Abstract
Transhepatic solute transport provides the osmotic driving force for canalicular bile formation. Choleretic and cholestatic agents affect bile formation, in part, by altering plasma membrane localizations of transporters involved in bile formation. These short-term dynamic changes in transporter location are highly regulated post-translational events requiring various cellular signaling pathways. Interestingly, both choleretic and cholestatic agents activate the same intracellular signaling kinases, such as phosphoinositide-3-kinase (PI3K), protein kinase C (PKC) and mitogen activated protein kinase (MAPK). An emerging theme is that choleretic and cholestatic effects may be mediated via different isoforms of these kinases. This is most evident for PKC-mediated regulation of plasma membrane localization of NTCP and MRP2 by conventional PKCα (cPKCα), novel PKCδ (nPKCδ), nPKCε, and atypical PKCζ (aPKCζ). Atypical PKCζ may mediate choleretic effects by inserting NTCP into the plasma membrane and nPKCε may mediate cholestatic effects by retrieving MRP2 from the plasma membrane. On the other hand, cPKCα and nPKCδ may be involved in choleretic, cholestatic and anticholestatic effects by inserting, retrieving and inhibiting retrieval of transporters, respectively. The effects of PKC isoforms may be mediated via phosphorylation of the transporters, actin binding proteins (radixin and MARCKS) and Rab proteins. Human NTCP plays an important role in the entry of hepatitis B and D viruses into hepatocytes and consequent infection. Thus, PKCs by regulating NTCP trafficking may also play an important role in hepatic viral infections.
I. Introduction
Bile provides an excretory route for endogenous and exogenous compounds. The coordinated function of transporters located at the sinusoidal and canalicular membranes results in the accumulation of solutes in the canalicular space providing the osmotic driving force for bile formation. Cholestasis accompanying many liver diseases (1, 2) results from inadequate solute transport across hepatocytes. During cholestasis, compounds normally excreted in the bile accumulate in the liver and blood resulting in adverse effects. It is becoming evident that cholestasis results from altered synthesis and localization of transporters involved in bile formation.
It is now well established that transporters involved in bile formation undergo transcriptional as well as post-translational regulation. The transcriptional regulation of hepatocellular transporters is mediated via nuclear receptor superfamily (3, 4) and assures long term adjustments of transporter functions. The post-translational regulations involve short-term rapid changes in plasma membrane (PM) localization of transporters (5–7) allowing for rapid changes in bile formation. A number of signaling pathways are involved in the regulation of these short-term changes.
Cyclic AMP, Ca2+, phosphoinositide-3-kinase (PI3K), protein kinase C (PKC), mitogen activated protein kinases (MAPKs), Rab proteins and protein phosphatases (PPs) have all been reported to be involved in short-term regulation (5–7). Paradoxically, both choleretic and cholestatic agents activate the same intracellular signaling kinases, such as PI3K, PKCs and MAPKs. A possible explanation for this paradox may be that choleretic and cholestatic effects result from activation of different isoforms of PI3K (8), PKC and p38-MAPK (9). These isoform specific effects are most evident for PKCs. While many transporters are involved in bile formation (2, 3, 5–7, 10), transporters shown to be regulated by PKC isoforms include Na+-taurocholate cotransporting polypeptide (NTCP, SLC10A1), organic anion transporting polypeptides (OATPs, SLCOs), bile salt export pump (BSEP, ABCB11) and multidrug resistance-associated protein 2 (MRP2, ABCC2 ).
II. Protein Kinase C isoforms
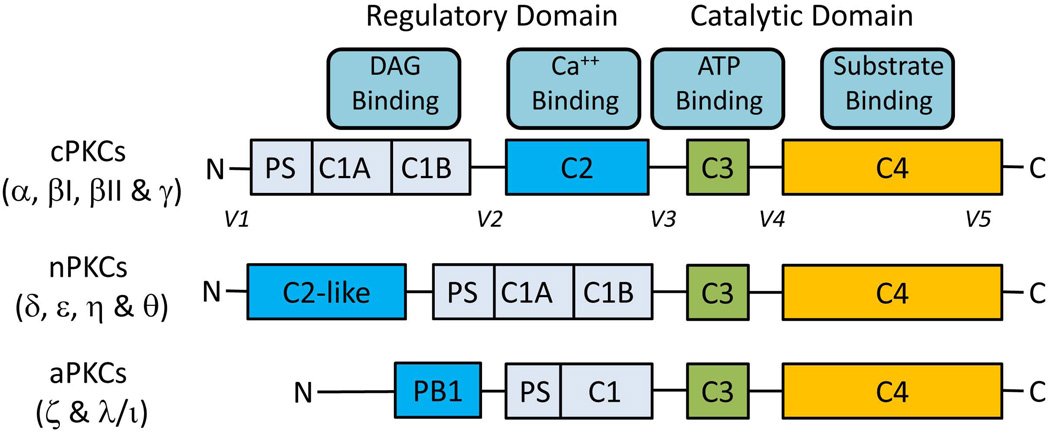
Protein kinase C belongs to a family of serine/threonine protein kinases and consists of at least 12 isoforms (11, 12): conventional (cPKCα, βI, βII and γ), novel (nPKCδ, ε, η and θ), and atypical (aPKCζ and λ/ι) isoforms (Fig. 1) . PKCs expressed in rat hepatocytes include cPKCα, nPKCδ, nPKCε, and aPKCζ with the presence of cPKCβII being controversial (13–15).
Fig. 1.
