Summary
Upon oxidative challenge the genome accumulates adducts and breaks that activate the DNA damage response to repair, arrest or eliminate the damaged cell. Thus, reactive oxygen species (ROS) generated by endogenous oxygen metabolism are thought to affect mutation frequency. However, few studies determined the mutation frequency when oxidative stress is reduced.
To test whether in vivo spontaneous mutation frequency is altered in mice with reduced oxidative stress and cell death rate, we crossed p66Shc knock out (p66KO) mice, characterized by reduced intracellular concentration of ROS and by impaired apoptosis, with a transgenic line harboring multiple copies of the lacZ mutation reporter gene as part of a plasmid that can be recovered from organs into E. coli to measure mutation rate. Liver and small intestine from 2- and 24- month old, lacZ (p66Shc+/+) and lacZp66KO mice, were investigated revealing no difference in overall mutation frequency but a significant increase of the frequency of size-change mutations in the intestine of lacZp66KO mice. This difference was further increased upon irradiation of mice with X-Ray. Additionally, we found that knocking down cyclophilin D, a gene that facilitates mitochondrial apoptosis acting downstream of p66Shc, increased the size-change mutation frequency in small intestine. Size-change mutations also accumulated in death-resistant embryonic fibroblasts from lacZp66KO mice treated with H2O2.
These results indicate that p66Shc plays a role in the accumulation of DNA rearrangements and suggest that p66Shc functions to clear damaged cells rather than affect DNA metabolism.
Keywords: longevity genes, mutagenesis, reactive oxygen species, cell death, mitochondria, mice
Introduction
Reactive oxygen species (ROS) produced by mitochondria and cytosolic oxidases are known inducers of oxidative stress that is thought to cause degenerative diseases and aging (Balaban et al., 2005; Sohal et al., 2012). DNA damage is involved in the deleterious effects of ROS as DNA mutations accumulate upon oxidant challenge (Packer et al., 1994) and with age (Lombard et al., 2005), whereas defects in DNA repair result in altered cellular survival and premature aging (Hasty et al., 2003). However, whether reduced intracellular levels of ROS affect mutation rate in vivo is unknown.
P66Shc is the largest isoform, almost ubiquitously expressed in vertebrates by the ShcA locus, it functions to regulate intracellular ROS levels and mitochondrial apoptosis (see for review: Luzi et al., 2000; Pellegrini et al., 2009). A fraction of p66Shc exists within the mitochondrial inter-membrane space (Orsini et al., 2004), where it oxidizes cytochrome c to form H2O2 (Giorgio et al., 2005; Pinton et al.; 2007; Gerz et al., 2008). Moreover, cytosolic p66Shc mediates the activation of the membrane oxidase activity (Khanday et al., 2006; Tomilov et al., 2010) and suppresses catalase (Nemoto et al., 2002) and MnSOD (Guo et al., 2009) expressions. Accordingly, cells from p66Shc−/− (p66KO) mice or p66Shc-depleted by RNAi have reduced ROS levels (immortalized fibroblasts, Nemoto et al., 2002 and Khanday et al., 2006; primary embryonic and adult fibroblasts, Trinei et al., 2002; endothelial cells, Zaccagnini et al., 2004; lymphocytes, Pacini et al., 2003; hepatocytes, Giorgio et al., 2005; adipocytes, Berniakovich et al., 2008; Neurons, Brown et al., 2010). In addition, p66KO mice show less intracellular and systemic oxidative damage (liver, spleen, Trinei et al., 2002; vessels, Napoli et al., 2003 and Cosentino et al., 2004; kidney, Pugliese et al., 2006; heart, Rota et al., 2006 and Carpi et al., 2009).
H2O2 generated by p66Shc triggers mitochondrial swelling through the opening of the mitochondrial permeability transition pore (Orsini et al., 2004, Giorgio et al., 2005, Pinton et al., 2007, Arany et al., 2010). Notably, a critical role of p66Shc in the propagation of apoptotic signals has been documented both in cell culture upon a variety of stimuli, including irradiation, oxidants, anticancer drugs, hyperglycemia, calcium overload, amyloid or HIV proteins (see for review Migliaccio et al., 2006) and has also been observed in vivo. In fact, p66KO mice show markedly reduced signs of tissue damage and apoptosis after ischemia (Zaccagnini et al., 2004; Carpi et al., 2009), hypercholesterolemic diet (Napoli et al., 2003), diabetes (Rota et al., 2006; Pugliese et al., 2006; Fadini et al., 2010), encephalitis (Su et al., 2012), hepatectomy (Haga et al., 2010) and challenges with angiothensin II (Graiani et al., 2005; Sun et al., 2010), paraquat (Migliaccio et al., 1999), ethanol (Koch et al., 2008) or chloride carbide (Giorgio et al., 2005). Consistently p66KO mice are resistant to degenerative diseases and show signs of retarded aging (Cosentino et al., 2004; Pugliese et al., 2006; Pesaresi et al., 2011).
To determine the effect of p66Shc on somatic mutations we investigated the mutant frequencies and spectra at the lacZ locus of primary mouse embryonic fibroblasts (MEFs) as well as liver and small intestine from mice, harboring lacZ reporter genes (Boerrigter et al., 1995), which have been crossed with p66KO mice.
