parameters, the scores were integrated into a single index value for each soil using a weighted additive approach initially suggested by Karlen and Stott [4], but modified later by Fernandes et al. [13]. The following weighted additive function was used for development of SQI-2 (Eq. 3):
| (3) |
where, RDC (root development capacity) is the rating for the soil’s ability to allow plant root development, WSC (water storage capacity) is the rating for the soil’s ability to store water, NSC (nutrient supply capacity) is the rating for the soil’s ability to supply nutrients. Weight 1, 2 and 3 are the respective numerical weights for each soil function (RDC, WSC, and NSC). The numerical weights were assigned to each soil function according to their importance in fulfilling the management goal(s) of maintaining soil quality. While the summation of all these numerical weights (Weight 1, 2 and 3) are supposed to be 1 and distributed evenly as 0.33, 0.33, and 0.34 [4], however, a modified approach was taken for the current study following Fernandes et al. [13], where lower weightage was given to the functional attribute which had lower number of representative indicators in the model. Similar to Fernandes et al. [13] weight values were arbitrarily chosen for RDC, WSC, and NSC as 0.4, 0.2, and 0.4 in the current study. The WSC received lower weight value than others because of low number of representative indicators (only AWC) [13] among all included parameters.
The soil parameters or indicators selected for (i) RDC were BD, PR, WSA, and GMD, (ii) WSC was AWC and (iii) NSC were pH, EC, C-Stock, and N-Stock. Within this network, the sub-weight values were given to each indicator based on their importance under the particular soil functional property, field versus laboratory measurements and scope of redundancy. The sub-weight values of different soil indicators or parameters were summed up to 1 under each soil functional property [13]. The rank of the subweight values were as follows: field measurement indicators (BD, PR) > laboratory measurement indicators (WSA, AWC, pH, EC) >> synthesized parameters (GMD, C-Stock, and N-Stock). Field indicators were given the maximum weightage as these were the most representative of the soil’s natural conditions and synthesized parameters received the lowest weightage to avoid data redundancy in the model. Thereafter, scaled SQI (SQI-2) of individual soil was computed by Eq. 2. An example of this model of development of SQI is shown in Table 3.
Table 3. Model of SQI-2.
| Soil function | Weight | Soil Indicators | Sub-weight | Scaled score | B×C | ∑ B×C | D×A | % | SQI |
| A | B | C | D | ||||||
| RDC | 0.4 | BD | 0.35 | 0.41 | 0.14 | 0.46 | 0.18 | 52.94 | 0.34 |
| PR | 0.35 | 0.52 | 0.18 | ||||||
| WSA | 0.20 | 0.54 | 0.11 | ||||||
| GMD | 0.10 | 0.34 | 0.03 | ||||||
| WSC | 0.2 | AWC | 1.00 | 0.06 | 0.06 | 0.06 | 0.01 | 2.94 | |
| NSC | 0.4 | pH | 0.30 | 0.79 | 0.24 | 0.38 | 0.15 | 44.12 | |
| EC | 0.30 | 0.25 | 0.08 | ||||||
| C-stock | 0.20 | 0.11 | 0.02 | ||||||
| N-stock | 0.20 | 0.18 | 0.04 |
Abbreviations: RDC: root development capacity, WSC: water storage capacity, NSC: nutrient storage capacity; all other abbreviations are same as Table 1.
Statistically modeled SQI (SQI-3)
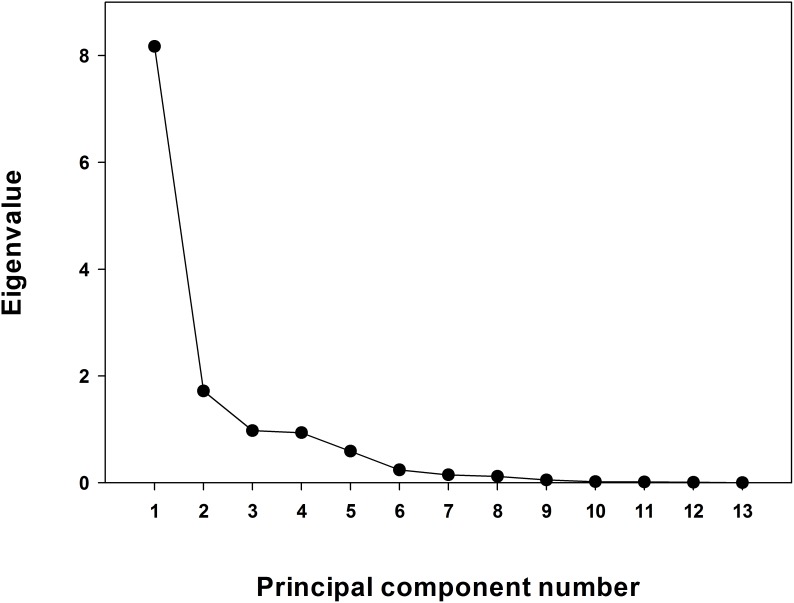
A statistics-based model was used to estimate SQI using principal component analysis (PCA) [8], [12], [14], [18], [30], [31]. The PCA-model is used to create a minimum data set (MDS) to reduce the indicator load in the model and avoid data redundancy [8]. The main difference between the first two versus the PCA method is that the first two rely mainly on subjective expert opinion and literature review, while the PCA method is more objective of using a number of statistical tools (multiple correlation, factor and cluster analyses) which could avoid any biasness and data redundancy by choosing an MDS using mathematical formulae [8], [32]. A relative advantage and disadvantage of each SQI method is explained in the later section. The preliminary function of PCA is to reduce the dimensionality of the entire data set consisting of a large number of interrelated variables, while retaining as much as possible of the variations present in the data set. This is achieved by transformation to a new set of variables, the principal components (PCs), which are uncorrelated, and ordered so that the first few retain most of the variation present in all of the original variables [33], [34]. In other words, the PCA method was chosen as a data reduction tool to select the most appropriate indicator(s) to represent and estimate SQI [18].