Primary structures of PKCs. There are four structurally conserved domains (C1-C4) in PKC isoforms divided into the N-terminal regulatory domain (C1-C2) and the C-terminal catalytic domain (C3-C4). The regulatory domain contains the binding sites for pseudosubstrate (PS), DAG (C1) and Ca++ (C2 or C2-like). The catalytic domain contains the binding sites of ATP (C3) and substrate (C4). C-regions (C1-C4) represent conserved domains and V-regions represent variable domains (V1-V5). The regulatory and the catalytic domains are separated by a flexible hinge domain (V3), which is cleaved by caspase-3 in apoptotic cells. Novel isoforms contain a C2-like domain which is unable to bind Ca++ and hence do not require Ca++ for activation. Atypical isozymes contain a variant of the C1 domain, which lacks the ligand-binding pocket for DAG and lacks C2 domain. As a result aPKCs are not regulated by DAG and Ca++; they are regulated by protein-protein interactions via PB1 domain. The intermolecular binding between PS and catalytic domain is highly regulated by membrane interactions, PKC conformation and phosphorylation.
All PKC isoforms undergo a maturation process requiring phosphorylation at three sites in the catalytic domain before they can be activated (11, 12). These phosphorylation events are constitutive and hence are not regulated for conventional and novel PKCs, but are dependent on agonists for atypical PKCs. Activation of the phosphorylated cPKCs and nPKC requires binding of DAG and/or Ca2+ and interaction with acidic membrane lipids. Since activated PKCs bind and phosphorylate substrates at the membrane (11), membrane translocation is often used as a read out for the activation of cPKCs and nPKCs. In contrast to cPKCs and nPKCs, protein-protein interaction leads to the activation of aPKCs (12).
PI3K plays an important role in the activation of some, but not all PKCs in hepatocytes. For example, activation of aPKCζ (16) and nPKCδ (17, 18) is PI3K dependent. Interestingly, taurolithocholate (TLC) induced activation of nPKCε is dependent on PI3K in rat hepatocytes (19), but not in a human hepatoma cell line (20). The activation of cPKCs in hepatocytes appears to be PI3K independent (21, 22). In addition to PI3K, activation of guanylyl cyclase by oxidative stress activates nPKCs in rat hepatocytes (23).
III. Role of PKC isoforms
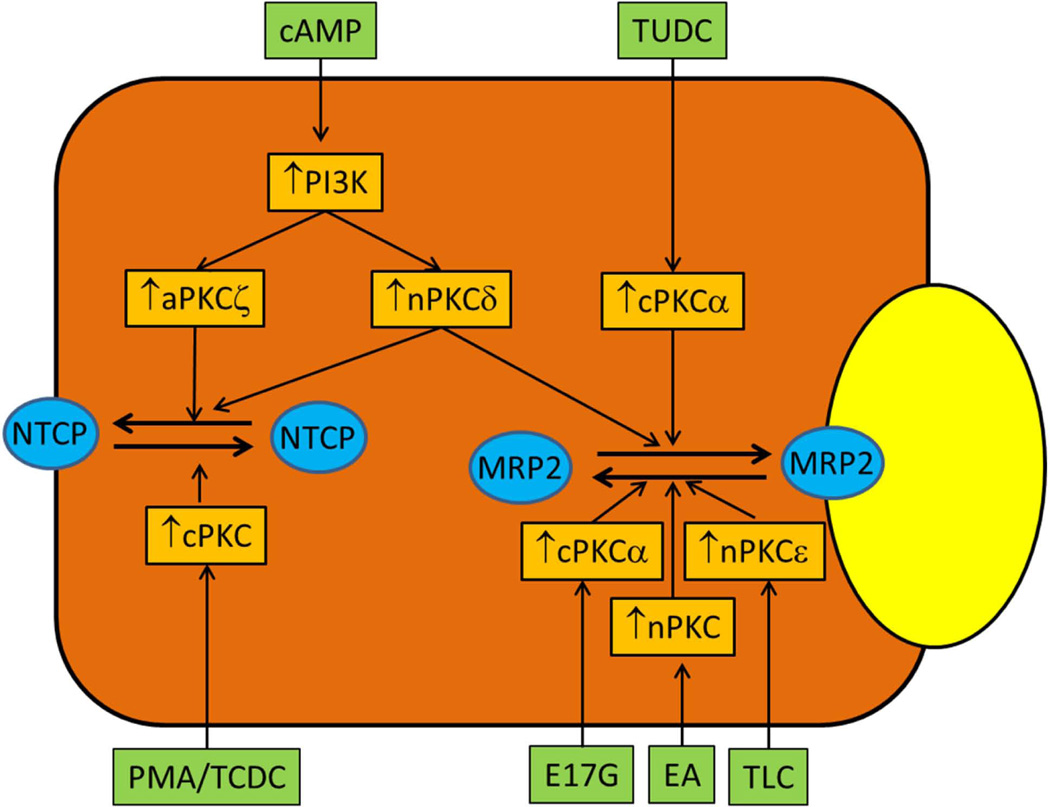
Initial studies using broad spectrum pharmacologic activators and inhibitors of cPKCs and nPKCs suggested that activation of PKCs produced cholestasis (5), inhibited basal and cAMP-induced increases in TC uptake (24) and decreased transport of solutes by MRP3 (25) and OATP (26). However, both choleretic and cholestatic agents activate PKCs (Table 1) and PKCs are implicated in cholestatic, anticholestatic and choleretic effects of bile acids and other agents. It is becoming evident that the opposing effects of choleretic and cholestatic agents may be mediated via different isoforms of PKCs. Studies to date would indicate that aPKCζ may mediate choleretic effects and nPKCε may mediate cholestatic effects, while cPKCα and nPKCδ may be involved in choleretic, cholestatic and anticholestatic effects by affecting insertion/retrieval of transporters involved in bile formation (Fig. 2).
Table 1.
