Abstract
Background and Objective
One of many limitations for cancer gene therapy is the inability of the therapeutic gene to transfect a sufficient number of tumor cells. Photochemical internalization (PCI) is a photodynamic therapy-based approach for improving the delivery of macromolecules and genes into the cell cytosol. The utility of PCI for the delivery of the GFP reporter gene on the same plasmid as a tumor suppressor gene (PTEN) was investigated in monolayers of U251 human glioma cells and muticell U87 glioma spheroids.
Materials and Methods
U251 monolayers or U87 spheroids were incubated in AlPcS2a and non-viral vector polyplexes for 18 hours. In all cases, light treatment was performed with a diode laser at a wavelength of 670 nm. The non-viral transfection agents, branched polyethylenimine (bPEI), or protamine sulfate (PS), were used with the plasmid constructs GFP/PTEN or GFP.
Results
PS/GFP polyplexes were much less toxic to the glioma cells compared to bPEI/GFP polyplexes but were highly inefficient at gene transfection if used alone. PCI resulted in a 5- to 10-fold increase in GFP protein expression compared to controls. PCI-bPEI/PTEN or PCI-PS/PTEN transfection of either U251 monolayers or U87 spheroids significantly inhibited their growth. but had no effect on MCF-7 cells containing a wild-type PTEN gene. In addition PCI-GFP transfection of gliomas cells had no effect on their growth pattern.
Conclusions
Collectively, the results suggest that AlPcS2a-mediated PCI can be used to enhance cell growth inhibition via transfection of tumor suppressor genes in glioma cells containing mutant PTEN genes.
Keywords: glioma, GBM, gene therapy, PDT, PCI, PTEN, photochemical internalization
Introduction
Glioblastoma multiforme (GBM) is the most deadly form of astrocytoma, which accounts for more than 60% of all primary malignant brain tumors [1]. Patients diagnosed with GBM have an average survival time of 12–14 months, and fewer than 3% are living 5 years after diagnosis. Despite advances in surgery, chemotherapy, and radiotherapy, the outcomes of patients with GBM have not significantly improved [2–5]. An important hallmark of GBM is the loss of heterozygosity on chromosome 10q. The PTEN gene, a phosphatase and tensin homolog, located in this region has been identified as a putative tumor suppressor gene [6,7]. The PTEN gene, has been mapped to chromosome 10q23.3, and is absent or mutated in majority of GBM [8–11]. Various functional studies of PTEN, including in vivo animal experimental model systems, have demonstrated that it plays a significant role in inducing G1 cell cycle arrest, apoptosis, and cell adhesion, migration, and differentiation [12–14]. A simplified graphic representation of the PTEN/PI3K/Akt signaling pathway is shown in Figure 1. Clearly, glioma is an attractive target for developing novel therapeutic approaches utilizing gene therapy. One, of many limitations for cancer gene therapy, is the inability of the therapeutic gene to transfect a sufficient number of tumor cells. In order for cells to take up and utilize macromolecules such as plasmid DNA the use of viral or non-viral gene delivery carriers is essential. Although viral vectors have been employed in many studies and several clinical protocols, non-viral gene carriers offer several advantages including reduced safety concerns [15,16]. Unfortunately many non-viral carriers, like the cationic polymer polyethylenimine (PEI) are relatively toxic and not well suited for in vivo use while biodegradable biologically available polymers, such as protamine, are much less efficient in gene transfer experiments compared with viral systems [17–20]. Methods to increase the transfection efficiency of biological gene vectors are therefore of interest.
Fig. 1.
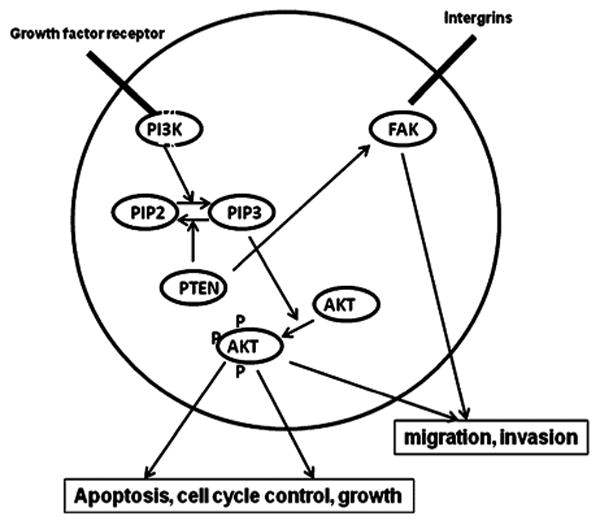
Graphic representation of the Pten/PI3K/Akt signaling pathway. PTEN has major implication in PI3 kinase (PI3K) signal transduction pathway and negatively controls PI3K phosphorylation. PTEN is implicated in cell cycle progression and cell death control through inhibition of PI3K-Akt signal transduction pathway and in the control of cell migration and spreading through its interaction with focal adhesion kinase (FAK).
Photochemical internalization (PCI) is a technique that utilizes the photochemical properties of photodynamic therapy (PDT) for the enhanced delivery of macromolecules into the cell cytosol. These macromolecules lack the ability to naturally permeate intracellular barriers such as the plasma and endocytic membranes [21,22]. The concept of PCI is based on using photosensitizers that localize in the membranes of endocytic vesicles. Upon illumination the photosensitizers react with oxygen causing membrane rupture releasing the trapped macromolecules into the cell cytosol, avoiding lysosomal degradation. Therefore, PCI which is a physical targeting technique, has been used in combination with adenovirus transfection introducing up to 20-fold increase in transgene expression in photochemically treated cells [23]. PCI has also been used to increase gene transfection with non-viral gene carriers [24,25]. The aim of the present research was designed to evaluate the effects of PCI on protamine sulfate (PS) or PEI-mediated wild-type PTEN gene restoration in PTEN mutated U87 and U251 human glioma cells.
