Many cancers demonstrate metabolic transformation, configuring cellular metabolism to support malignant growth [1]. Metabolic patterns commonly observed in cancer include increased lipogenesis [2–4] and aerobic glycolysis, the metabolism of glucose to lactate, despite the availability of oxygen for oxidative phosphorylation [5, 6]. Up-regulation of aerobic glycolysis in cancer is known as the Warburg effect and is understood to be a malignant adaptation that allows continued proliferation in diverse microenvironments. The importance of metabolic transformation to cancer pathogenesis, however, raises important questions: Are these metabolic programs unique to cancer cells? If not, what might be their physiologic origins? Recent studies of postnatal neurogenesis have identified a physiological role in neural development for metabolic patterns typically associated with cancer, including both increased lipogenesis [7, 8] and aerobic glycolysis [8, 9]. In contrast to the production of aberrant onco-metabolites such as 2-hydroxyglutarate, which requires IDH mutation [10–12], the lipogenic and glycolytic phenotypes frequently observed in cancer originate in the normal metabolic repertoire of neural progenitor cells. Neurogenesis, like cancer, involves rapid proliferation and these studies show that metabolic pathways in neural progenitors, as in cancer cells, are optimized for cell division. The pattern of increased lipogenesis and aerobic glycolysis manifest neural progenitors, is maintained in the progenitor-derived brain tumor medulloblastoma; in this cancer, developmentally-regulated metabolism is co-opted to support malignant growth. These studies provide a developmental perspective on the origins of cancer cell metabolism.
Medulloblastoma is the most common malignant brain tumor in children, and presents an ideal opportunity to examine cancer arising as a disruption of developmentally-regulated growth [13]. Medulloblastomas originate from the cerebellum, which is the most prominent site of neural progenitor proliferation in early post-natal life. Several lines of evidence link cerebellar neural progenitor proliferation to medulloblastoma pathogenesis. In the first year of life in humans, or the first 15 days of life in mice, cerebellar granule neuron progenitors (CGNPs) proliferate in a germinal matrix along the outside of the cerebellum called the external granule cell layer (EGL). This period of rapid proliferation is triggered by activation of the Sonic Hedgehog (Shh) signaling pathway [14]. While proliferation of cerebellar progenitors is virtually shut down once cerebellar development is complete, mutations in humans that aberrantly activate the Shh pathway predispose individuals to medulloblastoma formation; importantly, this process is recapitulated in transgenic mice [15–17]. Thus mice with conditional deletion of Patched (Ptc) or constitutively active alleles of Smoothened (Smo) allow the process of medulloblastoma tumorigenesis process to be examined prospectively, from CGNP proliferation forward to cancer [17, 18].
Along with induction of proliferation in the postnatal cerebellum, Shh signaling induces characteristic metabolic patterns in CGNPs, including decreased fatty acid oxidation [7], increased lipogenesis [7] and aerobic glycolysis [8, 9]. Despite the normoxic environment of the postnatal brain, Shh drives a shift in energy production away from oxidative reactions. This developmentally-programmed metabolic configuration of CGNPs persists in primary medulloblastoma in transgenic mice [7–9]. Studies of human patients, moreover, show that the glycolytic phenotype of the model is shared by the actual disease; medulloblastomas are readily detected by clinical 18FDG-PET studies [19, 20] and glucose uptake correlates inversely with patient survival [19]. Thus understanding the cellular and molecular mechanisms of metabolic configuration of neural progenitors places the metabolic patterns of medulloblastoma into a developmental context and may provide key insight in tumor pathogenesis.