Effect of choleretic and cholestatic agents on PKC isoforms in rat hepatocytes.
| PKC isoforms |
Choleretic agents | Cholestatic agents | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TC | TUDC | cAMP | GCDC | TCDC | TDC | TLC | E17G | EA | |
| cPKCα | ↑/− | ↑ | − | ↑ | ↑ | ↑ | ↓ | ↑ | − |
| nPKCδ | ↑/− | − | ↑ | ↑ | ↑ | ↑ | − | ND | ↑ |
| nPKCε | ↑/− | − | − | ↑ | ND | ND | ↑ | − | ↑ |
| aPKCζ | ND | − | ↑ | ND | ↑ | ND | − | ND | ND |
TC (15, 27, 83), TUDC (27, 83), GCDC(14), TCDC(83, 84), TDC (83) and TLC (19, 28, 45) have been shown to affect PKC isoform activity in hepatocytes. TC has been reported to activate (15, 83) cPKCα or to have no effect on cPKCα (27) in hepatocytes. E17G = Estradiol 17β-D-glucuronide (33), EA= ethacrynic acid (23), Activation (↑), inhibition (↓) or no effect (−); ND= not determined.
Figure 2.
Proposed regulation of NTCP and MRP2 by PKC isoforms. Activation of aPKCζ and nPKCδ by cAMP leads to translocations of NTCP and MRP2 to PM. Activation of cPKC (most likely cPKCα) by PMA and TCDC induces retrieval of NTCP from PM, while activation of cPKCα by TUDC facilitates MRP2 translocation to PM. Activations of cPKCα, nPKC and nPKCε have been implicated in MRP2 retrieval from PM by estradiol 17β-D-glucuronide (E17G), ethacrynic acid (EA) induced oxidative stress and taurolithocholate (TLC), respectively. Retrieval of BSEP by E17G, PMA and oxidative stress has been proposed to be mediated via cPKCs (not shown).
a. Role of cPKC/cPKCα
Tauroursodeoxycholate (TUDC) activates cPKCα in isolated rat hepatocytes (27) and reverses TLC-induced reduction in PM MRP2 via PKC-dependent mechanisms (28). Interestingly, TUDC-induced choleresis is not inhibited by Gö6976, an inhibitor of cPKCs (29), although cPKCα stimulates rat MRP2 activity, when coexpressed in Sf9 cells, (30). Thus, the anticholestatic and not the choleretic effect of TUDC may be mediated via cPKCα. Further studies showed that TUDC-mediated reversal of TLC-induced cholestasis is partially inhibited by combined inhibition of cPKC and protein kinase A (PKA) (31). It is suggested that the anticholestatic effect of TUDC may in part be mediated via cooperative post-translational cPKCα-/PKA-dependent mechanisms (31).
In contrast to potential anticholestatic effect, cPKCs/cPKCα have also been implicated in cholestasis. For example, cPKCs/cPKCα mediate BSEP retrieval induced by phorbol myristate acetate (PMA) (29) and oxidative stress produced by tertiary-butylhydroperoxide (t-BHP) (32), estradiol 17β-D-glucuronide (E17G)-induced retrieval of rat BSEP and MRP2 from the canalicular membrane (33) and retrieval of MRP2 and rat NTCP in HepG2 cells (21, 34, 35). On the other hand, TLC, a cholestatic bile acid, induces retrieval of NTCP (20) and MRP2, but inhibits cPKCα (28) in rat hepatocytes. These results suggest that the retrieval of NTCP and MRP2 by cholestatic agents/conditions may involve mediators in addition to cPKCα.
The opposing effects of cPKCs/cPKCα in bile formation may be due to interactions with different downstream regulators as suggested for the opposing effects of cPKCα in cell survival (11). It is possible that activation of cPKCs by choleretic and cholestatic agents involves translocation of cPKCs to different subcellular membranes resulting in activation/inhibition of different downstream effectors. One such target may be the estrogen receptor activated by cPKCα in E17G-induced cholestasis (36). Other potential downstream effectors of PKCs are discussed below.
b. Role of nPKCδ
Studies in rat hepatocytes suggest that nPKCδ is involved in hepatotoxicity induced by allyl alcohol (37) and 4-hydroxynonenal (38). However, a recent study using molecular activators and inhibitors of nPKCδ in HuH7-NTCP cells showed that activation of nPKCδ by GCDC actually induces a cytoprotective pathway by inhibiting JNK activation and down-regulating pro-apoptotic JNK/BIM pathway (39). In addition, nPKCδ mediates cAMP-induced translocation of NTCP and MRP2 to PM in HuH7-NTCP cells and rat hepatocytes (17, 18). Thus, nPKCδ may mediate both toxic and beneficial effects in hepatocytes. The underlying mechanism may be related to phosphorylation of nPKCδ.
Novel PKCδ can either positively or negatively regulate apoptosis depending on nPKCδ sites phosphorylated by various stimuli (40, 41). Phosphorylation at Tyr311 followed by caspase-3 mediated cleavage leads to the formation of the pro-apoptotic form of nPKCδ (11). On the other hand, the PI3K-dependent phosphorylation at Thr505 (42) is associated with cell survival effect (43). These studies raise the possibility that activation nPKCδ via Tyr311 phosphorylation may lead to cholestatic effects, while activation via Thr505 phosphorylation may lead to choleretic effects. Consistent with this hypothesis are the findings that activation of nPKCδ by cAMP (18) and the nPKCδ-mediated cytoprotective effect of GCDC (39) are associated with Thr505 and not Tyr311 phosphorylation in rat hepatocytes. However, whether differential phosphorylation dictates the effect of nPKCδ in bile formation and cholestasis remains to be established.