All the original observations (untransformed data) of each soil were included in the PCA model using SPSS, version 21.0 [35]. The PCs with high eigenvalues represented the maximum variation in the dataset [8], [12], [14], [18], [30], [31]. While most studies have assumed to examine PCs with eigenvalue >1.0 following Kaiser [36], the present study had the third and fourth components with eigenvalues of 0.98 and 0.89 and variances >5% (Table 4, Fig. 1). These were examined with first two PCs (eigenvalues 8.23, and 1.67) as prescribed for the cases of fewer than three components with eigenvalue >1.0 [8], [37]. Under a given PC, each variable had corresponding eigenvector weight value or factor loading (Table 2). Only the ‘highly weighted’ variables were retained to include in the MDS (Table 2). The ‘highly weighted’ variables were defined as the highest weighted variable under a certain PC and absolute factor loading value within 10% of the highest values under the same PC [37]. Thus, the bold-face values (Table 4: EC, BD, GMD, MWD, SOC, and N for PC-1, AWC for PC-2, WSA for PC-3, and pH for PC-4) were considered highly weighted eigenvectors and therefore were initially selected in the MDS. However, when more than one variable was retained under a particular PC, multivariate correlation matrix (Table 5) was used to determine the correlation coefficients between the parameters [8], [12]. If the parameters were significantly correlated (r>0.60, p<0.05), then the one with the highest loading factor was retained in the MDS and all others were eliminated from the MDS to avoid redundancy. Following this procedure, except SOC, all other eigenvectors from PC-1 were eliminated from MDS due to high and significant correlation between each other (Table 5). The non-correlated parameters under a particular PC were considered important and retained in the MDS [10], [12]. All the bold-faced and underlined soil parameters in Table 4 were selected in the final MDS.
Table 4. Results of principal component analyses (PCA).
| PC-1 | PC-2 | PC-3 | PC-4 | |
| Eigenvalues | 8.23 | 1.67 | 0.98 | 0.89 |
| % Variance | 63.34 | 12.87 | 7.56 | 6.82 |
| Cumulative variance | 63.3 | 76.2 | 83.8 | 90.6 |
| Eigen vectors or factor loading | ||||
| pH | 0.423 | 0.351 | –0.378 | 0.711 |
| EC | 0.925 | 0.113 | –0.153 | 0.147 |
| BD | –0.881 | –0.034 | 0.212 | 0.002 |
| WSA | 0.590 | –0.020 | 0.669 | 0.343 |
| GMD | 0.927 | 0.024 | 0.276 | –0.024 |
| MWD | 0.904 | 0.001 | 0.342 | 0.046 |
| PR | –0.770 | 0.268 | –0.131 | 0.092 |
| SOC | 0.975 | 0.073 | –0.109 | –0.025 |
| N | 0.972 | 0.050 | –0.103 | –0.053 |
| C-Stock | 0.831 | 0.412 | –0.107 | –0.268 |
| N-Stock | 0.722 | 0.440 | –0.067 | –0.385 |
| AWC | 0.555 | –0.782 | –0.189 | 0.057 |
| Water content | 0.624 | –0.693 | –0.199 | –0.066 |
Abbreviations are same as Table 1; PC: principal component; bold values under each component are highly weighted and underlined bold values are selected in minimum data set.
Figure 1. Scree plot of principal component analyses.
Table 5. Pearson correlation coefficients (r) for all soil indicators.
| Correlation (r) matrix | pH | EC | BD | WSA | GMD | MWD | PR | N | SOC | N-Stock | C-Stock | AWC |
| EC | 0.547 | |||||||||||
| BD | −0.394 | −0.88 | ||||||||||
| WSA | 0.248 | 0.483 | −0.338 | |||||||||
| GMD | 0.279 | 0.8 | −0.762 | 0.673 | ||||||||
| MWD | 0.286 | 0.782 | −0.725 | 0.734 | 0.975 | |||||||
| PR | −0.129 | −0.655 | 0.684 | −0.456 | −0.71 | −0.687 | ||||||
| N | 0.405 | 0.922 | −0.908 | 0.47 | 0.877 | 0.835 | −0.737 | |||||
| SOC | 0.438 | 0.93 | −0.914 | 0.473 | 0.881 | 0.842 | −0.731 | 0.995 | ||||
| N-Stock | 0.271 | 0.632 | −0.551 | 0.32 | 0.642 | 0.595 | −0.396 | 0.709 | 0.707 | |||
| C-Stock | 0.382 | 0.764 | −0.702 | 0.367 | 0.742 | 0.695 | −0.489 | 0.822 | 0.833 | 0.965 | ||
| AWC | 0.091 | 0.447 | −0.449 | 0.275 | 0.439 | 0.441 | −0.525 | 0.504 | 0.489 | 0.106 | 0.181 | |
| Water content (cm) | 0.092 | 0.487 | −0.485 | 0.282 | 0.501 | 0.496 | −0.557 | 0.558 | 0.546 | 0.28 | 0.333 | 0.967 |
| Significance level | ||||||||||||
| EC | <0.001 | |||||||||||
| BD | <0.001 | <0.001 | ||||||||||
| WSA | 0.018 | <0.001 | 0.002 | |||||||||
| GMD | 0.009 | <0.001 | <0.001 | <0.001 | ||||||||
| MWD | 0.007 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| PR | 0.141 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| N | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| SOC | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| N-Stock | 0.011 | <0.001 | <0.001 | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| C-Stock | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| AWC | 0.224 | <0.001 | <0.001 | 0.011 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.195 | 0.07 | |
| Water content (cm) | 0.22 | <0.001 | <0.001 | 0.009 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.01 | 0.003 | <0.001 |
Abbreviations are same as Table 1.