Materials and Methods
Cell Lines and Plasmid Construct
The PTEN mutated glioblastoma cell line U87and the breast tumor PTEN-wild type MCF-7 were obtained from the American Type Culture Collection (Manassas, VA). The U251 cell line originally obtained from the Department of Neuro-Oncology, The University of Texas M.D. Anderson Cancer Center. Both glioma lines have been demonstrated to have a mutated PTEN gene caused by sequence insertions or deletions resulting in frameshifts [26]. The tumor cells were grown as monolayers in DMEM medium (Invitrogen Corp., Carlsbad, CA) with 10% heat-inactivated fetal bovine serum (FBS), 50 mM HEPES buffer (pH 7.4), penicillin (100 U/ml), and streptomycin (100 mg/ml) at 37°C and 5% CO2. Two different plasmids, one coding for GFP and one encoding for both PTEN and GFP reporter gene (PTEN-GFP) were used in all transfection experiments. Both pcDNA3-EGFP plasmid (Addgene plasmid 13031, submitted by Dr. Doug Golenbock, University of Massachusetts) and PTEN-GFP plasmid (Addgene plasmid 13039, submitted by Dr. Alonzo Ross, University of Massachusetts) were purchased from Addgene (Cambridge, MA) transformed in Escherichia coli strain of DH5alpha. The stab culture received was streaked onto Ampicillin (100 μg/ml) containing Miller's LB agar (1.5%) for 24 hours to isolate single colony. A single colony was dispersed into Ampicillin containing Miller's LB Broth for 6 hours as preculture, followed by overnight expansion to desired volume. Bacteria cultures were collected at exponential growth phase and centrifuged at volume of 45 ml per tube at 7,000 rpm (Beckman J2-21 centrifuge with JA-20 rotor) for 30 minutes at 4°C. Bacteria pellets were stored at −20°C until ready to use. Plasmids were isolated with QIAGEN MidiKit in the ratio of 1 column per tube of bacteria pellet according to the manufacturer's instruction. Plasmid DNA concentrations were measured with a spectrophotometer (Nanodrop 1000 Therma Scientific, Wilmington, DE).
Gene Carriers-Branched Polyethylenimine (bPEI), Protamine Sulfate (PS)
bPEI, MW 25,000, (Sigma, St. Louis, MO) was used in all experiments. bPEI/DNA polyplexes were formed as follows. bPEI (6.5 μg) (Sigma–Aldrich, St. Louis, MO) was dispersed in 13 μl of deionized (DI) water and 5 μg of pDNA (plasmid DNA) was dispersed in 12 μl of DI water at pH of 8. pDNA was first added into microtubes along with 75 μl of Hank's Buffer (without calcium) and bPEI was drop-wise added. Resulting polyplexes were vortexed for at least 10 seconds and left undisturbed at room temperature for 15 minutes before use. A N/P ratio of the bPEI/DNA polyplexes of 5:1 was used in all experiments. PS/DNA polyplexes were formed as follows. Twenty microgram of pDNA was dispersed in 500 μl of DI water (Nuclease-free water Fisher) 400 μg of PS (Sigma–Aldrich) was dissolved in 500 μl of DI water. pDNA in water was drop-wise added to PS solution while vortexing. Resulting polyplex was left undisturbed for 30 minutes and refrigerated prior to use for efficient complexing. The size and surface charge of the prepared polyplex cores were characterized by dynamic light scattering and zeta-potential analysis (Malvern Instrument). A N/P ratio of the PS/DNA polyplexes of 10:1 was used in all experiments.
PCI-Mediated Gene Transfection
Both cell monolayers and multitumor cell spheroids were transfected in a similar manner. Photosensitizer AlPcS2a (1 μg/ml) and bPEI or PS/GFP or PTEN-GFP polyplexes were added to the cell cultures for 18 hours, followed by a triple wash. The cells were incubated for 4 hours in fresh medium to allow some of the photosensitizer to leach from the cell membrane. Light treatment at 670 nm at various fluence levels at a fluence rate of 5 mW/cm2 was administered from a diode laser (High Power Devices, North Brunswick, NJ). Light was coupled into a 200-μm diameter optical fiber containing a microlens at the output end. Controls consisted of cultures that contained bPEI or PS/GFP plasmids but were not exposed to light (dark controls).
PCI-GFP Transfection of Glioma Cells
U251 cells were cultured in 35-mm imaging dishes (FluoroDish, World Precision Instruments, Sarasota, FL) at 200,000 cells/well and allowed to grow overnight. PCI GFP transfection was carried out as described to a fluence level of 0.5 J/cm2 employing PTEN-GFP polyplexes. Following transfection the cells were incubated for an additional 24 hours and visualized using an inverted Zeiss laser-scanning microscope (LSM 410; Carl Zeiss, Jena, Germany). This system allows the differential visualization of cell nuclei using confocal and two-photon microscopy up to a depth of 100 μm with a field of view of 600 × 600 μm2.
Glioma Cell Growth Gene Treatment and Viability Assays
Two techniques were used for treatment and for assessing the results of gene transfection. The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; Promega, Madison, WI), was used to determine cell viability after treatment. For this, eight wells (in 96-well flat bottomed plates) for each set of treatment conditions were seeded with U251 or MCF-7 cells at a density of 5,000 cells/well and incubated for 24 hours prior to experimentation. Cells were only plated into every fourth well in the 12-well rows to reduce spill over light from the treated to the untreated cultures. Photosensitizer and GFP or PTEN-GFP polyplexes in DNA concentrations ranging from 0.5 to 2 μg/ml were then added and handled as described above. Light treatment at fluence levels of 0, 0.5, 0.75, or 1.5 J/cm2 at 5 mW/cm2 was administered through an opaque mask that only admitted light to one 8-well column at a time. Controls consisted of cultures that contained bPEI or PS/DNA plasmids but were not exposed to light (dark controls). Following transfection, incubation was continued for 48 hours after each treatment, at which point the culture medium was replaced with fresh clear buffer containing MTS reagents and incubated for a further 2 hours. The optical density was read using an ELx800uv Universal Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT).