c. Role of nPKCε
Studies in hepatocytes and hepatic cell lines show that TLC activates nPKCε (13, 20) and induces retrieval of BSEP (44) and MRP2 (28, 45) from PM. Knock down of nPKCε reverses TLC-induced retrieval of MRP2 in HuH7-NTCP cells (45). In addition, TUDC reverses TLC-induced cholestasis and MRP2 retrieval by inhibiting TLC-induced activation of nPKCε in perfused rat livers (19). Thus, nPKCε may mediate TLC-induced MRP2 retrieval. Rat MRP2 retrieval by oxidative stress induced by EA is mediated via nPKCs (23). It is likely that EA-induced MRP2 retrieval is mediated via nPKCε, since nPKCδ is involved in cAMP-induced MRP2 translocation to the membrane. Interestingly, TLC-induced inhibition of TC uptake is not mediated via nPKCε in HuH7-NTCP cells (20). Thus, the canalicular membrane may be the target of nPKCε and this is consistent with the finding that TLC translocates nPKCε to the canalicular membrane in rat hepatocytes (13).
TUDC inhibits TLC-induced nPKCε activation and reverses TLC-induced cholestasis (28, 46) and retrieval of MRP2 (19, 28, 46). Our unpublished studies show that cAMP can reverse TLC-induced MRP2 retrieval and nPKCε activation in hepatocytes. Thus, cAMP and TUDC may produce anticholestatic effects by reversing TLC-induced activation of nPKCε.
d. Role of aPKCζ
This atypical PKC isoform is a downstream effector of PI3K (11). The PI3K/aPKCζ pathway is involved in cAMP-induced NTCP translocation to the PM (16, 47) and NTCP-mediated transport of chenodeoxycholylglycylamidofluorescein (48) in rat hepatocytes. Since aPKCζ co-localizes with BSEP and MRP2 at the canalicular membrane (49), it may also be involved in the canalicular localization of these transporters.
IV. Beyond PKC isoforms
The cellular mechanisms by which PKC isoforms regulate hepatocellular transporters and hence bile formation, are incompletely understood. A number of likely mechanisms, however, can be proposed based on our current knowledge. These mechanisms include direct phosphorylation of transporters, modification of actin binding proteins (Radixin and MARCKS) and stimulation of Rab protein(s)-mediated vesicular transport, as discussed below.
a. Phosphorylation of transporters
Rat OATP1 is a serine-phosphoprotein (50), while rat NTCP is a serine/threonine phosphoprotein (51). Phosphorylation and dephosphorylation affect PM localization of rat OATP1 (52) and rat NTCP (53) by retrieving from and inserting into PM, respectively. Since cPKCs are involved in NTCP retrieval, cPKCs may mediate retrieval by phosphorylating NTCP. On the other hand, both BSEP and MRP2 are phosphorylated by cPKCs and nPKCs (30, 31, 54) raising the possibility that MRP2 retrieval by E-17G and TLC may involve phosphorylation of MRP2 by cPKCα and cPKCε, respectively. It is however unknown whether translocations of MRP2 and BSEP are regulated by phosphorylation as it has been suggested for NTCP (53) and OATP1 (52). Phosphorylation by cPKCα may also affect transport function of rat MRP2 (30). Thus, PM localization as well as function of transporters may be affected by PKC-mediated phosphorylation.
b. Interaction with actin binding proteins
Plasma membrane localization of a transporter requires close interactions between PM and the underlying cytoskeleton. Such interactions involve proteins that crosslink membrane proteins with F-actin (55–57). Since actin plays an important role in hepatobiliary transporter translocation (58–60), it is likely that actin interacting proteins are involved in their PM localization. Indeed, two such proteins, radixin, a member of Ezrin/Radixin/Moesin (ERM) protein family, and myristoylated alanine-rich C kinase substrate (MARCKS) have been suggested to play an important role in PM localization of MRP2.
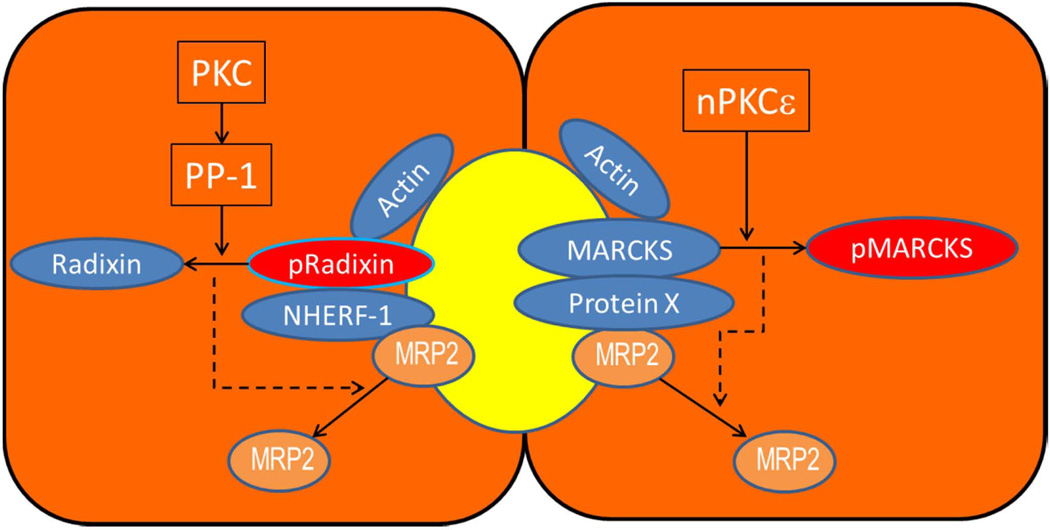
ERM proteins (61, 62) interact with specific membrane proteins directly or indirectly through adaptor molecules such as ERM-binding phosphoprotein 50 (EBP50) or sodium-hydrogen exchanger regulatory factor-1 (NHERF-1). Radixin, the dominant ERM protein in hepatocytes, is localized primarily at the canalicular membrane (63). The co-localization of radixin and MRP2 at the canalicular membrane is disrupted in cholestatic rats (64) and PBC patients (65). Knockdown and knockout of radixin lead to a reduction in membrane MRP2 as well as BSEP and MDR1 (66, 67). EBP50, which is localized at the apical membrane of hepatocytes and cholangiocytes (68), also binds to MRP2, and is needed for PM localization of MRP2 (69). Thus, the interaction of radixin with MRP2 (and possibly BSEP) via EBP50 and actin appears to be essential for maintaining the polarized targeting and retention of these transporters. This interaction is favored when radixin is activated by phosphorylation at Thr564 (55, 62). Studies to date would suggest that dephosphorylation of radixin by PKC-mediated activation of PP-1 may lead to MRP2 retrieval from the canalicular membrane (64, 70). Since cPKCα as well as nPKCε are involved in MRP2/BSEP retrieval (32, 33, 45), both isoforms are likely to be involved in radixin dephosphorylation (Fig. 3).