After selection of parameters for the MDS, all selected observations were transformed using linear scoring functions (less is better, more is better and optimum) as described in SQI-2 method. Once the selected observations were transformed in numerical scores (ranged 0–1), a weighted additive approach was used to integrate them into indices for each soil [8], [12]. Each PC explained a certain amount of variation in the dataset (Table 4), which was divided by the maximum total variation of the all PCs selected for the MDS to get a certain weightage value under a particular PC [8]. For example, the % variance (63.3) was divided by total cumulative variance (90.6) to obtain the weight value of 0.7 for PC-1 (Table 4). Thereafter, the weighted additive SQI was computed using Eq. 4:
| (4) |
Validation of SQI
The SQIs estimated from three different methods were validated against soybean (Glycin max) and corn (Zea mays) yield data of that particular year of collection of soil and crops by computing correlation coefficients [8]. The SQI values were also compared through pearson correlation to understand the effectiveness of each other.
Statistical analyses
All values are presented as means ± standard deviations of three field or laboratory measurements. Significant differences between treatments were analyzed using Tukey’s test in PROC GLM in SAS version 9.2 [38]. Treatment differences were deemed significant at p<0.05. The PCA was performed in SPSS version 21 [35]. Descriptive statistics and linear regressions were computed in Microsoft Excel [39] and all the figures were obtained using Sigmaplot Version 12.0 [40].
Results and Discussions
Most data of three farm sites have been presented and the detailed discussions of soil type, crop and management effects on soil properties are discussed elsewhere [20], [21]. The mean and standard deviation values of the soil parameters by depth under three soil types are listed in Table 1. Most properties of mineral soils (Ko and CrB) were significantly different compared to those of the organic soil (Mc) and except for AWC and water content two mineral soils’ characteristics were similar across soil profile up to 60 cm depth (Table 1). The water characteristics of Ko soil were significantly higher than those of CrB. On the other hand, organic soil (Mc) had much improved (lower BD and PR, and higher WSA, SOC, N concentrations and stock, AWC and water content) soil properties compared to those of the mineral counterparts in all four different depths (Ko and CrB). Impacts of all these parameters on overall soil quality in four soil depths calculated by three indexing methods are discussed in the following sections.
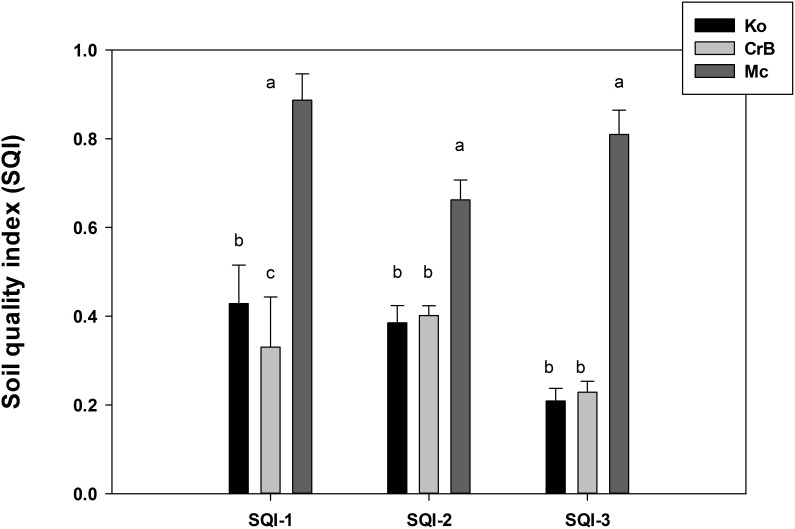
Under the framework of SQI-1, nine soil quality indicators were integrated numerically after scoring them primarily using the information from literature review. However, scoring data on some physicochemical indicators (e.g., C and N stocks) is scarce in the literature and thus, authors’ opinion was used in those cases. Further, different studies used different scoring on the same indicator based on soil type, and management goals. For an example, while Amacher and Perry [28] had eight different range classes and scores for pH, Feiza et al. [41] had only four classes and scores available for pH. Thus, scoring on indicators in the current study (Table 2) had importance on experts’ opinion although the knowledge was primarily based on available literature and authors’ experience [28], [42]. The high variability in the observations (Table 1) was reflected in the SQI-1 values. There was no statistical difference (p>0.05) across treatments (NCC versus CC, and CT versus NT) and depth under specific soil type (Table 6). Overall performance of indexing methods was determined from averaging SQI values obtained from calculations for each depth and presented in Fig. 2. The, SQI-1 was significantly higher in the following order: Mc>Ko>CrB (Fig. 2), indicating that soil quality was influenced more by soil type than management and sampling depth under the SQI-1 approach.