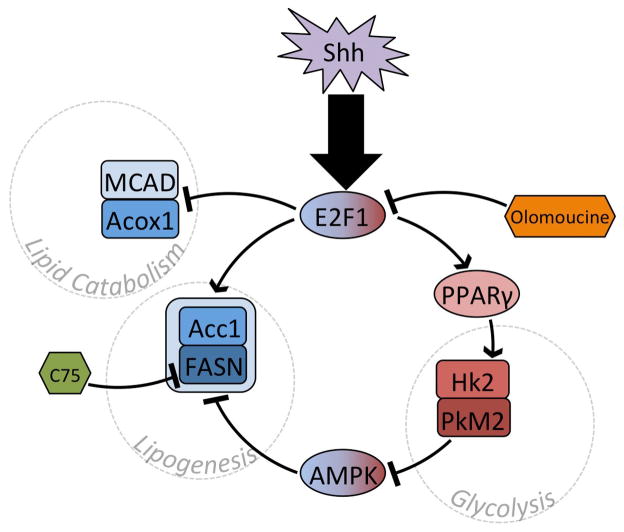
Bhatia et al demonstrated that Shh induced a metabolic switch from lipid consumption to lipid production [7]. After observing abundant lipid deposition in Shh-driven medulloblastomas in transgenic mice, the investigators examined whether fatty acid metabolism was altered by Shh in CGNPs, the normal cells from which these tumors originate. Bhatia et al found that CGNPs explanted into media containing Shh up-regulated key lipid synthesis enzymes, including Fatty Acid Synthase (FASN) and Acetyl-CoA Carboxylase (Acc1). Shh also caused down-regulation of enzymes required for lipid catabolism, including Acyl-CoA Oxidase 1 (Acox1) and Medium Chain Acyl-CoA Dehydrogenase (MCAD) [7]. These transcriptional changes seemed to be coupled with proliferation as they depended on the activity of the Rb-E2F axis; E2F1 knockdown blocked the induction of FASN and the suppression MCAD in Shh-treated CGNPs. Direct measurement of palmitate oxidation demonstrated that these transcriptional changes potently altered lipid metabolism; Shh reduced palmitate oxidation, which could be restored by the subsequent addition of E2F1 shRNA. Thus, as depicted in Figure 1, Shh shifted the lipid metabolism of CGNPs from catabolic to synthetic, and this shift was mediated by E2F1 and negatively regulated by Rb [7].
Figure 1.
Shh signaling regulates lipid metabolism and glycolysis in an integrated manner. Regulatory genes and effector proteins are denoted by ovals and rounded rectangles, respectively, whereas inhibitors are in hexagons. Entities involved in lipid or glucose metabolism are in blue or red. AMPK may switch-off lipogenesis when aerobic glycolysis is blocked and energy scarcity results.
Importantly, this metabolic switch was maintained in medulloblastoma, where it promoted tumor growth [7]. Treatment of medulloblastoma-bearing mice with inhibitors of either FASN or the CDK-Rb-E2F signaling pathway slowed medulloblastoma progression and prolonged mouse survival. Bhatia et al used the transgenic ND2:SmoA1 mouse line to generate animals with primary medulloblastoma. After tumor formation, these mice were injected daily for 2 weeks with either the CDK inhibitor olomoucine or with the FASN inhibitor C75. Both agents significantly extended animal survival by slowing tumor growth. Within treated tumors, C75 reduced lipid synthesis as expected. Similarly, olomoucine reduced intratumoral abundance of FASN [7]. Thus, direct inhibition of fatty acid synthesis exerted a significant anti-tumor effect and this effect was duplicated through CDK inhibition. Together these data show that lipogenic metabolism is both essential to tumor growth and derives from developmental physiology [7].
The same group, in a follow up investigation, demonstrated that the metabolic regulator, Peroxisome Proliferator-Activated Receptor-γ (PPARγ) plays a key role in shaping the metabolic response of CGNPs to Shh stimulation in a manner that persists after medulloblastoma formation [8]. The investigators show that E2F1 up-regulates PPARγ and that PPARγ in turn causes up-regulation of key glycolytic enzymes, Hexokinase 2 (Hk2) and Pyruvate Kinase M2 (PkM2), and of the glucose transporter Glut4. Inhibiting PPARγ in medulloblastoma-bearing ND2:SmoA1 mice decreased the expression of both Hk2 and PkM2, and reduced tumor glucose uptake, measured in vivo by 18FDG-PET scan. Similar to the inhibition of CDK and FASN, PPARγ inhibition reduced the rate of tumor growth and extended survival [8]. These findings further demonstrate that the proliferative and metabolic functions of neural progenitors are interconnected and jointly become subverted in medulloblastoma tumorigenesis. Moreover, as shown in Figure 1, this investigation demonstrates that changes in lipid and carbohydrate metabolizing enyzmes occur in concert, jointly regulated by common intracellular signals.