Results
LacZ size-change mutations accumulate in lacZp66KO MEFs following H2O2 treatment
We investigated the mutation frequency in MEFs obtained from C57Bl/6J lacZ homozygous / p66Shc+/+ (lacZ) and C57Bl/6J lacZ homozygous / p66Shc−/−(lacZp66KO) embryos. MEFs deleted for p66Shc underwent senescence after 5-6 passages as MEFs and were found to be resistant to apoptogenic stresses including H2O2 (Migliaccio et al., 1999; Nemoto et al., 2002; Giorgio et al., 2005 and Fig. 1A). Overall the lacZ mutant frequencies measured in untreated lacZ and lacZp66KO MEFs at passage 3 were similar at 7.3 ± 0.9 × 105 and 8.6 ± 2.0 × 105 respectively. To further characterize these mutations we subdivided them based on their restriction pattern. Those showing change in size in the plasmid insert are generally point mutations whilst those that altered the size of the insert by 50 bp or more (size-change mutations) are indicative of large genome rearrangements or deletions with one break point in the lacZ gene and another elsewhere in the mouse genome. This analysis showed the ratio of no-change mutations to genome rearrangements differed significantly (p-values<0.05) between the two groups with lacZp66KO MEFs exhibiting a slightly higher frequency of no-change mutations and lower frequency of size-change mutations at the lacZ locus with respect to lacZ MEFs (Fig.1B).
Figure 1. Frequency of mutations in lacZ and lacZp66KO MEFs.
(A) Cell survival of MEFs 24 hours after treatment with H2O2. (p-value<0.05 for differences among the two values). (B) Frequencies of no-change (open bars) and size-change (hatched bars) mutations (*p-value=0.0078, #p-value=0.0351, ^p-value=0.0613) of MEFs treated with H2O2. (C) Frequencies of mutations in floating cells/debris accumulated over 24 hours upon H2O2.
Averages and SD of three experiments using independent MEFs were shown.
Cells were then treated with 100 μM H2O2 and mutant frequencies were measured in the adherent cells recovered 6- and 24-hours after treatment. At 6 hours post treatment the mutant frequency in both lacZ and lacZp66KO MEFs (Fig. 1B) was elevated about 2.5-fold over untreated cells. Twenty four hours after treatment with H2O2, the mutant frequencies of LacZ MEFs were restored to wild-type levels (7.8 ± 1.0 × 105) whilst lacZp66KO MEFs at the showed significantly higher lacZ mutation frequency, 20.4 ± 3.4 × 105 (Fig. 1B). In general, the frequencies of both, size-change and no-change mutations were increased at 6 hours (Vijg et al., 2004, Busuttil et al., 2007) then decreased at 24 hours upon H2O2 in lacZ MEFs, whereas in lacZp66KO MEFs frequency of no-change mutations increased much less upon H2O2 whereas size-change mutation frequency increased and remained high till 24 hours (Fig. 1B). As expected (Migliaccio et al., 2006), the survival of H2O2 treated p66lacZ MEFs was higher than lacZ MEFs (Fig. 1A) and the number of detached cells collected from lacZp66KO plates was lower than lacZ. Floating debris present in the culture medium was harvested 24 hours post- H2O2 treatment by centrifugation. DNA was extracted and LacZ mutant frequency in the genomic extract derived from these pellets was determined. Both groups showed similar overall mutant frequencies, 25.3 ± 2.5 × 105 for the lacZ and 22.1 ± 3.0 × 105 for the lacZp66KO (average and SD from 3 independent samples each one from a different experiment and MEF preparation) (Fig. 1C) comparable (p-value=0.561) to the frequency (20.4 ± 3.4 × 105) observed in the LacZp66KO MEFs still attached to the dish at 24 hours post H2O2 treatment. Overall these results indicate that the genetic deletion of p66Shc in MEFs allows the accumulation of lacZ mutations, particularly of size-change mutations upon H2O2 challenge presumably because of increased resistance to cell death.
LacZ size-change mutations accumulate in lacZp66KO small intestine
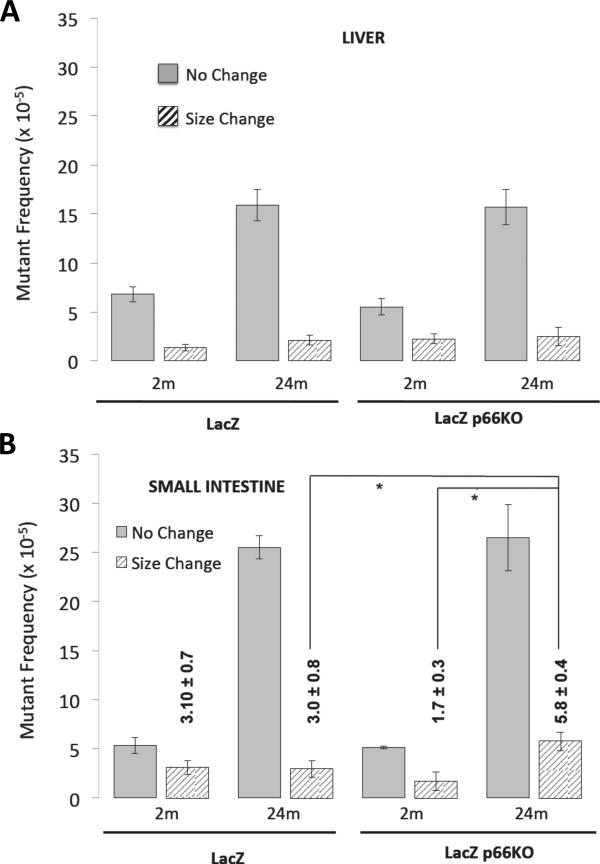
Spontaneous lacZ mutant frequencies were determined for liver and small intestine from 2- and 24-month old healthy lacZ and lacZp66KO mice.