Table 6. Treatment effects on soil quality index (SQI) under three soil types (Ko: Kokomo, CrB: Crosby silty loam, Mc: Muck) and four soil depths.
| Treatments | |||||||||
| Ko | NCC | CC | NCC | CC | NCC | CC | NCC | CC | |
| 0–10 cm | 10–20 cm | 20–40 cm | 40–60 cm | ||||||
| SQI-1 | 0.36a | 0.36a | 0.40a | 0.40a | 0.45a | 0.48a | 0.48a | 0.50a | |
| SQI-2 | 0.40a | 0.37a | 0.39a | 0.36a | 0.40a | 0.36a | 0.43a | 0.38a | |
| SQI-3 | 0.21a | 0.23a | 0.20a | 0.22a | 0.19a | 0.20a | 0.21a | 0.21a | |
| CrB | CT | NT | CT | NT | CT | NT | CT | NT | |
| 0–10 cm | 10–20 cm | 20–40 cm | 40–60 cm | ||||||
| SQI-1 | 0.36a | 0.38a | 0.36a | 0.17a | 0.45a | 0.29a | 0.38a | 0.26a | |
| SQI-2 | 0.42a | 0.40b | 0.40a | 0.37a | 0.41a | 0.40a | 0.41a | 0.40a | |
| SQI-3 | 0.26a | 0.24a | 0.24a | 0.22a | 0.22a | 0.22a | 0.22a | 0.21a | |
| Mc | CT | NT | CT | NT | CT | NT | CT | NT | |
| 0–10 cm | 10–20 cm | 20–40 cm | 40–60 cm | ||||||
| SQI-1 | 0.88a | 0.88a | 0.90a | 0.90a | 0.90a | 0.90a | 0.90a | 0.81a | |
| SQI-2 | 0.70a | 0.72a | 0.65a | 0.61a | 0.65a | 0.66a | 0.67a | 0.65a | |
| SQI-3 | 0.85a | 0.85a | 0.82a | 0.78a | 0.78a | 0.77a | 0.82a | 0.81a | |
Abbreviations: NCC: no cover crop, CC: cover crop, CT: conventional tillage, NT: no tillage.
Different letters under specific row and depth indicate significant differences at p<0.05 level, underline value is significant at p<0.1 level.
Figure 2. Overall soil quality index (SQI) values under different soil types.
Different letters indicate significant differences at p<0.05 level for particular indexing method.
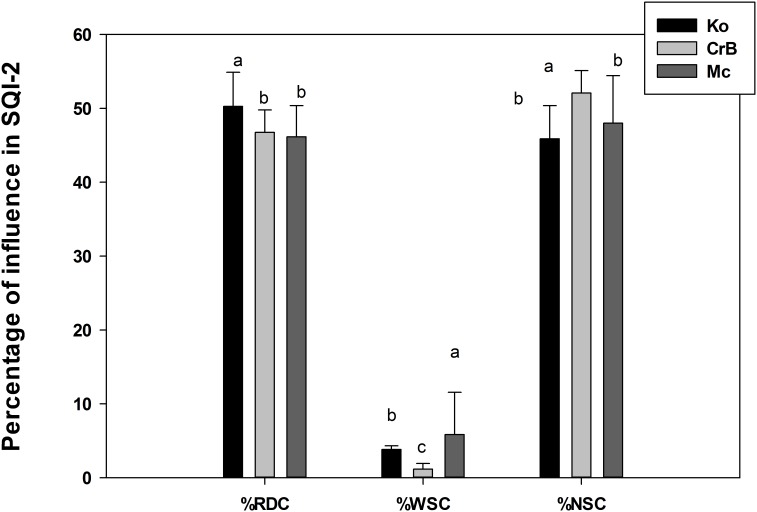
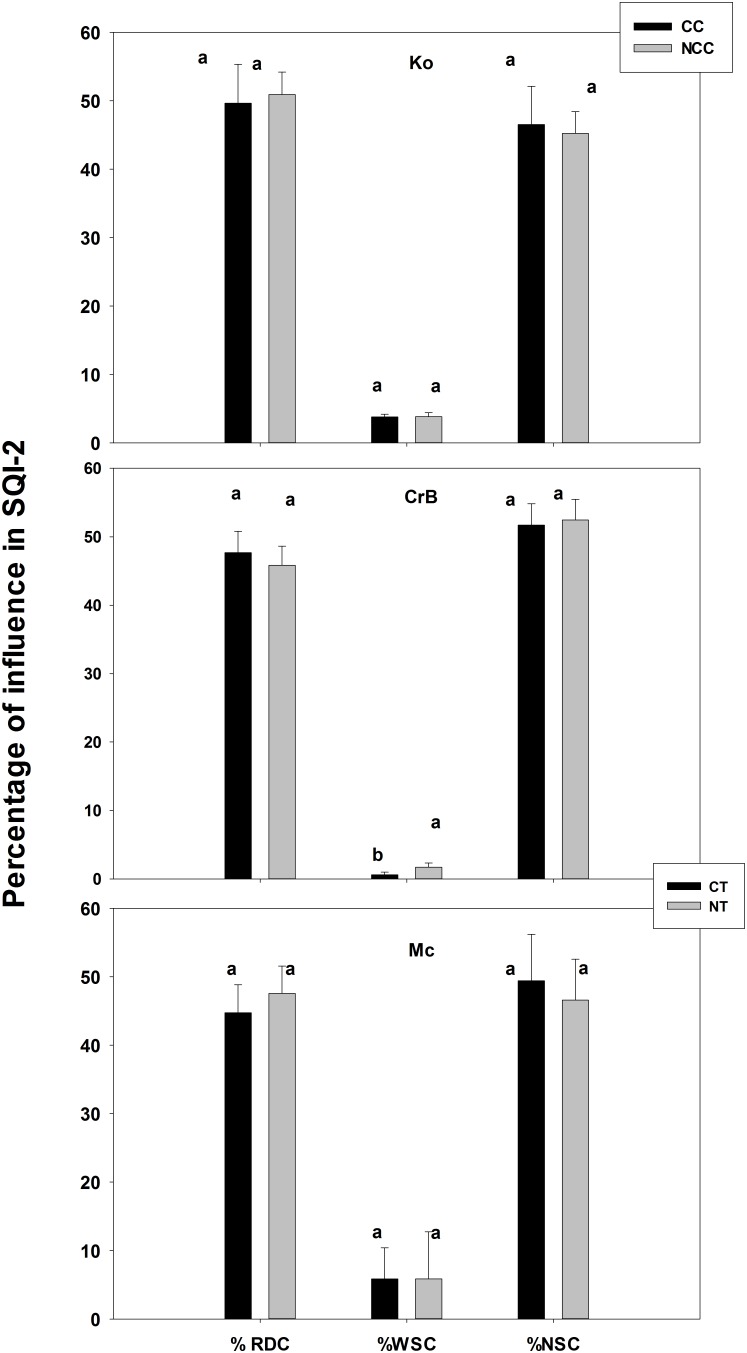
Overall percentage of various soil functional influences in SQI-2 is presented in Fig. 3 and depthwise percentage of influence in SQI-2 is presented in Fig. S1. Generally, mineral soils (Ko and CrB) did not have any depthwise significant changes for WSC but 0–10 cm layer of organic (Mc) soil had significantly higher influence of WSC in SQI-2 compared to lower depths. On the other hand, while both Ko and Mc had significantly higher influence of RDC in SQI-2 in the upper layers than the lower depths, the opposite trend was found for the NSC (Fig. S1). In the general composition of SQI-2, the main contributors were RDC, which ranged from 39–57% and NSC ranged from 35–59%. However, the WSC was relatively small and ranged merely from 0.1–12% (Fig. 3). The relative influence of root development functions in SQI-2 has been significantly higher in Ko than CrB, and Mc by 8 and 9%, respectively and the same for nutrient storage functions in SQI-2 has been significantly higher in CrB than Ko and Mc by 13 and 8%, respectively (Fig. 3). On the other hand, Mc soil had significantly higher water storage functional contribution to SQI-2 by 53 and 500%, respectively, compared to that of mineral (Ko and CrB) soils (Figs. 3). Thus, while the mineral soil quality was more sensitive to the functional attributes dedicated to root development and nutrient storage, muck soil quality was more influenced by soil attributes related to water storage under the scheme of SQI-2. Further, there was no significant effect on management practices on %RDC and %NSC to SQI-2 in different soil/management/crop combinations (Fig. 4). Note that CC/NCC management was used on field cultivated to soybean but CT/NT was used to soil under corn. However, contribution of WSC to SQI-2 for the case of CrB soil under NT management was significantly higher (178%) than that of CT practice (Fig. 4).
Figure 3. Percentage contribution of each soil function in SQI-2 under different soils.
Different letters indicate significant differences at p<0.05 level for particular soil function. Abbreviations: RDC: root development capacity, WSC: water storage capacity, NSC: nutrient storage capacity.
Figure 4. Percentage contribution of each soil function in SQI-2 under various management practices in different soil.