A non-biased metabolomic analysis determined the functional significance of Shh-mediated changes in the expression of metabolic enzymes. CGNPs were explanted into media with or without Shh and changes in media metabolite concentrations were measured over time. Shh increased lactate production and glucose utilization of CGNPs, without causing additional changes in nutrient utilization and metabolite production. Continuous, real-time measurement of media oxygen content showed that Shh did not increase the CGNP oxygen consumption rate, and that Shh-treated CGNPs retained significant unused capacity for mitochondrial respiration. In vivo studies, including quantitative measurement of 18FDG uptake and MR spectroscopy, further showed that glucose utilization and lactate production in the cerebellum were highest during the period of CGNP proliferation. 18FDG-PET also demonstrated that the glycolytic phenotype of CGNPs is preserved in ND2:SmoA1 medulloblastoma. These studies confirmed that Shh signaling increased aerobic glycolysis in CGNPs and that Shh-driven medulloblastomas inherited the metabolic phenotype of their progenitor cells of origin [9].
Importantly, these studies revealed that hexokinase isoforms Hk1 and Hk2 were expressed in mutually exclusive domains in the postnatal brain, defined by the presence or absence of proliferating progenitors. While proliferating CGNPs up-regulated Hk2, Hk1 was expressed by differentiated neurons throughout the brain. Hk1 was notably absent from the sites of postnatal progenitor proliferation, including the hippocampus, the subventricular zone, and the external granule layer of the cerebellum, the site of CGNP proliferation. These non-overlapping patterns suggested that the selective expression of Hk2, rather than Hk1, was integral to neural progenitor proliferation [9].
Insight into the functional significance of Hk2 expression in neural progenitors came from studies that conditionally deleted Hk2 in the brain. Mice with a floxed allele of Hk2 (Hk2f/f) were crossed with hGFAP-cre mice that express cre recombinase in brain stem cells during early brain development. While HK2 was deleted throughout the cerebellum, only CGNPs were directly affected because CGNPs are the primary source of Hk2 in the postnatal cerebellum [21]. Metabolomic analysis showed that CGNPs in hGFAP-cre;Hk2f/f mice generated markedly less lactate when exposed to Shh, confirming that Hk2 deletion blocked the induction of aerobic glycolysis by Shh [9].
Genetic deletion of Hk2 enabled functional studies to determine the role of aerobic glycolysis in both CGNP development and medulloblastoma tumorigenesis. Mice with conditional deletion of Hk2 were viable and fertile and had no overt neurologic deficits. Inspection of the cerebellar microanatomy, however, revealed disruption of the typically regular pattern of cell layering, and increased capillary formation disrupting the EGL. CGNPs typically migrate along a predictable route as they differentiate into neurons; in focal areas, Hk2-deficient CGNPs differentiated without completing migration, resulting in granule neurons positioned aberrantly on both sides of the Purkinje cell layer. The increased vascularity of the cerebellum in hGFAP-cre;Hk2 f/f mice suggested that Hk2-deficient CGNPs were increasingly dependent on oxidative metabolism and local tissue oxygenation, while the aberrant migration suggested disrupted timing of CGNP differentiation.
Further evidence that aerobic glycolysis negatively regulates terminal differentiation came from conditional deletion of Hk2 in medulloblastoma-prone mice. These mice were generated by crossing hGFAP-cre, Hk2f/f and SmoM2 mouse lines. Mice heterozygous for hGFAP-cre and SmoM2 alleles develop medulloblastoma with 100% frequency by P12 and typically die from tumor progression by P20 [22, 23]. In these mice, cerebellar development is profoundly altered as the posterior fossa becomes filled with rapidly growing tumor. Tumors with homozygous conditional deletion of Hk2 occurred in hGFAP-cre;SmoM2;Hk2f/f with similar incidence, but were markedly less malignant. Deletion of Hk2 significantly increased median survival from 18 to 30 days and in contrast to the 100% mortality of mice with medulloblastoma with intact Hk2, allowed 30% of tumor-bearing mice to survive long-term and to breed [9]. Similar survival benefits were demonstrated when Hk2 was deleted in mouse models of glioblastoma[24], and more recently, lung and breast cancer, confirming the broad relevance of Hk2 to solid tumors beyond developmental brain tumors [25].