Long-Term Survival Was Studied Using a Clonogenicity Assay
U251 cells were cultured in 35-mm dishes at 100,000 cells/well and allowed to grow overnight, followed by PCI transfection as described. Following irradiation, cells were washed with PBS, harvested by trypsinization, resuspended in MEM supplemented with serum, counted, and plated into 60-mm dishes per experimental group at 100–200 cells/dish. Cells were allowed to grow for 11–14 days whereupon they were stained with 0.5% crystal violet in 95% ethanol. Colonies containing more than 50 cells were scored as survivors. The number of colonies was normalized to a control group consisting of cells incubated in photosensitizer (dark control) but receiving no light treatment. All experiments were performed in quintuplicate and results are a combination of at least four separate experiments.
Multitumor Spheroid Generation and Transfection
Spheroids were formed by a modification of the centrifugation method [27,28]. 2.5 × 103 U87 cells in 200 μl of culture medium per well were alloquated into the wells of ultra-low attachment surface 96-well round-bottomed plates (Corning Inc., Corning, NY). The plates were centrifuged at 1,000g for 10 minutes. Immediately following centrifugation the tumor cells formed into a disk shape. The plates were maintained at 37°C in a 7.5% CO2 incubator for 48 hours to allow them to take on the usual three-dimensional spheroid form. Spheroids were formed in every fourth well in each of the 12-well rows and PCI gene transfection performed as previously described for the cell monolayers. Determination of spheroid size was carried out by averaging two measured perpendicular diameters of each spheroid using a microscope with a calibrated eyepiece micrometer and their volume calculated mathematically assuming a perfect sphere. Typically, 8–16 spheroids were followed in three individual trials for up to 21 days of incubation. Spheroid growth was monitored for an additional 2 weeks. Culture medium was changed every third day.
Statistical Analysis
Experimental data were analyzed using one-way ANalysis Of VAriance (ANOVA) using an Excel spreadsheet at the significance level P < 0.05 and presented as mean with standard error unless otherwise noted.
Results
Since PCI is optimal with a light fluence level that allows 70–80% survival, we performed AlPcS2a PDT at increasing light doses. Live/dead assay of U251 following PDT is shown in Figure 2a and b for 0 and 0.75 J/cm2, respectively. Fluence levels of 1.5 J/cm2 proved highly toxic killing more than 80% of the cells. Fluence level of 0.5–0.75 J/cm2 seemed optimal.
Fig. 2.
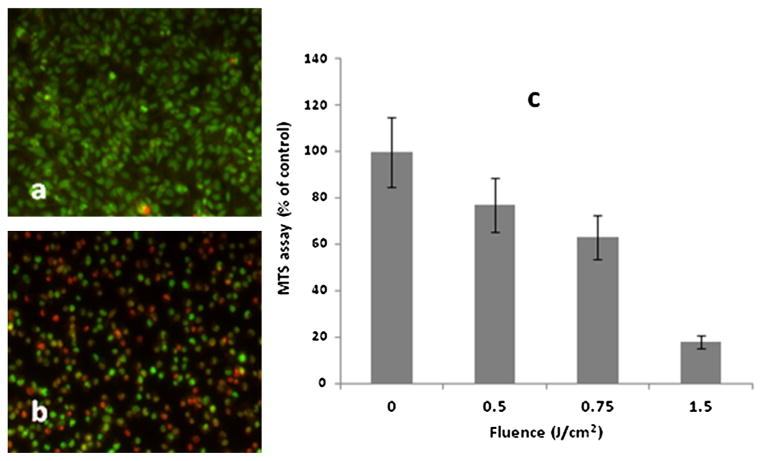
Live dead assay of U251 cell monolayers following AlPcS2a PDT at 0J (a) and 0.75 J/cm2 (b). The cells were stained using a combination of Hoechst 33342 and Ethidium Homodimer 1 and were visualized using an inverted Zeiss laser-scanning microscope Simultaneously detected blue and red emissions were isolated by using BP 390–465 IR and BP 565–615 IR band pass filters, respectively. Fluorescent images were pseudo-colored blue and red. Live: blue cells, dead: red cells. c: AlPcS2a-mediated PDT at increasing fluence levels. MTS assay 24 hours following light treatment. The results are shown as a percentage of dark controls ± SE (dark control = 100%) (c). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/lsm]
Toxcicity of bPEI and PS DNA Polyplexes
To determine the direct toxicity of the two types of gene carriers used here, 5,000 U251 cells/well in a 96-well plate were incubated in media (200 μl/well) containing either bPEI/GFP or PS/GFP polyplexes at various concentrations for 18 hours, followed by replacement with fresh media. After incubation for an additional 48 hours at 378°C, cytotoxicity was determined by conventional MTS assay. If not complexed with a plasmid, bPEI proved very toxic at all of the concentrations tested in contrast to PS (data not shown). Even when complexed with plasmid, bPEI was clearly of a much greater toxicity compared to PS polyplexes (Fig. 3).
Fig. 3.