Figure 3.
Role of radixin and MARCKS in PM localization of MRP2. Phosphorylated radixin (active form) stabilizes MRP2 in the membrane by binding to actin and NHERF-1, which binds MRP2. MARCKS also binds actin and possibly MRP2 via an unknown protein X. Dephosphorylation of radixin (inactive form) by PP-1 activated by cPKCα/cPKCε and phosphorylation of MARCKS by nPKCε result in the loss of their binding to actin leading to retrieval from PM. Removal of radixin and MARCKS from PM results in the retrieval of MRP2 (dotted line), mostly likely due to the loss of PM anchoring proteins for MRP2. Similar mechanisms may also be involved in BSEP retrieval (not shown).
MARCKS plays a key role in endocytosis (71) and its phosphorylation by cPKCs and nPKCs results in MARCKS retrieval from PM and in F-actin disassembly (56). Studies in rat hepatocytes and HuH7-NTCP cells suggest that MRP2 retrieval by TLC involves activation of nPKCε followed by MARCKS phosphorylation and consequent detachment of MARCKS from the membrane (45). Similarly, retrieval of MRP2 by oxidative stress and E-17G may also involve cPKCα-mediated phosphorylation of MARCKS (Fig. 3).
c. Rab proteins mediated vesicular transport
Rab proteins cycle between GTP-bound active form and GDP-bound inactive form and are involved in vesicle trafficking (72, 73). Among various Rab proteins, Rab5 is involved in the apical sorting of BSEP (74) while Rab4 and Rab11 are involved in rapid and slow recycling from early and late endosomes, respectively, to the PM (73). Rab11 is expressed in apical vesicular populations in the liver (75). There are limited studies suggesting a role for PKCs in Rab4-mediated NTCP translocation to PM.
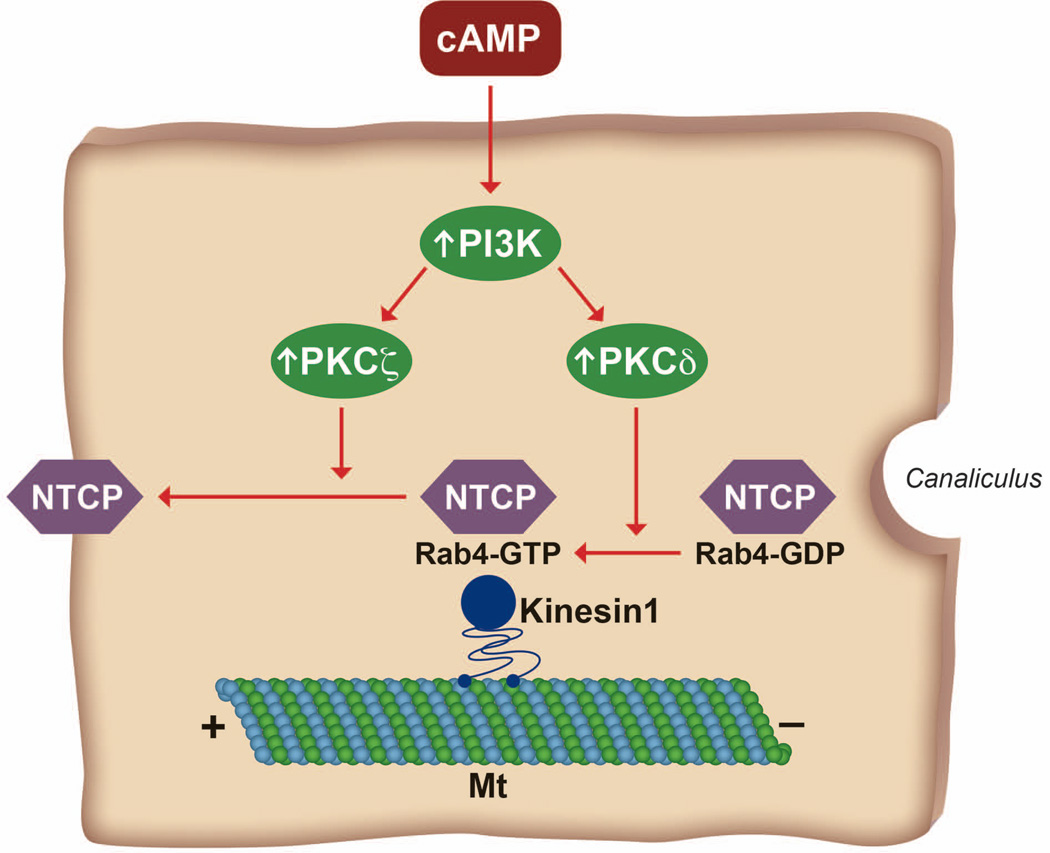
Cyclic AMP-induced translocation of NTCP to PM is mediated via PI3K-dependent activation of nPKCδ and aPKCζ (16, 17, 47, 76) and nPKCδ activates Rab4 (17). NTCP is enriched in rat liver endocytic vesicles and co-localizes with Rab4 (47). PKCζ increases motility of NTCP containing vesicles along the microtubules (47). Other studies show that cAMP induced NTCP translocation is dependent on PI3K (58) and actin cytoskeleton and microtubules (58, 59). In addition, a Rab effector protein kinesin-1 is involved in NTCP-containing vesicles movement (47). Taken together, these results may suggest that activation of PI3K/PKCδ by cAMP leads to the activation of Rab4 followed by recruitment of kinesin-1 and increased motility of NTCP/Rab4 containing vesicles along the microtubules to PM (Fig. 4).