Different letters indicate significant differences at p<0.05 level for particular soil function. Abbreviations: RDC: root development capacity, WSC: water storage capacity, NSC: nutrient storage capacity; NCC: no cover crop, CC: cover crop, CT: conventional tillage, NT: no tillage.
Correlation coefficients of SQIs to each other and depthwise SQIs and dry grain and straw are given in Table 7. Crop yield is obviously related to soil quality but soil quality in different layers may not be same and can influence crop yield accordingly. Data on SQI variation for different soil layers are scarce and thus no comparison to the data presented in the current study (Table 6) was possible. However, while numerical values of SQI were almost unchangeable for different depths at Mc site, SQI generally variable with depth in Ko and CrB sites (Table 6). One goal of this study was to validate SQI against crop yield as only a few studies have done so with some exceptions [8], [14]. Challenge lies in establishing a correlation between crop yield versus SQI calculated either depthwise or in combination of soil layers as standard methodology is not yet available and was not given much attention to date. For example, one can have only one crop yield value for multiple soil layers and thus to date crop yield has been correlated only against SQI calculated from surface layer. Low (ranged from 0.11–0.32) and high (ranged from 0.83–0.96) correlation coefficients of SQI and crop yield across a number of soil/crop combinations were reported [8], [31]. The reasons attributed to low correlation between yield and SQI were: (i) the assumptions that the indicators considered good for a soil quality may not always lead to desired outcomes and flexibility in scoring functions needed [8], (ii) indicators which may not directly relate to crop performance were given more weightage by the method employed, or (iii) some key soil indicators were not included in the MDS due to the design of the study [9], [43]. Nevertheless, in order to evaluate the index performance in the current study, SQIs were correlated against end point variable (i.e., yield) at variable depths (Table 7). In the present study, depth of soil sampling had no significant effect in SQI values (Table 6) and thus the individual SQI values were integrated by combining 0–20 cm and 0–60 cm profiles by averaging 0–10, and 10–20 cm for obtaining values for 0–20 cm depth and 0–10, 10–20, 20–40 and 40–60 cm for obtaining values for 0–60 cm depths and correlated the combined values with crop yield (Table 7). In the present study, grain and biomass yields were all significantly (p<0.05, r = 0.65–0.79) correlated under any SQI methods employed indicating that either of these methods was successful in predicting crop yield (Table 7). However, correlation coefficients in Table 7 are derived from the complete datasets including both corn and soybean yield but the correlation was considerably low (r<0.20 for soybean grain and r<0.60 for corn grain; data not shown), when dataset was further divided into corn and soybean. As discussed above, low correlation of SQI and crop yield was observed before and one of the listed reasons was some key soil indicators were not included in the dataset as per the experimental design and objective [8]. Crop yield is invariably related to soil fertility status and thus SQI may not always lead to high correlation with yield if some of the related characterizations are not observed as identified before [9], [43] which is supported by the data of the current study (Table 7). Thus, the low correlation coefficient values may be because soil fertility (ion exchange capacities, macro and micro nutrients) and microbial (microbial biomass, microbial C, and N, and soil respiration) aspects were not monitored in the current studied sites, especially in the case of soybean grain yield. Nevertheless, relatively higher correlation coefficient was observed in the 0–10 cm soil layer than the deeper soil profile (Table 7) probably due to higher plant root density in the top layer of the soil [44].