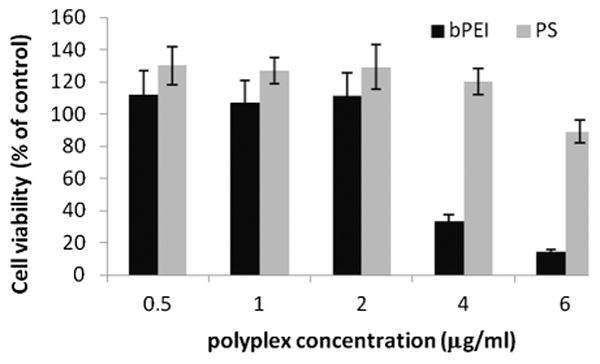
U251 cell viability (MTS assay) for increasing concentrations of bPEI/GFP or PS/GFP polyplexes. The results show the average of eight replicate cultures repeated two times.
Effects of PCI on GFP Transfection with bPEI/PS DNA Polyplexes
In order to optimize the various parameters for effective PCI-mediated gene transfection, experiments were carried out on U251 monolayers employing the PTEN-GFP plasmid with either PS or bPEI gene carriers. Various PEI/DNA (N/P) ratios and concentrations of bPEI were investigated as the delivery carrier and a N/P ratio of 5:1 proved optimal at a plasmid concentration of 1 μg/ml. Fluorescent microscopy of U251 monolayers showed a clear increase in GFP positive cells employing either gene carrier with the addition of PCI treatment and a 10-fold increase in transfection rate for PS/GFP could be demonstrated (Fig. 4a). PCI increased the number of GFP positive cells from 15% to 27% for bPEI/GFP and from 3% to 37% for PS/GFP, respectively (Fig. 4b), which is comparable to virus vectors.
Fig. 4.
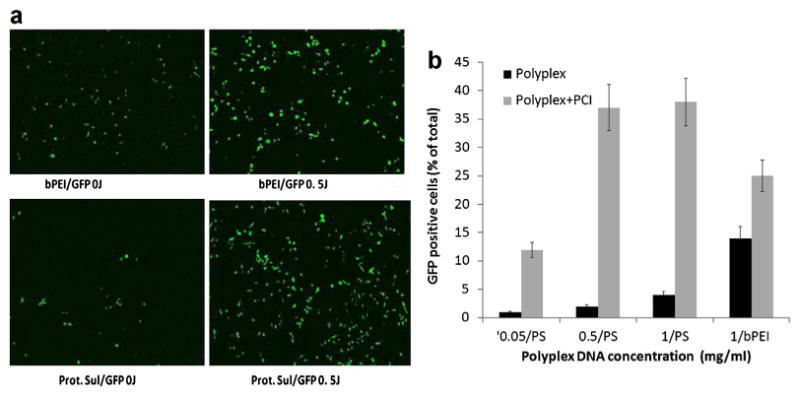
Fluorescent microscopy of GFP production. (a) Effects of PCI on GFP reporter gene transfection with bPEI or PS polyplexes on monolayers of U251 cells. DNA complexed with bPEI, N/P ratio 5:1, DNA concentration 1 μg/ml, PCI light treatment 0.5 J/cm2. b: Percentage transfected cells; number GFP positive/total cell count. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/lsm]
PTEN Restoration Inhibition of Proliferation and Colony Formation of Glioblastoma Cells
Cell growth inhibition following PTEN gene transfer was investigated in U251 glioma cells transfected using bPEI/PTEN or PS/PTEN polyplexes, either alone or combined with PCI. Plasmids encoding, respectively, the GFP gene alone and GFP/PTEN were used in these experiments. Cell growth inhibition following PTEN-GFP or GFP gene transfection was investigated using the MTS assay. Figure 5 shows the results of the MTS assay performed 48 hours following light treatment. The results are presented as a percentage of dark or PDT controls that received no plasmids respectively. In the absence of light treatment, no significant inhibition in cell growth (P > 0.05) was observed in the PS/PTEN groups at all of the DNA concentrations tested (Fig. 5a). Growth inhibition in the absence of light was seen only at the highest bPEI/PTEN concentration (Fig. 5c). In contrast, following light treatment significant growth inhibition (P < 0.001) was observed for PTEN gene transfection at DNA concentrations of 1 or 2 μg/ml for both PS and bPEI. As can be seen from Figure 5b no significant inhibition of cell growth was observed for the PS/GFP transfection both in the absence or presence of light treatment. Similar results were obtained using the bPEI/GFP polyplex (Fig. 5d).
Fig. 5.
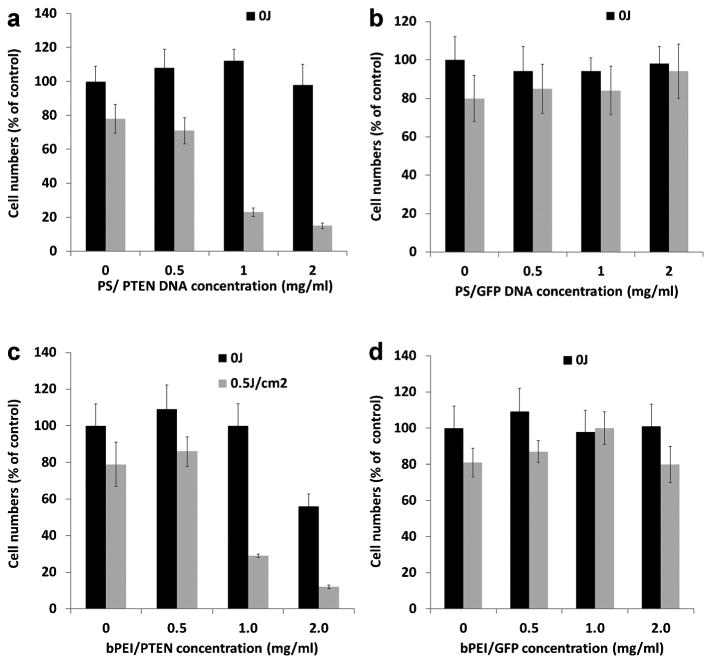
Effects of PCI on growth of U251 cell monolayers. Eighteen-hour incubation with photosensitizer and polyplexes. Light treatment fluence level 0.5 J/cm2. a: PS/PTEN-GFP, (b) PS/GFP, (c) bPEI/PTEN-GFP, and (d) bPEI/GFP. MTS assay 48 hours post-light treatment. Values are average percentages of non-treated controls.