In the liver of young mice, overall lacZ mutant frequency was similar (p-values>0.05) in lacZ (8.2 ± 1.1 × 105) and lacZp66KO (7.8 ± 1.1 × 105) mice. Whilst we observed an age related increase in mutant frequency this was the same in both lacZ (18.0 ± 1.4 × 105) and lacZp66KO (18.2 ± 2.1 × 105) mice. Furthermore, characterization of the mutations did not reveal differences between age-matched lacZ and lacZp66KO mice (Fig. 2A).
Figure 2. Frequency of mutations in liver and small intestines of young and old lacZ and lacZp66KO mice.
Frequencies of no-change (open bars) and size-change (hatched bars) mutations in liver (A) and small intestine (B) of 2 and 24 -month old lacZ and lacZp66KO mice (*p-value<0.05; n=8-10 mice).
The overall lacZ mutant frequency in the small intestine of young mice was also similar between the two groups: 8.5 ± 0.6 × 105 for the lacZ and 6.9 ± 0.5 × 105 for the lacZp66KO. In older mice the small intestine mutant frequency increased significantly up to 28.5 ± 1.8 × 105 for lacZ and 32.3 ± 2.1 × 105 for p66KO animals. Analysis of size-change and no-change mutations, although the majority of mutations present in the small intestine were due to point mutations (Dolle` et al., 2006); revealed that there was a significant increase (p-value=0.0401) in the frequency of size-change mutations in the older lacZp66KO mice (5.8 ± 0.6 × 105)(Fig. 2B) compared to the younger counterparts (1.7 ± 0.7 × 105)(Fig. 2B). Thus, deletion of p66Shc in mice results in the accumulation of size-change mutations in small intestine, as determined using the lacZ mutation reporter system.
X-Ray exposure enriches for no-change mutations in the small intestine of lacZ mice particularly
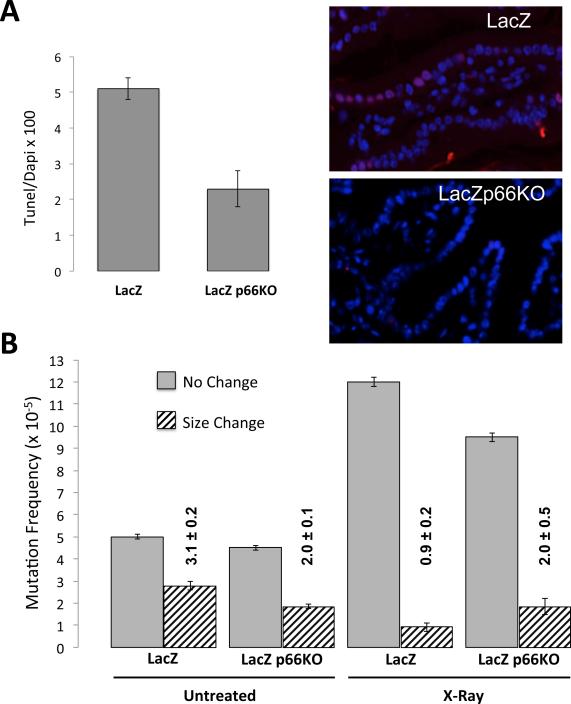
To test the effect of p66Shc loss on mutant frequency induced by DNA damaging treatment in vivo we determined lacZ mutation frequencies in small intestines of lacZ and lacZp66KO young mice 24 hours after exposure to 4 Gy X-Ray. Both strains of mice survived (100% up to 2 months in control experiments) this treatment despite an initial drop in their white blood cells (from 4.76 ± 0.18 103/μl to 1.10 ± 0.21 103/μl 24 hours following irradiation in wild-type and from 4.60 ± 0.15 103/μl to 0.92 ± 0.08 103/μl in p66KO mice). Notably, the number of apoptotic cells, detected in the epithelium of small intestine by TUNEL assay 24 hours after irradiation, was higher (p-value=0.0370) in the lacZ mice (10%) compared to the lacZp66KO mice (6%) (Fig. 3A).
Figure 3. Frequency of mutations in small intestine of lacZ and lacZp66KO mice following X-ray radiation.
(A) Percentage of TUNEL positive cells (n= 3-4 mice; p-value<0.05 for differences among the two groups) and representative image of TUNEL assay in small intestine sections from lacZ and lacZp66KO mice. (B) Frequencies of no-change (open bars) and size-change (hatched bars) mutations in small intestine of lacZ and lacZp66KO mice exposed to X-rays.
The analysis of lacZ mutations revealed that X-Ray increased the frequency of no-change mutations significantly both in lacZ and lacZp66KO small intestines, up to 12.6 ± 1.1 × 105 and 9.3 ± 1.9 × 105 respectively. However, the frequency of size-change mutations decreased, from 3.1 ± 0.2 × 105 to 0.9 ± 0.2 × 105 in the lacZ small intestine (p-value=0.0031), but remained unchanged in lacZp66KO to 2.0 ± 0.5 × 105 (Fig. 3B).
Thus, while deletion of p66Shc in mice does not affect the increase of no-change mutations, it does influence the clearance of size-change mutations induced by X-Ray irradiation.
Like p66Shc, deletion of cyclophilin D increases size-change mutations in small intestine
To study the possible role of apoptosis in causing the differences observed between lacZ and lacZ-p66ShcKO, we checked the mutant frequency in cyclophilin D knock out (CypDKO) mice, characterized by a reduced rate of mitochondrial apoptosis in different tissues (Baines et al., 2005; Du et al., 2008; Palma et al., 2009; Fujimoto et al., 2010). Mechanistically, CypD favors opening of the mitochondrial permeability transition pore, triggering mitochondrial swelling and apoptosis downstream to p66Shc (Giorgio et al., 2005).