Table 7. Pearson correlation coefficients (r) of soil quality index (SQI) versus crop yield and correlations between different SQI values which were averaged up to a certain depth; all numbers are significant at p<0.05 level.
| SQI-1 | Dry Grain | Dry Straw |
| 0–60 cm | 0.65 | 0.67 |
| 0–20 cm | 0.71 | 0.71 |
| 0–10 cm | 0.73 | 0.71 |
| SQI-2 | Dry Grain | Dry Straw |
| 0–60 cm | 0.75 | 0.73 |
| 0–20 cm | 0.75 | 0.72 |
| 0–10 cm | 0.79 | 0.75 |
| SQI-3 | Dry Grain | Dry Straw |
| 0–60 cm | 0.76 | 0.74 |
| 0–20 cm | 0.76 | 0.74 |
| 0–10 cm | 0.78 | 0.76 |
| SQI-1 | SQI-2 | |
| SQI-2 | 0.92 | |
| SQI-3 | 0.93 | 0.97 |
Additionally, SQI computed from different methods were also highly correlated with each other (Table 7) suggesting that (i) a relatively easy and user-friendly SQI (SQI-1) can be computed to evaluate and compare soil quality, which is similarly useful to other approaches, (ii) giving certain appropriate weightage on scores (SQI-2) could be similarly useful in predicting particular soil quality and (ii) a minimum group of carefully selected soil indicators (SQI-3) may be used to evaluate soil quality across various soil types and management practices. As all three SQI methods were significantly correlated to each other, it is difficult to conclude which one is the best approach, however, it highly depends on factors such as design of the study, choice of soil parameters included in the model to compute SQI and end point variable (environmental aspects versus crop yield). The advantage of using SQI-1 is that the soil quality could be assessed after measuring any number (low to high) of soil parameters and this procedure is relatively easier compared to other methods as the scoring requires literature review and expert opinions only. The disadvantage of SQI-1 is that it is subjective and relies mainly on researcher’s point of view. On the other hand, advantage of SQI-2 is that it includes weightage based on the design of the study, system or the dataset to offset the subjectivity of the approach present in SQI-2. However, the disadvantage of SQI-2 is that it requires multiple numbers of soil parameters under different soil functional systems which may be expensive and time consuming in practical case. The SQI-3 is advantageous in the aspect of its ability to predict soil quality based on a reduced dataset with low number of soil parameters. Additionally, it is mostly objective approach as the statistical procedure would select a low number of soil parameters needed to calculate SQI based on the variances present in the whole dataset. So, in a long-term aspect within a particular soil/crop system, SQI-3 can be used effectively once it evaluated the most influential soil parameters required to assess soil quality of a particular soil/crop/management scenario. However, current data suggest that relative higher SQI values were obtained for Ko and Mc by SQI-1 than SQI-2 or SQI-3 and for CrB by SQI-2 than SQI-1 or SQI-3 (Fig. 2). Although SQI-1 and SQI-2 had higher values than SQI-3 in different scenarios, however, based on the current experimental design, and selected soil parameters SQI-3 successfully predicted crop yield relatively higher [Table 7 and also when grouped by crops (data not shown)] than SQI-1 and 2. Depth had little influence in any SQIs in the present study and additional chemical and biological parameters are also needed to strengthen the validity of SQI. Thus, SQI-3 appears to be the best method among the three under long-term scenario, especially due to its objective approach, relative higher correlations with crop yield and lower number of indicator selection which is more cost and time-effective over time than other approaches.