Clonogenicity Assay
Anchorage-dependent colony formation is an important phenotype of malignant cells, correlating with their tumorgenicity, and invasive potentials. Clonogenic survival assays using either PS or bPEI gene carriers on U251 cells demonstrated that PCI enhanced PTEN restoration significantly inhibited colony size and number compared to control cultures that received no light (Table 1). In the absence of light treatment (0J), significant growth inhibition was seen only with bPEI as gene carrier but not with PS.
Table 1. Inhibition of U251 Colony Formation by PCI-PTEN Protamine Sulfate (PS) or bPEI Polyplexes as Gene Carrier.
| PTEN polyplexes concentration | PCI dark control | PCI 0.5 J/cm2 | PCI 0.75 J/cm2 |
|---|---|---|---|
| None | 100 ± 8.3a | 86 ± 6.7 | 72 ± 7.1 |
| PS 1.0 mg/ml | 98 ± 7.1 | 27 ± 1.9b | 23 ± 1.8b |
| PS 2.0 mg/ml | 91 ± 8.2 | 21 ± 1.8b | 16 ± 1.2b |
| bPEI 1.0 mg/ml | 80 ± 6.3 | 45 ± 3.9b | 36 ± 2.6b |
| bPEI 2.0 mg/ml | 63 ± 4.7b | 28 ± 1.9b | 18 ± 1.4b |
PS, protamine sulfate; bPEI, branched polyethylenimine.
Average value ± SE as a % of non-treated dark control.
Significant inhibition compared to control.
In order to ascertain the effects of PCI-mediated PTEN transfection into cells known to have wild-type PTEN, similar monolayer experiments employing the MTS assay were performed with the breast tumor cell line MCF-7 and the gene carrier polyplex PS/PTEN-GFP. As can be seen from Figure 6, in contrast to the significant inhibitory effects of PCI-mediated PTEN transfection into U251 glioma cells, a slight stimulatory effect on cell viability was observed at all DNA concentrations evaluated.
Fig. 6.
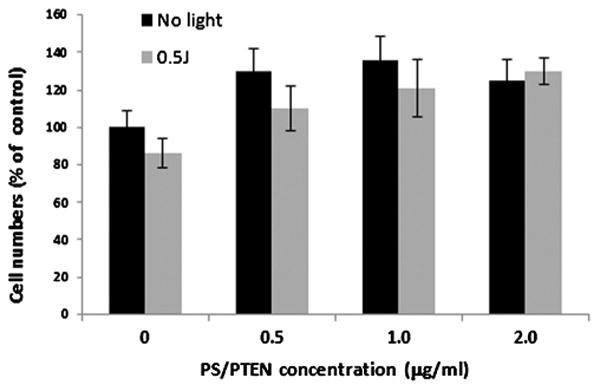
Effects of PCI on growth of MCF-7 cell monolayers. Eighteen-hour incubation with photosensitizer and polyplexes, PS/PTEN-GFP. Light treatment fluence level 0.5 J/cm2; DNA concentration as shown. MTS assay 48-hour post-light treatment. Values are averaged percentages of non-treated controls.
PTEN Inhibition of U87 Tumor Spheroids Growth
U87 glioma spheroids were incubated for 18 hours with the PS/PTEN-GFP (DNA concentration 4 μg/ml) together with AlPcS2a, and following 4-hour incubation in fresh medium, treated with 1.5 J of laser light. Control spheroids received the same incubation but received no light. The dramatic effects of PCI are shown in the Two-photon micrograph (Fig. 7). No GFP producing cells were evident in the dark controls as opposed to the light treated cultures where GFP positive cells were clearly evident both at the periphery and deep within the spheroid.
Fig. 7.
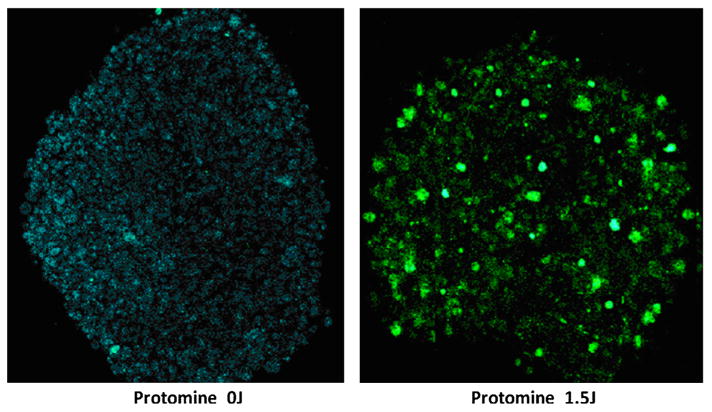
Two photon micrograph of GFP transfected U87 spheroids. PS/PTEN-GFP DNA concentration 4 μg/ml. a: The spheroids receiving no light showed only a faint auto fluorescence. b: PCI light treatment 1.5 J/cm2 showed greatly enhanced GFP production. Spheroid diameter 400 μm. Images were acquired at a depth of approximately 100 μm. The field of view for images was 600 × 600 μm2. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/lsm]
To ascertain the effects of PCI-PTEN transfection, on the growth and development of U87 spheroids experiments were performed consisting of four groups: (1) untreated control, (2) AlPcS2a-PDT, (3) PS/PTEN-GFP, and (4) AlPcS2a-PCI PS/PTEN-GFP. 1.5 J/cm2 radiant exposure and 1 μg/ml AlPcS2a were used in all experiments. Similar to the monolayer results light doses representing 80–90% spheroid survival (1.5 J/cm2) was used in order to optimize the PCI effect. Figure 8 shows the average spheroid volume as a percentage of untreated controls measured after a 2-week period, from a starting diameter of 400 μm at treatment. PTEN-GFP DNA concentration of 2 and 4 μg/ml were used. Three identical experiments were performed with 16 spheroids in each group per experiment. As seen in the figure, the average volume of both the PDT and PS/PTEN-treatment groups was not significantly different from control levels. In contrast, the average volume of the PCI-PS/PTEN transfected spheroids were significantly reduced (P < 0.05) at both DNA concentrations.