Figure 4.
Postulated role of PI3K/PKCδ, PI3K/PKCζ in Rab4-mediated insertion of NTCP into PM. NTCP containing vesicles co-localize with Rab4, which cycles between GTP bound (Rab4-GTP) active form and GDP bound (Rab4-GDP) inactive form. Activation of PKCδ leads to the conversion of inactive Rab4-GDP to active Rab4-GTP followed by recruitment of kinesin-1, which moves NTCP containing vesicles towards the plus end of microtubules (Mt). This movement is further facilitated by PKCζ.
Rab11 is required for bile canalicular formation in WIF-B9 cells (77), regulates canalicular localization of BSEP (78) and mediates TUDC- and cAMP-induced translocation of MRP2 to PM (79). Since Rab11 activation involves PKC-mediated phosphorylation (80), and cAMP and TUDC activate nPKCδ (18) and cPKCα (28) respectively, it can be speculated that phosphorylation (and activation) of Rab11 by cPKCα and nPKCδ may be involved in MRP2 translocation to the PM. Further studies are needed to establish a role for Rab11 phosphorylation by PKCs in MRP2 translocation.
VI. Future Perspectives
Our understanding of regulation of hepatocellular transporters involved in bile formation has been steadily increasing. However, it is still unclear how activation of the same PKC isoform can lead to opposing effects. Whether the opposing effects are due to different subcellular localization leading to activation/inhibition of different downstream mediators remains to be established. We are beginning to elucidate the signaling pathways by which PKC isoforms regulate PM localization of hepatocellular transporters. While phosphorylation of transporters, actin binding proteins (radixin and MARCKS) and Rab proteins by PKCs has been suggested, further studies are needed to confirm these hypotheses. Our current understanding of the role of PKC isoforms is based primarily on studies using chemical inhibitors of PKC isoforms. Future studies should include other approaches, such as knockout, knockdown, constitutively active and dominant negative mutants, to establish the role of PKC isoforms and their downstream effectors. Human NTCP plays an important role in the entry of hepatitis B and D viruses into hepatocytes and consequent infection (81). The viral entry may involve PKC-mediated endocytosis of NTCP (82). This new functional implication of NTCP underscores the importance of understanding signaling pathways involved in PM localization of transporters. A better understanding of the underlying signaling should allow us to target specific pathways to limit or enhance solute transport and thereby limit hepatic toxicity/infection or enhance hepatic functions as needed.
ACKNOWLEDGEMENT
This work was supported in part by grants from NIH (DK033436 and DK090010). The author gratefully acknowledges critical comments and editorial suggestions by Drs. Cynthia R.L. Webster, Christopher Schonhoff and SeWon Park.
REFERENCES
- 1.Stapelbroek JM, van Erpecum KJ, Klomp LW, Houwen RH. Liver disease associated with canalicular transport defects: current and future therapies. J Hepatol. 2010;52:258–271. doi: 10.1016/j.jhep.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Maillette de Buy WL, Beuers U. Bile salts and cholestasis. Dig Liver Dis. 2010;42:409–418. doi: 10.1016/j.dld.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Halilbasic E, Claudel T, Trauner M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol. 2013;58:155–168. doi: 10.1016/j.jhep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anwer MS. Cellular Regulation of hepatic bile acid transport in health and cholestasis. Hepatology. 2004;39:581–589. doi: 10.1002/hep.20090. [DOI] [PubMed] [Google Scholar]
- 6.Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3:1035–1078. doi: 10.1002/cphy.c120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crocenzi FA, Zucchetti AE, Boaglio AC, Barosso IR, Sanchez Pozzi EJ, Mottino AD, et al. Localization status of hepatocellular transporters in cholestasis. Front Biosci. 2012;17:1201–1218. doi: 10.2741/3981. [DOI] [PubMed] [Google Scholar]
- 8.Hohenester S, Gates A, Wimmer R, Beuers U, Anwer MS, Rust C, et al. Phosphatidylinositol-3-kinase p110gamma contributes to bile salt-induced apoptosis in primary rat hepatocytes and human hepatoma cells. J Hepatol. 2010;53:918–926. doi: 10.1016/j.jhep.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schonhoff CM, Webster CR, Anwer MS. Cyclic AMP stimulates Mrp2 translocation by activating p38{alpha} MAPK in hepatic cells. Am J Physiol Gastrointest Liver Physiol. 2010;298:G667–G674. doi: 10.1152/ajpgi.00506.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann AF. The enterohepatic circulation of bile acids in mammals: form and functions. Front Biosci. 2009;14:2584–2598. doi: 10.2741/3399. [DOI] [PubMed] [Google Scholar]