Conclusions
The data presented support the following conclusions: The SQI calculated by three different methods indicated that studied muck soil has significantly higher soil quality than that of mineral soils under on-farm conditions. The SQI was affected more by management and soil type than by depths in the studied on-farm sites. The SQI computed using three established methods were all significantly correlated to each other indicating that relatively easy and user-friendly SQI (SQI-1) can be similarly useful to evaluate soil quality, appropriate weightage on scores can predict soil’s quality (SQI-2) with high performance but requires a number of soil parameters under different soil functional components and carefully chosen MDS with small numbers of soil variables (SQI-3) may adequately evaluate its quality. All three SQIs were highly and significantly correlated with crop yield. In general, however, analyses by crop type (corn and soybean) revealed that correlation was low (r<0.20 or 0.60 for soybean or corn grain yield, respectively; data not shown) suggesting choice of crop-specific and key soil parameters used in computing SQI. Under the current experimental on-farm conditions SQI-1 (for Ko and Mc) and SQI-2 (for CrB) values were higher than SQI-3, however, SQI-3 can be regarded as the best and easiest model given its relatively higher success to predict crop yield and objectivity approach with lower number of indicator selection ability which should be regarded as a relatively less expensive procedure over time compared to SQI-1 and 2. In addition, in order to effectively predict particular crop yield one must include soil fertility and microbial parameters in the model of SQI.
Supporting Information
Percentage contribution of each soil function in SQI-2 under different soils in four soil layers; different letters indicate significant differences at p<0.05 level for particular soil function. Abbreviations: RDC: root development capacity, WSC: water storage capacity, NSC: nutrient storage capacity.
(TIF)
Acknowledgments
Authors would like to thank Dr. Shiguo Jiang for his help with PCA and Mr. Basant Rimal for his assistance in the laboratory. Authors would also like to thank three anonymous reviewers and an academic editor for their constructive suggestions to improve the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are presented in the paper.
Funding Statement
This research was part of a collaborative project supported by the USDA-NIFA, award number: 2011-68002-30190, “Cropping Systems Coordinated Agricultural Project (CAP): Climate Change, Mitigation, and Adaptation in Corn-based Cropping Systems” (sustainablecorn.org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Doran J, Coleman D, Bezdicek D, Stewart B (1994) A framework for evaluating physical and chemical indicators of soil quality.
- 2. Doran JW, Parkin TB (1994) Defining and assessing soil quality. SSSA special publication 35: 3–3. [Google Scholar]
- 3. Karlen DL, Mausbach MJ, Doran JW, Cline RG, Harris RF, et al. (1997) Soil Quality: A Concept, Definition, and Framework for Evaluation (A Guest Editorial). Soil Sci Soc Am J 61: 4–10. [Google Scholar]
- 4.Karlen DL, Stott DE (1994) A framework for evaluating physical and chemical indicators of soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA, editors. Defining soil quality for a sustainable environment. Madison, WI: Soil Science Society of America. pp. 53–72.
- 5. Stocking MA (2003) Tropical Soils and Food Security: The Next 50 Years. Science 302: 1356–1359. [DOI] [PubMed] [Google Scholar]
- 6.Diack M, Stott D (2001) Development of a soil quality index for the Chalmers Silty Clay Loam from the Midwest USA. Purdue University: USDA-ARS National Soil Erosion Research Laboratory. pp. 550–555.
- 7.Larson WE, Pierce FJ (1991) Conservation and enhancement of soil quality. Evaluation of Sustainable Land Management in the Developing World. International Board for Soil Research and Management, Bangkok, Thailand.
- 8. Andrews S, Karlen D, Mitchell J (2002) A comparison of soil quality indexing methods for vegetable production systems in Northern California. Agriculture, Ecosystems & Environment 90: 25–45. [Google Scholar]
- 9. Bhardwaj AK, Jasrotia P, Hamilton SK, Robertson GP (2011) Ecological management of intensively cropped agro-ecosystems improves soil quality with sustained productivity. Agriculture Ecosystems & Environment 140: 419–429. [Google Scholar]
- 10. Andrews SS, Carroll CR (2001) Designing a soil quality assessment tool for sustainable agroecosystem management. Ecological Applications 11: 1573–1585. [Google Scholar]
- 11. Andrews SS, Karlen DL, Cambardella CA (2004) The soil management assessment framework: A quantitative soil quality evaluation method. Soil Science Society of America Journal 68: 1945–1962. [Google Scholar]
- 12. Andrews SS, Mitchell JP, Mancinelli R, Karlen DL, Hartz TK, et al. (2002) On-Farm Assessment of Soil Quality in California's Central Valley. Agron J 94: 12–23. [Google Scholar]
- 13. Fernandes JC, Gamero CA, Rodrigues JGL, Mirás-Avalos JM (2011) Determination of the quality index of a Paleudult under sunflower culture and different management systems. Soil and Tillage Research 112: 167–174. [Google Scholar]
- 14. Mandal UK, Ramachandran K, Sharma K, Satyam B, Venkanna K, et al. (2011) Assessing Soil Quality in a Semiarid Tropical Watershed Using a Geographic Information System. Soil Science Society of America Journal 75: 1144–1160. [Google Scholar]
- 15. Zobeck TM, Halvorson AD, Wienhold B, Acosta-Martinez V, Karlen DL (2008) Comparison of two soil quality indexes to evaluate cropping systems in northern Colorado. Journal of Soil and Water Conservation 63: 329–338. [Google Scholar]
- 16. Zornoza R, Mataix-Solera J, Guerrero C, Arcenegui V, Mataix-Beneyto J, et al. (2008) Validating the effectiveness and sensitivity of two soil quality indices based on natural forest soils under Mediterranean conditions. Soil Biology and Biochemistry 40: 2079–2087. [Google Scholar]
- 17. Jokela W, Posner J, Hedtcke J, Balser T, Read H (2011) Midwest Cropping System Effects on Soil Properties and on a Soil Quality Index. Agronomy Journal 103: 1552–1562. [Google Scholar]
- 19.Brady NC, Weil RR (1984) The nature and properties of soils New York: Macmillan.