Fig. 8.
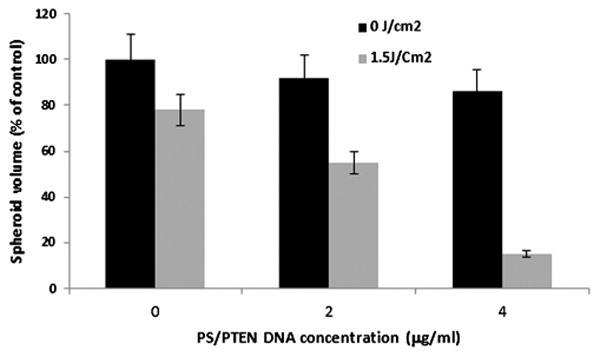
Inhibition of U-87 spheroid growth by PCI-mediated transfection of PS/PTEN-GFP gene. Average volume growth measured as a percentage of control spheroids following 2 weeks of incubation time post-treatment. PS/PTEN–GFP polyplexes at 2 or 4 μg/ml. Light treatment, 1 μg/ml AlPcS2a incubation for 18 hours. Wavelength of 670 nm: radiant exposure of 1.5 J/cm2; irradiance of 5 mW/cm2. Each data point represents the mean of three experiments (16 spheroids per trial). Error bars denote standard errors. ** Significant (P < 0.05) compared to non-PCI treated PS/PTEN-GFP transfection.
Discussion
The mechanisms leading to GBM development are not well understood but animal studies support the hypothesis that inactivation or mutations of tumor suppressor genes in neural stem cells (NSC), transforming them to tumor stem cells (TSC), is required and sufficient to induce glial cancers [29]. The ability to enhance the non-viral insertion of functioning suppressor genes into glioma cells by PCI, formed the basis of the study presented here. The aim of PCI in this context is the site-specific restoration of normal suppressor gene expression in limited areas of the brain. This would lead to the growth inhibition of the nests of tumor cells or migrating TSCs sequestered in the margins of the resection cavity following surgical removal of bulk tumor, while minimizing damage to the surrounding brain tissue. The rapid attenuation of light in the brain limits the efficacy of PCI to the margins surrounding the resection cavity and should lead to minimal side effects since the effect is localized to the irradiated area. This is a blessing to normal cells but a limitation of light-based therapies in general.
In well over 1,000 publications, the first from 1997 [7], the function and mechanism of the PTEN gene, not only in inducing cell cycle arrest and programming apoptosis, but also in other aspects of cell physiology including the regulation of cell adhesion, migration, and infiltration has been examined in great detail (for review see [12]). In light of this detailed previous documentation we have not reexamined the various mechanisms by which PTEN exerts its effects, but have concentrated on the ability of PCI to specifically enhance its suppressor function in cell monolayers and spheroid growth assays. The results of the experiments presented here could demonstrate that PCI significantly enhanced non-viral gene transfection using protamine as gene carrier based on increased reporter gene expression. More importantly PCI-enhanced transfection of PS/PTEN polyplexes, into both PTEN mutant cell lines U251 and U87 spheroids, greatly inhibited their growth potential compared to conventional bPEI transfection alone. PCI-PTEN transfection significantly inhibited both the growth of U251 monolayers and their transforming ability to produce secondary colonies (Table 1), as well as significantly inhibiting the growth of U87 multi cell tumor spheroids. The inhibitory effects of PCI enhanced PTEN transfection was specific since PCI increased transfection of the neutral GFP gene, had no effect on cell growth. Successful gene therapy for cancer is dependent upon the specificity of the treatment to be aimed at the neoplastic cells, while proving neutral towards normal cells. The results of transfecting cells containing endogenous wild-type PTEN (MCF-7) with exogenous wild-type PTEN with and without PCI clearly indicated that this was the case. In fact a low level stimulatory effect on MCF-7 cell viability was observed. This might be due to the presence of protamine, which was used as a gene carrier in these experiments. Despite its low transfection efficiency it is nuclear protein that helps DNA packaging in sperm cells and has been reported to act as a transfection accelerator for gene delivery [30,31].