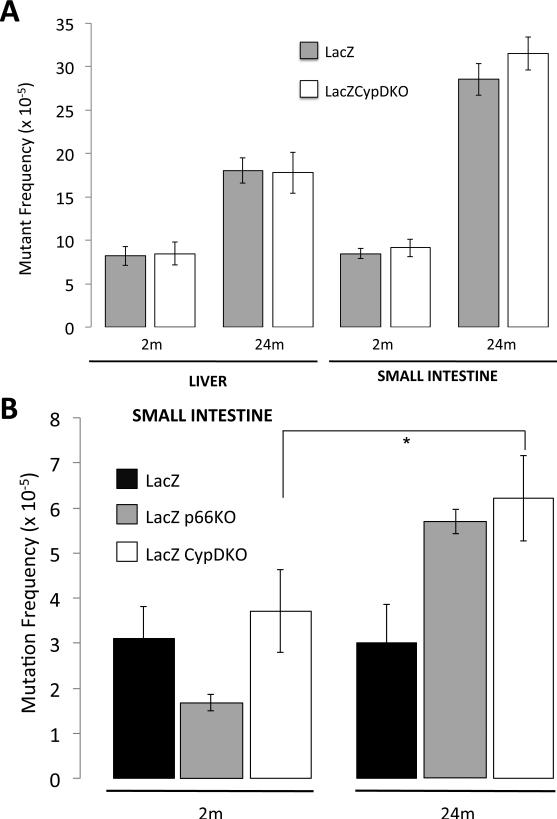
We have crossed C57Bl/6J CypDKO mice with C57Bl/6J lacZ mice and measured the mutant frequency rates in liver and small intestine from 2- and 24- month old C57Bl/6J lacZ CypD+/+ (code-named lacZ) and C57Bl/6J lacZ CypD−/− (code-named lacZCypDKO) mice. We have obtained organs from 5 mice per group and repeated the lacZ mutation analysis 8 different times for each single sample of genomic DNA extracted.
Results revealed that the deletion of CypD did not affect (p-value>0.6) the overall lacZ mutation frequency in liver in either of the 2 age groups studied (Fig. 4A). In the small intestine lacZCypDKO mice showed slightly higher frequency overall when compared to lacZ littermate controls. Further characterization of the mutants showed this increase to be solely due to a significant (p-value=0.0309) increase in size-change (Fig. 4B), as observed in the LacZP66KO mice.
Figure 4. Frequencies of mutations in liver and small intestine of young and old lacZCypDKO and lacZ mice.
(A) Overall lacZ mutant frequency in liver and small intestine of 2- and 24-month of lacZ (grey bars) and lacZCypDKO (white bars) mice. (B) Frequencies of size-change mutations in small intestine of 2- and 24-month of lacZ, lacZp66KO and lacZCypDKO mice (*p-value=0.0309).
P66Shc deletion does not affect spontaneous tumor incidence
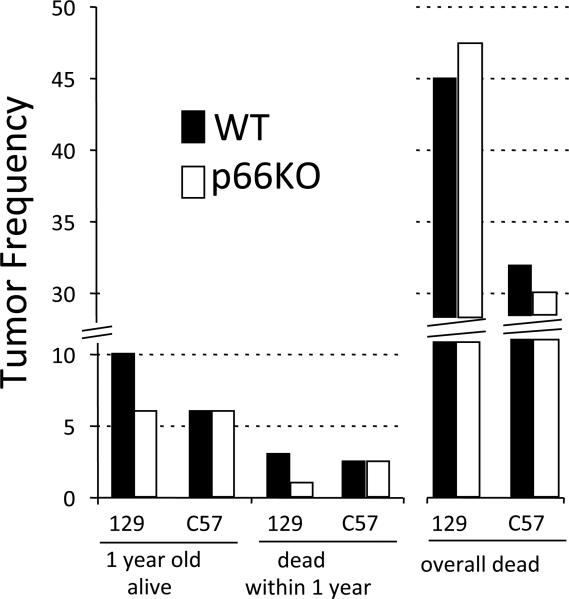
Decreased apoptosis and accumulation of mutations are expected to increase risk of tumors. Thus, we studied spontaneous tumor incidence in p66KO mice in two different strains, 129Sv and C57Bl/6J, and compared to wild-type (WT) controls. Malignant and non-malignant tumor incidence in mice euthanized at the age of 1 year is modest in WT (5/50 in 129 strain (10%) and 3/50 in C57 strain, (6%)) as well as in p66KO (3 /50 in 129 strain (6%) and 3/50 in C57 strain (6%); Fig. 5) genotypes. Approximately 5% of the mice died spontaneously within the first year irrelevant of their genotype. Necropsies conducted on these animals indicated that 3% (3/100) of WT 129 and 2.5% (2/80) of WT C57 mice as well as 1.0% (1/100) of 129 p66Shc−/−and 2.5% (2/80) of C57 p66Shc−/− had developed tumor masses (Fig. 5).
Figure 5. Spontaneous tumor incidence in p66KO mice.