- 20.Mukherjee A, Lal R (2014) Long and short-term tillage effects on quality of organic and mineral soils under on-farm conditions in Ohio, Submitted. Soil and Tillage Research.
- 21.Mukherjee A, Lal R (2014) Short-term effects of cover cropping on quality of a Typic Argiaquolls in Central Ohio, Submitted.
- 22.Grossman RB, Reinsch TG (2002) Bulk density and linear extensibility. In: Dane JH, Topp GC, editors. Methods of soil analysis, Part 4: Physical methods. Madison, WI: SSSA. pp. 201–254.
- 23. Herrick JE, Jones TL (2002) A dynamic cone penetrometer for measuring soil penetration resistance. Soil Science Society of America Journal 66: 1320–1324. [Google Scholar]
- 24. Yoder RE (1936) A direct method of aggregate analysis of soils and a study of the physical nature of erosion losses. J Am Soc Agron 28: 337–351. [Google Scholar]
- 25. Youker RE, McGuinness JL (1957) A short method of obtaining mean weight-diameter values of aggregate analyses of soils. Soil Science 83: 291–294. [Google Scholar]
- 26.Dane JH, Hopmans JH (2002) Water retention and storage. In: Dane JH, Topp GC, editors. Methods of soil analysis Part 4, SSSA Book Ser 5. Madison, WI.: SSSA. pp. 671–717.
- 27. Chhabra A, Palria S, Dadhwal VK (2003) Soil organic carbon pool in Indian forests. Forest Ecology and Management 173: 187–199. [Google Scholar]
- 28.Amacher MC, Perry CH (2007) Soil vital signs: A new Soil Quality Index (SQI) for assessing forest soil health.
- 29. Liebig MA, Varvel G, Doran J (2001) A simple performance-based index for assessing multiple agroecosystem functions. Agronomy Journal 93: 313–318. [Google Scholar]
- 30. Li Q, Xu MX, Liu GB, Zhao YG, Tuo DF (2013) Cumulative effects of a 17-year chemical fertilization on the soil quality of cropping system in the Loess Hilly Region, China. Journal of Plant Nutrition and Soil Science 176: 249–259. [Google Scholar]
- 31. Masto RE, Chhonkar PK, Singh D, Patra AK (2008) Alternative soil quality indices for evaluating the effect of intensive cropping, fertilisation and manuring for 31 years in the semi-arid soils of India. Environmental monitoring and assessment 136: 419–435. [DOI] [PubMed] [Google Scholar]
- 32. Doran JW, Parkin TB (1996) Quantitative indicators of soil quality: a minimum data set. SSSA special publication 49: 25–38. [Google Scholar]
- 33.Dunteman GH (1989) Principal components analysis: Sage.
- 34.Jolliffe I (2005) Principal component analysis: Wiley Online Library.
- 35.SPSS (2014) IBM SPSS, Version 21.0, Chicago, USA.
- 36. Kaiser HF (1960) The application of electronic computers to factor analysis. Educational and Psychological Measurement 20: 141–151. [Google Scholar]
- 37. Wander MM, Bollero GA (1999) Soil quality assessment of tillage impacts in Illinois. Soil Science Society of America Journal 63: 961–971. [Google Scholar]
- 38.SAS (2012) SAS Institute Inc.; SAS version 9.2.
- 39.Microsoft (2007) Microsoft Excel. Redmond, Washington.
- 40.Sigmaplot (2012) Scientific Software Solutions Internationals; Sigmaplot version 12.
- 41. Feiza V, Feiziene D, Kadziene G, Lazauskas S, Deveikyte I, et al. (2011) Soil state in the 11th year of three tillage systems application on a cambisol. Journal of Food, Agriculture & Environment 9: 1088–1095. [Google Scholar]
- 42.Lal R (1994) Methods and guidelines for assessing sustainable use of soil and water resources in the tropics; Washington D.C.: USDA/SMSS Technical Monograph 21.
- 43. Zornoza R, Mataix-Solera J, Guerrero C, Arcenegui V, García-Orenes F, et al. (2007) Evaluation of soil quality using multiple lineal regression based on physical, chemical and biochemical properties. Science of The Total Environment 378: 233–237. [DOI] [PubMed] [Google Scholar]
- 44. Maurya PR, Lal R (1980) Effects of No-Tillage and Ploughing on Roots of Maize and Leguminous Crops. Experimental Agriculture 16: 185–193. [Google Scholar]
- 45. Brzezinska M, Sokolowska Z, Alekseeva T, Alekseev A, Hajnos M, et al. (2011) Some characteristics of organic soils irigated with municipal wastewater. Land Degradation & Development 22: 586–595. [Google Scholar]
- 46.Sokoowska Z (2011) Effect of phosphates on dissolved organic matter release from peat-muck soils.
- 47. Walczak R, Rovdan E, Witkowska-Walczak B (2002) Water retention characteristics of peat and sand mixtures. International Agrophysics 16: 161–166. [Google Scholar]
- 48.Carter MR (2006) Quality critical limits and standardization. In: Lal R, editor. Encyclopedia of Soil Science. New York: Marcel Dekker. pp. 1412–1415.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percentage contribution of each soil function in SQI-2 under different soils in four soil layers; different letters indicate significant differences at p<0.05 level for particular soil function. Abbreviations: RDC: root development capacity, WSC: water storage capacity, NSC: nutrient storage capacity.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are presented in the paper.