Polycations, such as PEI, exert weak base properties resulting in a proton–sponge effect facilitating endosomal release. This ability is dependent on PEI concentration and N/P ratios. However, as seen in Figure 5, PEI displays only moderate endosomolytic activity since gene transfection was clearly enhanced by PCI. Although bPEI is an effective transfection agent, it is more toxic than PS and is not well suited for in vivo applications. In agreement with the results reported here protamine/DNA polyplexes alone have been reported to have relatively low-transfection efficiency [19,32]. In contrast PCI of PS/DNA polyplexes demonstrated a dramatic increase in transfection rate for the reporter GFP gene as well as significantly inhibiting glioma cell growth at optimum PS/DNA concentrations (Figs. 4 and 5). Some possible reasons of this finding may lie in the strong hydrophilicity of protamine, which makes it difficult to cross cell membranes, inhibiting endosomal escape. It has previously been shown that PS polyplexes enter cells in similar amounts compared to bPEI [32]. The difference in transfection efficiency between these two gene carriers is most probably due to increased endosomal escape by bPEI/DNA polyplexes. Since PCI greatly enhances endosomal escape, the dramatic effects of PCI shown in Figure 5 for PS/DNA polyplexes support this interpretation. PCI technology has previously been demonstrated to successfully facilitate the release of macromolecules entrapped in cytoplasmic vesicles by destroying the cytoplasmic membrane [33–35]. The cytotoxic effect of PCI of PTEN gene was stronger than expected from the fraction of cells expressing GFP, even when taking the PDT effect into account. PCI of PTEN plasmid/PS resulted in 77% cell kill (Table 1) while only 35% of the cells expressed GFP (Fig. 4). This larger than expected toxic response is likely from differential expression of the two genes (GFP and PTEN) carried by the same vector. Further experiments are ongoing to pinpoint these mechanisms.
Multi-cell tumor spheroids represent a useful in vitro bridge between monolayer cell culture and animal models. In comparison to monolayer cultures, a significant advantage of tumor spheroids is that their micro-environment more closely mimics the in vivo situation and therefore, concentrations of nutritients and oxygen, gene expression and the biological behavior of the cells are likely similar to that encountered in tumor cells in situ. In addition, cells within spheroids have an expression pattern of adhesion molecules that mimics the in vivo situation and produce an extracellular matrix. Since the PCI results obtained on monolayer cultures demonstrated superior transfection efficacy of PS over bPEI as a transfection agent and PS is relatively non-toxic compared to bPEI, U87 MTS experiments where therefore carried out using PS exclusively as gene carrier. In many studies of the effects of gene therapy on cancer, gene transfer was achieved employing stable models using viral (or non-viral) vectors and subsequent selection of stable cell clones or animal models. Most gene transfer techniques yield transient gene expression, which would limit their therapeutic application since in vivo no selection of stable transfectants would occur. The ability of iterative therapy with a low toxicity gene carrier like PS activated by PCI would therefore constitute a considerable advantage. Repetitive treatment protocols employing viral vectors are usually ineffective due to immunological response to the viral carrier.
In addition to absence or mutation of the PTEN gene, another hallmark of GBM is an increased expression of both vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR). In recent studies, stable doxycycline-regulated or Lipofectamine-induced expression of wild-type PTEN in U251 GBM cells reduced proliferation, arrested the cell cycle at G0/G1 stage, and promoted apoptosis via inhibition of PIK/AKT signaling pathways [36,37]. In addition these authors reported that combined gene therapy, using up regulation of wild-type PTEN reconstruct and VEGF or EGFR siRNA knock down, could demonstrate increased inhibition of tumor growth both in vitro and in vivo compared to PTEN reconstruction alone. PCI has been shown to enhance siRNA-mediated silencing of EGFR in the human epidermoid carcinoma cell line A431 compared to siRNA therapy alone [34]. We are presently exploring PCI enhanced combined PTEN/EGFR siRNA gene transfection as a potential therapy modality for glioma.
Acknowledgments
This work was supported by grants from the Norwegian Radium Hospital Research Foundation, and the American Society for Lasers Medicine and Surgery (ASLMS). Portions of this work were made possible through access to the Laser Microbeam and Medical Program (LAMMP) and the Chao Cancer Center Optical Biology Shared Resource at the University of California, Irvine.
Contract grant sponsor: Norwegian Radium Hospital Research Foundation; Contract grant sponsor: American Society for Lasers Medicine and Surgery.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008 [Google Scholar]
- 2.Kleihues P, Cavenee WK. Pathology and genetics of tumor of the nervous system. Lyon (France): IARC Press; 2000. pp. 9–54. [Google Scholar]
- 3.Sposto R, Ertel IJ, Jenkin RD, Boesel CP, Venes JL, Ortega JA, Evans AE, Wara W, Hammond D. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: Results of a randomized trial. A report from the Childrens Cancer Study Group. J Neurooncol. 1989;7(2):165–177. doi: 10.1007/BF00165101. [DOI] [PubMed] [Google Scholar]
- 4.Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71(8):2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Stewart LA. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 6.Endersby R, Baker SJ. PTEN signaling in brain: Neuropathology and tumorigenesis. Oncogene. 2008;27(41):5416–5430. doi: 10.1038/onc.2008.239. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 8.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100(4):387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 9.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15(4):356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 10.Li DM, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci USA. 1998;95(26):15406–15411. doi: 10.1073/pnas.95.26.15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furnari FB, Huang HJ, Cavenee WK. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58(22):5002–5008. [PubMed] [Google Scholar]