Histologic examination of Hk2 deleted medulloblastomas showed that Hk2-deficiency dramatically increased the proportion of SmoM2-expressing CGNPs that differentiated appropriately, leading to relative normalization of cerebellar architecture. Staining for endothelial marker CD31 demonstrated increased vascularization in Hk2-deficient tumors. Proliferating cells localized predominantly in the perivascular regions; between regions of perivascular proliferation tumor cells exited the cell cycle and up-regulated the differentiation marker p27. The increased angiogenesis of Hk2-deficient tumors and the increased dependence of proliferative behavior on vascular proximity, suggested that growth restriction occurred when these Hk2-deficient tumors exceeded the limits of the compensatory effect of neovascularization. The presence of energy scarcity in Hk2-deficient tumors was assessed by studies of the phosphorylation of the intracellular energy sensor, AMP-activated Kinase (AMPK). Consistent with energy scarcity, in Hk2-deficient tumors AMPK was consistently phosphorylated, as was the AMPK target, Acc1 [9].
How does AMPK activation in Hk2-deficient tumors impair malignant growth? As shown in Figure 1, the metabolic switch from lipid catabolism to lipid synthesis, identified by Bhatia et al as required for tumor growth, [7, 8] may be the underlying mechanism that is targeted by AMPK. While Bhatia et al showed that lipogenesis is regulated in CGNPs by E2F1 and PPARγ, AMPK-Acc1 signaling has been shown to down-regulate lipid production in diverse cell types [26] and may similarly affect the lipid metabolism of CGNPs. AMPK activation in Hk2-deletion medulloblastoma increased the inhibitory phosphorylation of Acc1 [9], which would be predicted to block lipogenesis by inhibiting production of malonyl-CoA [27], leading to increased fatty acid oxidation [28]. Thus lipogenic metabolism may be a key mechanism of tumor pathogenesis that is supported by Hk2-dependent glycolysis. By preventing AMPK activation, Hk2-mediated aerobic glycolysis may enhance tumor growth not only by generating energy with less oxygen, but also by allowing lipogenesis to proceed. If so, pharmacologic agents that directly activate AMPK may be able to exploit this mechanism to inhibit tumor growth.
The induction of PkM2 by Shh, noted by Bhatia et al may be another critical link between Shh-driven glycolysis and Shh-driven lipid synthesis. We have recently found that Smo-driven medulloblastomas in mice, like CGNPs, up-regulate PkM2 (unpublished data). While pyruvate kinase plays an essential role in glucose metabolism, there are key differences in the activity of the PkM1 and PkM2 isoforms. In contrast to PkM1, the PkM2 isoform is subject to down-regulation of enzymatic activity and this regulation may be essential to its growth promoting effect [29]. By poorly catalyzing the conversion of phosphoenolpyruvate into pyruvate, PkM2 may increase the supply of glycolytic intermediaries upstream of phosphoenolpyruvate that can be diverted into alternative fates, including lipid production and nucleotide biosynthesis [30, 31].