Tumor frequencies in 129Sv and C57/Bl6J backgrounds as indicated WT (Black bars) and p66KO (white bars): i) euthanized 1-year old mice (n=50 per strain) (p > 0.5 for both strain or p66Shc mutation effects) ii) mice that died spontaneously within one year of age (<1year; n=100 and n=80 for the 129Sv and C57Bl6J backgrounds respectively; p = 0.6212 for the effect of p66Shc mutation in 129Sv background); iii) mice that died spontaneously, regardless of their age [n (number of mice observed) =180 and n= 190, 129Sv and C57Bl6/J WT respectively and n= 270 and n= 290, 129Sv and C57Bl/6J p66KO, collected over a period of 8 years; p= 0.5631 for the effect of p66Shc mutation in 129Sv background and p = 0.5467 for the effect of p66Shc mutation in C57Bl/6J background].
Finally, overall tumor incidence was evaluated in mice that died spontaneously irrespective of age (see Supplementary Table 1 for the age of death of these mice) and was found to be similar (p-value>0.5) in WT and p66Shc−/− animals (45% and 48% in 129 background WT and p66KO respectively, and 32% and 30% in C57 background WT and p66ShcKO respectively; Fig. 5). Most of mice were affected by lymphoma. In particular, hyperplasia of spleen and/or thymus was observed in almost 35% of the mice regardless the mutation of p66Shc. In older animals, lesions in organs such as liver and kidneys were found frequently as lymphomas. Then, lung adenomas, osteosarcomas, colon carcinomas and ovary cystoadenomas were detected. Sporadically, salivary and harderian glands developed tumor masses. No evidence of altered tumor spectrum in p66ShcKO resulted.
Notably, usual mortality rate and tumor incidence were found in our C57Bl/6J CypDKO mouse colony as well (data not shown).
Therefore, p66Shc deletion does not affect spontaneous tumor incidence.
Discussion
In this study we have measured mutant frequency of lacZ transgene when p66Shc is deleted and consequently intracellular levels of ROS and apoptosis are reduced. Results revealed that size-change mutations rather than no-change mutations accumulate particularly in MEFs and small intestine from p66KO mice.
ROS induce DNA adducts and breaks that activate the genome damage response to induce repair, cell cycle arrest and eventually clear the damaged cell (Halliwell and Gutteridge, 2007).
Indeed, studies utilizing p66KO mice as well as other transgenic animal models have shown that intracellular levels of ROS negatively correlate with stress resistance and life span (Orr et al., 1994; Migliaccio et al., 1999; Mitzui et al., 2002; Andrews et al., 2008; Csiszar et al., 2008; Perez Rivero et al., 2008). Previous studies utilising the lacZ transgenic reporter mice reported an increase of point mutations in short living and cancer prone mice deficient for the ROS scavenger enzyme Sod1 (Busuttil et al., 2005) whereas caloric restriction and suppression of the somatotroph axis, that promote longevity, was shown to reduce the frequency of lacZ mutations (Garcia et al., 2008; Dongwei et al., 2011).
Here we report that, the overall lacZ mutation frequency was unaffected by the deletion of p66Shc indicating that the amount of ROS generated by p66Shc, although relevant to trigger mitochondrial apoptosis, are not genotoxic. Indeed, p66KO mice showed normal tumor incidence.
However, the deletion of p66Shc changes the type of mutations that are accumulated over time and upon stress, as size-change mutations were significantly higher in p66KO mice compared to WT, both in the small intestine from old and young irradiated mice and stressed MEFs.
Interestingly, lacZ and p66KO MEFs killed by H2O2 showed similar frequency of size-change mutations. Thus, the reason of the difference observed in the size-change frequency of MEFs treated with H2O2 may rely on the reduced susceptibility to die of the p66KO cells regardless the damage. In agreement with this hypothesis we have observed that also the deletion of cyclophilin D, which facilitates apoptosis through the opening of mitochondrial permeability transition pore, similar to p66Shc, increases size-change mutations in the small intestine of old mice (Fig. 4B).
The fact that the small intestine but not the liver showed differences in both mouse mutants as compared to the wt, although p66Shc and CypD are expressed in the liver, suggest that tissues with high turnover particularly feel the effect of p66Shc and CypD, thus supporting the hypothesis that impaired apoptosis plays a role.
Finally, p66Shc deletion protects from stress and degenerative diseases but may affect robustness of some tissues allowing the accumulation of particularly mutated cells. To what extent these cells guarantee tissue function is questionable. Recently, we have found that p66Shc deletion is counter-selected when mice are maintained in harsh conditions (open field under cold and competition for food) that mimic wild, indicating that p66Shc is essential for fitness under naturally stressful conditions but redundant in protected environments (Giorgio et al., 2012). So, p66KO mice can survive to accumulate a peculiar spectrum of mutations only in laboratory conditions. In conclusion, the particular spectrum of LacZ mutations in p66KO discloses the importance of cell death rather than the overall redox balance for mutagenesis and suggests that specific genetic sets, in favorable environments, determine somatic mutations.
Experimental Procedures
Animals
Mice were bred in the certified IFOM-IEO campus animal facility in accordance with national and institutional guidelines.
Mice were housed in an air-conditioned room (temperature 21 ±1 °C, relative humidity 60 ± 10%) with a white-red light cycle (lights on from 07:00 to 19:00) and with ad libitum food availability (2018S Teklad Global 18% Protein Rodent Diet, provided by Harlan Teklad) and drinking water (autoclaved tap water). Group housing (4 animals per cage) was chosen to improve animal welfare. Home cages were Plexiglas boxes (42 × 27 × 14 cm) with sawdust as bedding. All the in vivo experiments were performed in accordance with Italian laws and regulations.