- 11.Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci. 2009;14:2386–2399. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beuers U, Probst I, Soroka C, Boyer JL, Kullak-Ublick GA, Paumgartner G. Modulation of protein kinase C by taurolithocholic acid in isolated rat hepatocytes. Hepatology. 1999;29:477–482. doi: 10.1002/hep.510290227. [DOI] [PubMed] [Google Scholar]
- 14.Jones BA, Rao YP, Stravitz RT, Gores GJ. Bile salt-induced apoptosis of hepatocytes involves activation of protein kinase C. Am J Physiol. 1997;272:G1109–G1115. doi: 10.1152/ajpgi.1997.272.5.G1109. [DOI] [PubMed] [Google Scholar]
- 15.Stravitz RT, Rao YP, Vlahcevic ZR, Gurley EC, Jarvis WD, Hylemon PB. Hepatocellular protein kinase C activation by bile acids: implications for regulation of cholesterol 7 alpha-hydroxylase. Am J Physiol. 1996;271:G293–G303. doi: 10.1152/ajpgi.1996.271.2.G293. [DOI] [PubMed] [Google Scholar]
- 16.McConkey M, Gillin H, Webster CR, Anwer MS. Cross-talk between protein kinases Czeta and B in cyclic AMP-mediated sodium taurocholate co-transporting polypeptide translocation in hepatocytes. J Biol Chem. 2004;279:20882–20888. doi: 10.1074/jbc.M309988200. [DOI] [PubMed] [Google Scholar]
- 17.Park SW, Schonhoff CM, Webster CR, Anwer MS. Protein kinase Cdelta differentially regulates cAMP-dependent translocation of NTCP and MRP2 to the plasma membrane. Am J Physiol Gastrointest Liver Physiol. 2012;303:G657–G665. doi: 10.1152/ajpgi.00529.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schonhoff CM, Gillin H, Webster CR, Anwer MS. Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology. 2008;47:1309–1316. doi: 10.1002/hep.22162. [DOI] [PubMed] [Google Scholar]
- 19.Beuers U, Denk GU, Soroka CJ, Wimmer R, Rust C, Paumgartner G, et al. Taurolithocholic acid exerts cholestatic effects via phosphatidylinositol-3 kinase-dependent mechanisms in perfused rat livers and rat hepatocyte couplets. J Biol Chem. 2003;278:17810–17818. doi: 10.1074/jbc.M209898200. [DOI] [PubMed] [Google Scholar]
- 20.Schonhoff CM, Yamazaki A, Hohenester S, Webster CR, Bouscarel B, Anwer MS. PKC{epsilon}-dependent and -independent effects of taurolithocholate on PI3K/PKB pathway and taurocholate uptake in HuH-NTCP cell line. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1259–G1267. doi: 10.1152/ajpgi.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muhlfeld S, Domanova O, Berlage T, Stross C, Helmer A, Keitel V, et al. Short-term feedback regulation of bile salt uptake by bile salts in rodent liver. Hepatology. 2012;56:2387–2397. doi: 10.1002/hep.25955. [DOI] [PubMed] [Google Scholar]
- 22.Boaglio AC, Zucchetti AE, Sanchez Pozzi EJ, Pellegrino JM, Ochoa JE, Mottino AD, et al. Phosphoinositide 3-kinase/protein kinase B signaling pathway is involved in estradiol 17beta-D-glucuronide-induced cholestasis: complementarity with classical protein kinase C. Hepatology. 2010;52:1465–1476. doi: 10.1002/hep.23846. [DOI] [PubMed] [Google Scholar]
- 23.Sekine S, Ito K, Horie T. Oxidative stress and Mrp2 internalization. Free Radic Biol Med. 2006;40:2166–2174. doi: 10.1016/j.freeradbiomed.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Grüne S, Engelking LR, Anwer MS. Role of intracellular calcium and protein kinases in the activation of hepatic Na+/taurocholate cotransport by cyclic AMP. J Biol Chem. 1993;268:17734–17741. [PubMed] [Google Scholar]
- 25.Chandra P, Zhang P, Brouwer KL. Short-term regulation of multidrug resistance-associated protein 3 in rat and human hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1252–G1258. doi: 10.1152/ajpgi.00362.2004. [DOI] [PubMed] [Google Scholar]
- 26.Guo GL, Klaassen CD. Protein kinase C suppresses rat organic anion transporting polypeptide 1- and 2-mediated uptake. J Pharmacol Exp Ther. 2001;299:551–557. [PubMed] [Google Scholar]
- 27.Beuers U, Throckmorton DC, Anderson MS, Isales CM, Thasler W, Kullak-Ublick GA, et al. Tauroursodeoxycholic acid activates protein kinase C in isolated rat hepatocytes. Gastroenterology. 1996;110:1553–1563. doi: 10.1053/gast.1996.v110.pm8613063. [DOI] [PubMed] [Google Scholar]
- 28.Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, et al. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology. 2001;33:1206–1216. doi: 10.1053/jhep.2001.24034. [DOI] [PubMed] [Google Scholar]
- 29.Kubitz R, Saha N, Kuhlkamp T, Dutta S, vom DS, Wettstein M, et al. Ca2+-dependent protein kinase C isoforms induce cholestasis in rat liver. J Biol Chem. 2004;279:10323–10330. doi: 10.1074/jbc.M306242200. [DOI] [PubMed] [Google Scholar]
- 30.Ito K, Wakabayashi T, Horie T. Mrp2/Abcc2 transport activity is stimulated by protein kinase Calpha in a baculo virus co-expression system. Life Sci. 2005;77:539–550. doi: 10.1016/j.lfs.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 31.Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut. 2008;57:1448–1454. doi: 10.1136/gut.2007.140871. [DOI] [PubMed] [Google Scholar]