The expression of the less active PkM2 isoform rather than the constitutively active PKM1, in both Shh-stimulated CGNPS and medulloblastoma suggests that pyruvate kinase activity may have an inhibitory effect on growth. Recent work by Anastasiou et al demonstrated that PkM2 activators, DASA-58 and TEPP-46, can block lipogenesis and inhibit the growth of PkM2-expressing cancers [32]. Unlike the activation of PkM2 by endogenous FBP, the activating effect of these agents could not be inhibited by phosphotyrosine signaling. Cells treated with DASA-58 reduced the flux of glucose-derived carbons into acetyl-CoA and generally lowered de novo lipid synthesis. Similarly, TEPP-46 treatment reduced the intracellular concentrations of acetyl-CoA, lactate, ribose phosphate, and serine, important precursors for lipid metabolism. Moreover, xenograft tumors from TEPP-46-treated mice recapitulated the metabolic profile found in vitro and exhibited delayed latency and smaller size than vehicle-treated mice. These findings show that increasing PkM2 activity alters glucose and lipid metabolism and reduces tumor growth. Conversely, Israelsen et al showed that conditional deletion of PkM2 actually accelerates tumorigenesis in an in vivo model of lymphoma [33]. Within PkM2-deficient tumors, a subpopulation of non-proliferating cells expressed PkM1. Proliferative tumor cells, however, expressed no PkM1 and completely lacked PkM2. Together these studies show that pyruvate kinase activity correlates inversely with malignant growth.
The switch to PkM2 expression in CGNPs may similarly promote neural progenitor growth, again demonstrating a developmental origin for a metabolic pattern typically associated with cancer. As depicted in Figure 2, in Shh-stimulated CGNPs, low PkM2 activity, operating downstream of high Hk-2 driven glycolytic flux, may divert glycolytic intermediates from lactate generation, into lipogenesis. PkM2 activation may reduce this diversion of glycolytic intermediates, reducing lipid synthesis required for growth. As medulloblastomas up-regulate PkM2, agents that increase PkM2 enzymatic activity may be able to block the lipogenic metabolism of these tumors. Through this mechanism, PkM2 activation, like AMPK activation, may offer a novel approach to medulloblastoma therapy by disrupting metabolic patterns that support tumor growth.
Figure 2.
By providing variable resistance to glycolytic flux, PkM2, induced by Shh, is ideally positioned to divert glycolytic intermediates, generated downstream of Shh-induced Hk2, to alternate metabolic pathways, such as lipogenesis, that support developmental and malignant growth. A) Low PkM2 activity may channel glycolytic intermediates to alternate fates while allowing some production of lactate. B) Hk2 deletion reduces glycolytic flux upstream of PkM2, preventing both lactate generation and alternate uses of glycolytic intermediates. C) Pharmacologic activation of PkM2 activation may increase lactate generation while acting like Hk2 deletion to block the growth-promoting effects of aerobic glycolysis.
By up-regulating glycolytic enzymes Hk2 and PkM2, and lipid synthesis enzymes FASN and Acc1, while down-regulating lipid catabolizing enzymes including Acox1, Shh configures CGNPs for both aerobic glycolysis and lipid synthesis. These processes operate in tandem to satisfy the ATP needs of the cell while carrying out a net conversion of glucose into lactate and lipids. CGNPs integrate developmental regulation of lipid synthesis and aerobic glycolysis in order to support physiological proliferation during brain development. These same metabolic patterns become activated in medulloblastoma tumorigenesis and function similarly to support malignant growth.
An important question is the relevance of these findings beyond cerebellar development and its disruption in medulloblastoma tumorigenesis. Transcriptional analyses of patient-derived samples demonstrate that medulloblastoma is a heterogeneous group of tumors, with at least 4 molecular subtypes [34–38]. The SHH subgroup of medulloblastoma makes up approximately 25% of the cases of this rare tumor. Do observations of metabolic regulation in cerebellar progenitors and medulloblastoma have relevance for other cell types during development and for other cancers?
Rather than being unique to CGNPs, aerobic glycolysis is likely to be a common feature of neural progenitors throughout the neuraxis. While Shh-dependent proliferation is unique to cerebellar progenitors, Hk1 is absent in all progenitor regions of the postnatal brain [9], suggesting a molecular switch to Hk2 may be activated by diverse growth factors. Similarly, PkM2 is known to integrate diverse growth factor signaling pathways into a common output of reduced PK activity [39, 40]. Thus the activation of aerobic glycolysis by CGNPs in response to Shh may a general response of progenitor cells to the specific growth factors that drive them to proliferate. As a group, tumors of neural progenitors, including retinoblastoma, Ewing sarcoma and neuroblastoma comprise the largest set of solid tumors in children; the set of tumors recapitulating the metabolism of their cells of origin is thus likely to be broad.