LacZ+/+ line 30 mice in a C57Bl/6J background (Dolle et al., 1996) were derived from the colony of one of the authors (J.V.) at the Buck Institute for Research and Aging, in Novato, US.; CypDKO p66Shc−/− were obtained by sequential backcross of original p66Shc mutants (Migliaccio et al., 1999) with WT C57Bl/6J (G>12 with selection of polymorphism); C57Bl/6J CypDKO founders were kindly provided by Prof. Mike Forte and Paolo Bernardi. LacZp66KO mice used for the experiments were littermates generated from the crosses of G1 double heterozygous lacZ+/-p66Shc +/- obtained from the GO initial cross of homozygous lacZ+/+ and p66shc−/−founders. LacZCypDKO were obtained following the same scheme of crosses.
Mice were sacrificed by cervical dislocation at 2- and 24- months of age and tissues harvested and stored till required.
For total body irradiation experiments, 10 week old lacZ (n=3) and lacZ-p66ShcKO (n=4) were irradiated with 4.0 Gy X-Ray using a Gilardoni CHF 320G X-ray generator (Gilardoni S.p.A.Italy) operated at 250 kVp, 15 mA. Mice were euthanized 24 hours after irradiation by cervical dislocation.
Small intestine was cut 0.5 cm below stomach till 1 cm above cecum. It was voided and cleaned with PBS. Liver was isolated, separated from the gallbladder. Organs were washed once in PBS and immediately fixed for paraffin blocks preparations or snap frozen on dry ice. Frozen samples were stored at −80°C until use.
Cells
Embryos from lacZ and lacZp66KO mice were isolated from pregnant female at day 13.5 post coitum as determined by assessment of vaginal plug and washed twice in PBS. Then, embryos were transferred in a Petri dish and each embryo was separated from placental membranes, amniotic sac, head and primordial blood organs. Embryos were finely chopped with scissors, re-suspended in trypsin-EDTA (0.5%, LONZA; 1mL/embryo) and incubated at 37°C for 15min. Cells were re-suspended with a pipette in 10mL complete medium (Dulbecco's Modified Eagle Medium containing 10% North American fetal bovine serum, 100U/mL Penicillin and 100γ/mL Streptomycin, 2mM L-Glutamine) and divided in 10cm Petri dishes (1 dish/embryo). Cells were cultured in a humidified incubator at 37°C 20%O2 10% CO2. Medium was replaced on the following day. Then, within 2-3 days, cells were seeded in 15cm Petri dishes and then frozen at the second passage. After thawing passage 3 cells were re-plated in 15cm dishes for the experiments (density 2×106 cells/dish) and then treated, when they reached at 80% confluency, for 6 hours with 100 μM H2O2 (Sigma-Aldrich) in serum free medium. At the indicated time points (6 and 24 hours), dishes were washed with PBS and cells were harvested and counted to assess the number of surviving cells and for DNA extraction. Alternatively, debris accumulated in the culture medium over 24 hours upon treatment was pelleted by centrifugation at 1500g at 4C for 10 minutes. DNA was subsequently extracted and analyzed for LacZ mutations.
LacZ mutation assay
DNA was isolated from MEFs and adult tissues as described (Dollé et al., 1996) with minor modifications. Briefly, frozen cell pellets were resuspended in lysis buffer (10 mM Tris-HCl, pH 8.0, 10 mM EDTA, 150 mM NaCl), whereas frozen tissues were homogenized in an appropriate volume (4.5mL for the liver and 9mL for the small intestine) of lysis buffer with a dounce-homogenizer. RNAse A (Sigma-Aldrich) was added to a final concentration of 120μg/mL and samples were incubated 1hour at 37°C with agitation. Then, SDS and proteinase K (Sigma-Aldrich) were added to a final concentration of 1% and 0.5mg/mL respectively. Tissues were digested overnight at 55 °C with agitation, DNA was extracted once with phenol–chloroform– isoamyl alcohol (25: 24: 1), followed by addition of 1/5 volume of 8 M potassium acetate and extraction twice with 1 volume of chloroform. DNA was precipitated by addition of isopropanol, washed with 70% ethanol and solubilized in 10 mM Tris-HCl, pH 8.0. DNA concentration was determined by measuring the OD at 260 nm. Plasmids were rescued as described (Boerrigter et al., 1995). Briefly, genomic DNA was digested with HindIII and LacZ plasmid was isolated using magnetic beads, pre-coated with LacI repressor protein. Plasmids were then ligated and transferred to E. coli C (ΔlacZ, GalE-) by electro-transformation. 1:1000 of transformants was plated in medium with 5-bromo-4-chloro-indolyl-β-D-galactopyranoside (X-ga l ) t o determine the total number of plasmids rescued. The remainder was plated in the presence of high concentrations of the lactose analogue Phenyl-b-D-galactoside (pgal); this only allows cells harboring a mutant lacZ gene to grow. Mutants are characterized using restriction analysis as described (Boerrigter et al., 1995).
Briefly, mutant colonies from selective plates were picked and grown overnight in LB medium in a 96-well plate. One microlitre was plated onto an X-gal plate to identify galactose insensitive mutants and one microlitre was added to a PCR reaction to amplify a 4252 bp region of lacZ. Primers used were: pUR4923-F 5’-TGG AGC GAA CGA CCT ACA CCG AAC TGA GAT-3’ pUR3829-R 5’-ATA GTG TAT GCG GCG ACC GAG TTG CTC TTG- 3’) The PCR product was then digested with AvaI and separated on a 1% agarose gel. Samples were classified as no-change (point mutations or small insertions/deletions up to 50bp) or size-change (rearragements) based on their restriction pattern. Point mutations, after AvaI digestion, exhibit a restriction pattern similar to that of the wild-type pUR288 plasmid (3 fragments of 1992 bp, 1443 bp and 818 bp, see Supplementary Fig. 1) whilst mutations that resulted in an altered restriction pattern were considered size-change mutants (intra-genic size-changes or genomic rearrangements).