- 12.Knobbe CB, Merlo A, Reifenberger G. Pten signaling in gliomas. Neuro Oncol. 2002;4(3):196–211. [PMC free article] [PubMed] [Google Scholar]
- 13.Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96(5):2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochem Biophys Acta. 2008;1784(1):150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Somia N, Verma IM. Gene therapy: Trials and tribulations. Nat Rev Genet. 2000;1(2):91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 16.Han S, Mahato RI, Sung YK, Kim SW. Development of biomaterials for gene therapy. Mol Ther. 2000;2(4):302–317. doi: 10.1006/mthe.2000.0142. [DOI] [PubMed] [Google Scholar]
- 17.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture in vivo: Polyethylenimine. Proc Natl Acad Sci USA. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toncheva V, Wolfert MA, Dash PR, Oupicky D, Ulbrich K, Seymour LW, Schacht EH. Novel vectors for gene delivery formed by self-assembly of DNA with poly(Llysine) grafted with hydrophilic polymers. Biochim Biophys Acta. 1998;1380(3):354–368. doi: 10.1016/s0304-4165(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Guo S, Li Z, Liu L, Gu J. Synthesis and characterization of stearyl protamine and investigation of their complexes with DNA for gene delivery. Colloids Surf B Biointerfaces. 2009;73(1):36–41. doi: 10.1016/j.colsurfb.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Sun X, Zhang N. Cationic polymer optimization for efficient gene delivery. Mini Rev Med Chem. 2010;10(2):108–125. doi: 10.2174/138955710791185109. [DOI] [PubMed] [Google Scholar]
- 21.Berg K, Folini M, Prasmickaite L, Selbo PK, Bonsted A, Engesæter BØ, Zaffaroni N, Weyergang A, Dietze A, Mælandsmo GM, Wagner E, Norum OJ, Høgset A. Photochemical internalization: A novel tool for drug delivery. Curr Pharm Biotechnol. 2007;8(6):362–372. doi: 10.2174/138920107783018354. [DOI] [PubMed] [Google Scholar]
- 22.Selbo PK, Weyergang A, Hogset A, Norum OJ, Berstad MB, Vikdal M, Berg K. Photochemical internalization provides time and space-controlled endolysosomal escape of therapeutic molecules. J Controlled Release. 2010;148(1):2–12. doi: 10.1016/j.jconrel.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Høgset A, Engesaeter BO, Prasmickaite L, Berg K, Fodstad O, Maelandsmo GM. Light induced adenovirus gene transfer, an efficient and specific gene delivery technology for cancer gene therapy. Cancer Gene Ther. 2002;9(4):365–371. doi: 10.1038/sj.cgt.7700447. [DOI] [PubMed] [Google Scholar]
- 24.Ndoye A, Dolivet G, Høgset A, Leroux A, Fifre A, Erbacher P, Berg K, Behr JP, Guillemin F, Merlin JL. Eradication of p53-mutated head and neck squamous cell carcinoma xenografts using nonviral p53 gene therapy and photochemical internalization. Mol Ther. 2006;13(6):1156–1162. doi: 10.1016/j.ymthe.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Maurice-Duelli A, Ndoye A, Bouali S, Leroux A, Merlin JL. Enhanced cell growth inhibition following PTEN nonviral gene transfer using polyethylenimine and photochemical internalization in endometrial cancer cells. Technol Cancer Res Treat. 2004;3(5):459–465. doi: 10.1177/153303460400300507. [DOI] [PubMed] [Google Scholar]
- 26.Furnari FB, Lin H, Huang HS, Cavenee WK. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Acad Nat Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen. 2006;11(8):922–932. doi: 10.1177/1087057106292763. [DOI] [PubMed] [Google Scholar]
- 28.Mathews MS, Blickenstaff JW, Shih EC, Zamora G, Vo V, Sun CH, Hirschberg H, Madsen SJ. Photochemical internalization of bleomycin for glioma treatment. Biomed Opt. 2012;17(5):058001. doi: 10.1117/1.JBO.17.5.058001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Llaguno SA, Chen J, Kwon CH, Parada LF. Neural and cancer stem cells in tumor suppressor mouse models of malignant astrocytoma. Cold Spring Harb Symp Quant Biol. 2008;73:421–426. doi: 10.1101/sqb.2008.73.005. [DOI] [PubMed] [Google Scholar]
- 30.El-Aneed A. An overview of current delivery systems in cancer gene therapy. J Control Release. 2004;94(1):1–14. doi: 10.1016/j.jconrel.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Braun RE. Packaging paternal chromosomes with protamine. Nat Genet. 2001;28(1):10–12. doi: 10.1038/ng0501-10. [DOI] [PubMed] [Google Scholar]
- 32.Cho SK, Kwon YJ. Polyamine/DNA polyplexes with aciddegradable polymeric shell as structurally and functionally virus-mimicking nonviral vectors. J Control Release. 2011;150(3):287–297. doi: 10.1016/j.jconrel.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Prasmickaite L, Høgset A, Tjelle TE, Olsen VM, Berg K. Role of endosomes in gene transfection mediated by photochemical internalization (PCI) J Gene Med. 2000;2(6):477–488. doi: 10.1002/1521-2254(200011/12)2:6<477::AID-JGM137>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 34.Hogset A, Prasmickaite L, Selbo PK, Hellum M, Engesaeter BO, Bonsted A, Berg K. Photochemical internalization in drug and gene delivery. Adv Drug Deliv Rev. 2004;56(1):95–115. doi: 10.1016/j.addr.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira S, Fretz MM, Høgset A, Storm G, Schiffelers RM. Photochemical internalization enhances silencing of epidermal growth factor receptor through improved endosomal escape of siRNA. Biochim Biophys Acta. 2007;1768(5):1211–1217. doi: 10.1016/j.bbamem.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Shen X, Guo C, Zhu H, Zhou L, Zhu Y, Wang H, Zheng Y, Huang L. Phosphatase and tensin homolog reconstruction and vascular endothelial growth factor knockdown synergistically inhibit the growth of glioblastoma. Cancer Biother Radiopharm. 2010;25(6):713–721. doi: 10.1089/cbr.2010.0821. [DOI] [PubMed] [Google Scholar]
- 37.Han L, Zhang AL, Xu P, Yue X, Yang Y, Wang GX, Jia ZF, Pu PY, Kang CS. Combination gene therapy with PTEN and EGFR siRNA suppresses U251 malignant glioma cell growth in vitro and in vivo. Med Oncol. 2010;27(3):843–852. doi: 10.1007/s12032-009-9295-8. [DOI] [PubMed] [Google Scholar]