The role of N-Myc in mediating Shh-induced glycolysis in CGNPs supports the general relevance of metabolic configuration to progenitor function. N-Myc, and the homolog C-Myc, are essential regulators of neural and hematopoietic progenitor function [41, 42]. They are also among the common oncogenes activated in human cancer [43, 44], including all 4 medulloblastoma subtypes [45]. Disruption of Myc-Max interaction in CGNPs treated with the inhibitor 10058-F4 blocked the induction of aerobic glycolysis by Shh and prevented Shh-induced proliferation [9]. These findings are consistent with the documented role of c-Myc in up-regulating glycolysis in both normal tissues and in cancer [46, 47]. Moreover, disruption of N-Myc-Max interaction with the same agent in neuroblastoma reduced tumor growth while altering metabolism [48]. Thus while the metabolic and proliferative behaviors induced by Shh may be specific to CGNPs, the pattern of growth factor-induced metabolic configuration may be generalizable to diverse progenitors and progenitor-derived cancers. N-Myc and C-Myc proteins may be common effectors of metabolic transformation that operate downstream of diverse mitogenic signals, typified by Shh, that are cell-type specific.
The phenomenon of postnatal cerebellar neurogenesis degenerating into medulloblastoma in mice presents an ideal system in which to study the developmental origins of cancer metabolism. Because the cells of origin of these tumors are identifiable and accessible, the molecular regulation of energy metabolism in these progenitors can be discerned in detail. Moreover, the patterns of metabolism in cells of origin and in resulting tumors can be compared. Importantly, Shh-pathway activation drives medulloblastoma tumorigenesis through downstream oncogenes, including N-myc, that are relevant to diverse cancers in both children and adults. Understanding the mechanisms that regulate the metabolism of cerebellar progenitors during development may thus lead to novel, metabolic approaches to anti-cancer therapy.
Acknowledgments
TRG is supported by grants from the National Institutes of Health (NIH; 1K08NS077978-01), the St. Baldrick’s Foundation, and the American Institute for Cancer Research.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. British journal of cancer. 2009;100(9):1369–72. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature reviews Cancer. 2007;7(10):763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 4.Abramson HN. The lipogenesis pathway as a cancer target. Journal of medicinal chemistry. 2011;54(16):5615–38. doi: 10.1021/jm2005805. [DOI] [PubMed] [Google Scholar]
- 5.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang CV. Links between metabolism and cancer. Genes & Development. 2012;26(9):877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia B, et al. Mitogenic Sonic hedgehog signaling drives E2F1-dependent lipogenesis in progenitor cells and medulloblastoma. Oncogene. 2011;30(4):410–22. doi: 10.1038/onc.2010.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia B, et al. Hedgehog-mediated regulation of PPARgamma controls metabolic patterns in neural precursors and shh-driven medulloblastoma. Acta Neuropathologica. 2012;123(4):587–600. doi: 10.1007/s00401-012-0968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gershon TR, et al. Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer & Metabolism. 2013:1. doi: 10.1186/2049-3002-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, et al. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(20):5562–71. doi: 10.1158/1078-0432.CCR-12-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang L, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2010;465(7300):966. doi: 10.1038/nature09132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimmer MR, Weiss WA. Childhood tumors of the nervous system as disorders of normal development. Current Opinion in Pediatrics. 2006;18(6):634–8. doi: 10.1097/MOP.0b013e32801080fe. [DOI] [PubMed] [Google Scholar]
- 14.Wechsler-Reya RJ, Scott MP. Control of Neuronal Precursor Proliferation in the Cerebellum by Sonic Hedgehog. Neuron. 1999;22(1):103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 15.Zurawel RH, et al. Analysis of PTCH/SMO/SHH pathway genes in medulloblastoma. Genes, Chromosomes and Cancer. 2000;27(1):44–51. doi: 10.1002/(sici)1098-2264(200001)27:1<44::aid-gcc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Zurawel RH, et al. Evidence that haploinsufficiency of Ptch leads to medulloblastoma in mice. Genes Chromosomes Cancer. 2000;28(1):77–81. [PubMed] [Google Scholar]
- 17.Hallahan AR, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Research. 2004;64(21):7794–800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 18.Oliver TG, et al. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development. 2005;132(10):2425–2439. doi: 10.1242/dev.01793. [DOI] [PubMed] [Google Scholar]