For each experimental set, from 96 up to 200 mutants were analyzed.
TUNEL Assay
To determine the percentage of apoptotic cells in the small intestine of lacZ and lacZp66ShcKO mice, sections of 4 μm paraffin-embedded tissue samples, deparaffinized and dehydrated, were processed for the immunohistochemical detection of apoptosis by carrying out In situ Cell Death Detection assay (Roche Diagnostic) following the manufacturer's instructions.
Statistical analysis
Student's t-test and Fisher's exact test was performed to test for the significance of a difference between two normally distributed averages from independent samples.
Supplementary Material
Acknowledgments
We thank Mike Forte of the Oregon Health & Science University-USA and Paolo Bernardi of the University of Padova-Italy for sharing the CypDKO mice, Mirella Trinei of the EIO for many helpful discussions and advice on bacterial handling and Costanza Savino and Massimo Stendardo of the EIO for help in taking care of the mice.
This work was supported by AG020438 and AG017242 grants awarded to J.V., National Institutes of Health Grant 1P01AG025532-01A1 to PG.P. and Italian Association for Cancer Research (AIRC) investigator grant awarded to M.G..
References
- Andrews ZB, Horvath TL. Uncoupling protein-2 regulates lifespan in mice. Am J Physiol Endocrinol Metab. 2009;296:E621–7. doi: 10.1152/ajpendo.90903.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany I, Faisal A, Clark JS, Vera T, Baliga R, Nagamine Y. p66shc-mediated mitochondrial dysfunction in renal proximal tubule cells during oxidative injury. Am J Physiol Renal Physiol. 2010;298:F1214–21. doi: 10.1152/ajprenal.00639.2009. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Berniakovich I, Trinei M, Stendardo M, Migliaccio E, Minucci S, Bernardi P, Pelicci PG, Giorgio M. p66Shc-generated oxidative signal promotes fat accumulation. J Biol Chem. 2008;283:34283–93. doi: 10.1074/jbc.M804362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerrigter ME, Dollé ME, Martus HJ, Gossen JA, Vijg J. Plasmid-based transgenic mouse model for studying in vivo mutations. Nature. 1995;377:657–9. doi: 10.1038/377657a0. [DOI] [PubMed] [Google Scholar]
- Brown JE, Zeiger SL, Hettinger JC, Brooks JD, Holt B, Morrow JD, Musiek ES, Milne G, McLaughlin B. Essential role of the redox-sensitive kinase p66shc in determining energetic and oxidative status and cell fate in neuronal preconditioning. J Neurosci. 2010;30:5242–52. doi: 10.1523/JNEUROSCI.6366-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busuttil RA, Garcia AM, Cabrera C, Rodriguez A, Suh Y, Kim WH, Huang TT, Vijg J. Organ-specific increase in mutation accumulation and apoptosis rate in CuZn-superoxide dismutase-deficient mice. Cancer Res. 2005;65:11271–5. doi: 10.1158/0008-5472.CAN-05-2980. [DOI] [PubMed] [Google Scholar]
- Busuttil RA, Garcia AM, Reddick RL, Dollé ME, Calder RB, Nelson JF, Vijg J. Intra-organ variation in age-related mutation accumulation in the mouse. PLoS One. 2007;2:e876. doi: 10.1371/journal.pone.0000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpi A, Menabò R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim Biophys Acta. 2009;1787:774–80. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–94. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollé ME, Martus HJ, Gossen JA, Boerrigter ME, Vijg J. Evaluation of a plasmid-based transgenic mouse model for detecting in vivo mutations. Mutagenesis. 1996;11:111–8. doi: 10.1093/mutage/11.1.111. [DOI] [PubMed] [Google Scholar]
- Dollé ME, Busuttil RA, Garcia AM, Wijnhoven S, van Drunen E, Niedernhofer LJ, van der Horst G, Hoeijmakers JH, van Steeg H, Vijg J. Increased genomic instability is not a prerequisite for shortened lifespan in DNA repair deficient mice. Mutat Res. 2006;596:22–35. doi: 10.1016/j.mrfmmm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini GP, Albiero M, Menegazzo L, Boscaro E, Pagnin E, Iori E, Cosma C, Lapolla A, Pengo V, Stendardo M, Agostini C, Pelicci PG, Giorgio M, Avogaro A. The redox enzyme p66Shc contributes to diabetes and ischemia-induced delay in cutaneous wound healing. Diabetes. 2010;59:2306–14. doi: 10.2337/db09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Chen Y, Polonsky KS. Targeting cyclophilin D and the mitochondrial permeability transition enhances beta-cell survival and prevents diabetes in Pdx1 deficiency. Proc Natl Acad Sci U S A. 2010;107:10214–9. doi: 10.1073/pnas.0914209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Busuttil RA, Calder RB, Dollé ME, Diaz V, McMahan CA, Bartke A, Nelson J, Reddick R, Vijg J. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech Ageing Dev. 2008;129:528–33. doi: 10.1016/j.mad.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz M, Fischer F, Wolters D, Steegborn C. Activation of the lifespan regulator p66Shc through reversible disulfide bond formation. Proc Natl Acad Sci U S A. 2008;105:5705–9. doi: 10.1073/pnas.0800691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–33. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Giorgio M, Berry A, Berniakovich I, Poletaeva I, Trinei M, Stendardo M, Hagopian K, Ramsey JJ, Cortopassi G, Migliaccio E, Nötzli S, Amrein I, Lipp HP, Cirulli F, Pelicci PG. The p66Shc knocked out mice are short lived under natural condition. Aging Cell. 2012;11:162–8. doi: 10.1111/j.1474-9726.2011.00770.x. [DOI] [PubMed] [Google Scholar]