- 19.Gururangan SMRCP, et al. [18F]Fluorodeoxyglucose-Positron Emission Tomography in Patients with Medulloblastoma. Neurosurgery. 2004;55(6):1280–1289. doi: 10.1227/01.neu.0000143027.41632.2b. [DOI] [PubMed] [Google Scholar]
- 20.Tripathi M, et al. Demonstration of diffuse leptomeningeal metastasis in a treated case of medulloblastoma with F-18 FDG PET/CT. Clinical nuclear medicine. 2009;34(8):530–2. doi: 10.1097/RLU.0b013e3181abb72e. [DOI] [PubMed] [Google Scholar]
- 21.Gershon T, Crowther AJ, Liu H, Miller CR, Deshmukh M. Cerebellar granule neuron progenitors are the source of Hk2 in the postnatal cerebellum. Cancer & Metabolism. 2013;1(15) doi: 10.1186/2049-3002-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schüller U, et al. Acquisition of Granule Neuron Precursor Identity Is a Critical Determinant of Progenitor Cell Competence to Form Shh-Induced Medulloblastoma. Cancer Cell. 2008;14(2):123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao J, et al. A Novel Somatic Mouse Model to Survey Tumorigenic Potential Applied to the Hedgehog Pathway. Cancer Research. 2006;66(20):10171–10178. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf A, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011 doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patra KC, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24(2):213–28. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardie DG. Minireview: The AMP-Activated Protein Kinase Cascade: The Key Sensor of Cellular Energy Status. Endocrinology. 2003;144(12):5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 27.Ruderman NB, Saha AK, Kraegen EW. Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology. 2003;144(12):5166–71. doi: 10.1210/en.2003-0849. [DOI] [PubMed] [Google Scholar]
- 28.Foster DW. Malonyl-CoA: the regulator of fatty acid synthesis and oxidation. The Journal of Clinical Investigation. 2012;122(6):1958–9. doi: 10.1172/JCI63967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 30.Eigenbrodt E, et al. Double role for pyruvate kinase type M2 in the expansion of phosphometabolite pools found in tumor cells. Critical reviews in oncogenesis. 1992;3(1–2):91–115. [PubMed] [Google Scholar]
- 31.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. The international journal of biochemistry & cell biology. 2011;43(7):969–80. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Anastasiou D, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nature chemical biology. 2012;8(10):839–47. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Israelsen WJ, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155(2):397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones DT, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–5. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Northcott PA, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kool M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathologica. 2012;123(4):473–84. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellison DW, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121(3):381–96. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson MC, et al. Genomics Identifies Medulloblastoma Subgroups That Are Enriched for Specific Genetic Alterations. Journal of Clinical Oncology. 2006;24(12):1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 39.Hitosugi T, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Science signaling. 2009;2(97):ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christofk HR, et al. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452(7184):181–6. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 41.Laurenti E, et al. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell stem cell. 2008;3(6):611–24. doi: 10.1016/j.stem.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes & Development. 2002;16(20):2699–712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8(12):976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 44.Eilers M, Eisenman RN. Myc‚Äôs broad reach. Genes & Development. 2008;22(20):2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roussel MF, Robinson GW. Role of MYC in Medulloblastoma. Cold Spring Harbor perspectives in medicine. 2013;3(11) doi: 10.1101/cshperspect.a014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller DM, et al. c-Myc and cancer metabolism. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(20):5546–53. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dang CV. MYC, Metabolism, Cell Growth, and Tumorigenesis. Cold SpringHarbor perspectives in medicine. 2013;3(8) doi: 10.1101/cshperspect.a014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zirath H, et al. MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(25):10258–63. doi: 10.1073/pnas.1222404110. [DOI] [PMC free article] [PubMed] [Google Scholar]