- Graiani G, Lagrasta C, Migliaccio E, Spillmann F, Meloni M, Madeddu P, Quaini F, Padura IM, Lanfrancone L, Pelicci P, Emanueli C. Genetic deletion of the p66Shc adaptor protein protects from angiotensin II-induced myocardial damage. Hypertension. 2005;46:433–40. doi: 10.1161/01.HYP.0000174986.73346.ba. [DOI] [PubMed] [Google Scholar]
- Guo J, Gertsberg Z, Ozgen N, Steinberg SF. p66Shc links alpha1-adrenergic receptors to a reactive oxygen species-dependent AKT-FOXO3A phosphorylation pathway in cardiomyocytes. Circ Res. 2009;104:660–9. doi: 10.1161/CIRCRESAHA.108.186288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S, Morita N, Irani K, Fujiyoshi M, Ogino T, Ozawa T, Ozaki M. p66(Shc) has a pivotal function in impaired liver regeneration in aged mice by a redox-dependent mechanism. Lab Invest. 2010;90:1718–26. doi: 10.1038/labinvest.2010.119. [DOI] [PubMed] [Google Scholar]
- Halliwell and Gutteridge Free radicals in Biology and Medicine. Oxford Bioscience. 2007 [Google Scholar]
- Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–9. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Koch OR, Fusco S, Ranieri SC, Maulucci G, Palozza P, Larocca LM, Cravero AA, Farre' SM, De Spirito M, Galeotti T, Pani G. Role of the life span determinant P66(shcA) in ethanol-induced liver damage. Lab Invest. 2008;88:750–60. doi: 10.1038/labinvest.2008.44. [DOI] [PubMed] [Google Scholar]
- Luzi L, Confalonieri S, Di Fiore PP, Pelicci PG. Evolution of Shc functions from nematode to human. Curr Opin Genet Dev. 2000;10:668–74. doi: 10.1016/s0959-437x(00)00146-5. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Pelicci PG. Apoptosis and aging: role of p66Shc redox protein. Antioxid Redox Signal. 2006;8:600–8. doi: 10.1089/ars.2006.8.600. [DOI] [PubMed] [Google Scholar]
- Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, Ishizaki-Koizumi S, Hirakawa T, Inoue T, Yoder J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4:693–6. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–30. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Packer L. Oxygen Radicals in Biological Systems Part D. Methods Enzymol. 1994;234:33–44. [Google Scholar]
- Palma E, Tiepolo T, Angelin A, Sabatelli P, Maraldi NM, Basso E, Forte MA, Bernardi P, Bonaldo P. Genetic ablation of cyclophilin D rescues mitochondrial defects and prevents muscle apoptosis in collagen VI myopathic mice. Hum Mol Genet. 2009;18:2024–31. doi: 10.1093/hmg/ddp126. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Baldari CT. Apoptosis and oxidative stress-related diseases: the p66Shc connection. Curr Mol Med. 2009;9:392–8. doi: 10.2174/156652409787847254. [DOI] [PubMed] [Google Scholar]
- Pérez-Rivero G, Ruiz-Torres MP, Díez-Marqués ML, Canela A, López-Novoa JM, Rodríguez-Puyol M, Blasco MA, Rodríguez-Puyol D. Telomerase deficiency promotes oxidative stress by reducing catalase activity. Free Radic Biol Med. 2008;45:1243–51. doi: 10.1016/j.freeradbiomed.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Pesaresi MG, Amori I, Giorgi C, Ferri A, Fiorenzo P, Gabanella F, Salvatore AM, Giorgio M, Pelicci PG, Pinton P, Carrì MT, Cozzolino M. Mitochondrial redox signalling by p66Shc mediates ALS-like disease through Rac1 inactivation. Hum Mol Genet. 2011;20:4196–208. doi: 10.1093/hmg/ddr347. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del Sal G, Pelicci PG, Rizzuto R. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–63. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Lüscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med. 2012;52:539–55. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su KG, Savino C, Marracci G, Chaudhary P, Yu X, Morris B, Galipeau D, Giorgio M, Forte M, Bourdette D. Genetic inactivation of the p66 isoform of ShcA is neuroprotective in a murine model of multiple sclerosis. Eur J Neurosci. 2012;353:562–71. doi: 10.1111/j.1460-9568.2011.07972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Xiao L, Nie J, Liu FY, Ling GH, Zhu XJ, Tang WB, Chen WC, Xia YC, Zhan M, Ma MM, Peng YM, Liu H, Liu YH, Kanwar YS. p66Shc mediates high-glucose and angiotensin II-induced oxidative stress renal tubular injury via mitochondrial-dependent apoptotic pathway. Am J Physiol Renal Physiol. 2010;299:F1014–25. doi: 10.1152/ajprenal.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomilov AA, Bicocca V, Schoenfeld RA, Giorgio M, Migliaccio E, Ramsey JJ, Hagopian K, Pelicci PG, Cortopassi GA. Decreased superoxide production in macrophages of long-lived p66Shc knock-out mice. J Biol Chem. 2010;285:1153–65. doi: 10.1074/jbc.M109.017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J. Impact of genome instability on transcription regulation of aging and senescence. Mech Ageing Dev. 2004;125:747–53. doi: 10.1016/j.mad.2004.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.