- 32.Perez LM, Milkiewicz P, Elias E, Coleman R, Sanchez Pozzi EJ, Roma MG. Oxidative stress induces internalization of the bile salt export pump, Bsep, and bile salt secretory failure in isolated rat hepatocyte couplets: a role for protein kinase C and prevention by protein kinase A. Toxicol Sci. 2006;91:150–158. doi: 10.1093/toxsci/kfj113. [DOI] [PubMed] [Google Scholar]
- 33.Crocenzi FA, Sanchez Pozzi EJ, Ruiz ML, Zucchetti AE, Roma MG, Mottino AD, et al. Ca(2+)-dependent protein kinase C isoforms are critical to estradiol 17beta-D-glucuronide-induced cholestasis in the rat. Hepatology. 2008;48:1885–1895. doi: 10.1002/hep.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubitz R, Huth C, Schmitt M, Horbach A, Kullak-Ublick G, Haussinger D. Protein kinase C-dependent distribution of the multidrug resistance protein 2 from the canalicular to the basolateral membrane in human HepG2 cells. Hepatology. 2001;34:340–350. doi: 10.1053/jhep.2001.25959. [DOI] [PubMed] [Google Scholar]
- 35.Stross C, Helmer A, Weissenberger K, Gorg B, Keitel V, Haussinger D, et al. Protein kinase C induces endocytosis of the sodium taurocholate cotransporting polypeptide. Am J Physiol Gastrointest Liver Physiol. 2010;299:G320–G328. doi: 10.1152/ajpgi.00180.2010. [DOI] [PubMed] [Google Scholar]
- 36.Barosso IR, Zucchetti AE, Boaglio AC, Larocca MC, Taborda DR, Luquita MG, et al. Sequential activation of classic PKC and estrogen receptor alpha is involved in estradiol 17β-D-glucuronide-induced cholestasis. PLoS One. 2012;7:e50711. doi: 10.1371/journal.pone.0050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maddox JF, Roth RA, Ganey PE. Allyl alcohol activation of protein kinase C delta leads to cytotoxicity of rat hepatocytes. Chem Res Toxicol. 2003;16:609–615. doi: 10.1021/tx025655n. [DOI] [PubMed] [Google Scholar]
- 38.Castello L, Marengo B, Nitti M, Froio T, Domenicotti C, Biasi F, et al. 4-Hydroxynonenal signalling to apoptosis in isolated rat hepatocytes: the role of PKC-delta. Biochim Biophys Acta. 2005;1737:83–93. doi: 10.1016/j.bbalip.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Webster CR, Anwer MS. Activation of protein kinase C delta protects against bile acid induced apoptosis by suppression of a pro-apototic JNK/BIM pathway [Abstract] Hepatology. 2013;58(4 suppl):286A. [Google Scholar]
- 40.Jackson DN, Foster DA. The enigmatic protein kinase Cdelta: complex roles in cell proliferation and survival. FASEB J. 2004;18:627–636. doi: 10.1096/fj.03-0979rev. [DOI] [PubMed] [Google Scholar]
- 41.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c delta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- 42.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphinositide 3-kinase through the protein kinsae PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 43.Gliki G, Wheeler-Jones C, Zachary I. Vascular endothelial growth factor induces protein kinase C (PKC)-dependent Akt/PKB activation and phosphatidylinositol 3'-kinase-mediates PKC delta phosphorylation: role of PKC in angiogenesis. Cell Biol Int. 2002;26:751–759. doi: 10.1016/s1065-6995(02)90926-1. [DOI] [PubMed] [Google Scholar]
- 44.Crocenzi FA, Mottino AD, Sanchez Pozzi EJ, Pellegrino JM, Rodriguez Garay EA, Milkiewicz P, et al. Impaired localisation and transport function of canalicular Bsep in taurolithocholate induced cholestasis in the rat. Gut. 2003;52:1170–1177. doi: 10.1136/gut.52.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schonhoff CM, Webster CR, Anwer MS. Taurolithocholate-induced MRP2 retrieval involves MARCKS phosphorylation by protein kinase C in HUH-NTCP Cells. Hepatology. 2013;58:284–292. doi: 10.1002/hep.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholmerich J, Baumgartner U, Miyai K, Gerok W. Tauroursodeoxycholate prevents taurolithocholate-induced cholestasis and toxicity in rat liver. J Hepatol. 1990;10:280–283. doi: 10.1016/0168-8278(90)90133-c. [DOI] [PubMed] [Google Scholar]
- 47.Sarkar S, Bananis E, Nath S, Anwer MS, Wolkoff AW, Murray JW. PKCzeta is required for microtubule-based motility of vesicles containing the ntcp transporter. Traffic. 2006;7:1078–1091. doi: 10.1111/j.1600-0854.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 48.Murray JW, Thosani AJ, Wang P, Wolkoff AW. Heterogeneous accumulation of fluorescent bile acids in primary rat hepatocytes does not correlate with their homogenous expression of ntcp. Am J Physiol Gastrointest Liver Physiol. 2011;301:G60–G68. doi: 10.1152/ajpgi.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stross C, Keitel V, Winands E, Haussinger D, Kubitz R. Expression and localization of atypical PKC isoforms in liver parenchymal cells. Biol Chem. 2009;390:235–244. doi: 10.1515/BC.2009.031. [DOI] [PubMed] [Google Scholar]
- 50.Xiao Y, Nieves E, Angeletti RH, Orr GA, Wolkoff AW. Rat organic anion transporting protein 1A1 (Oatp1a1): purification and phosphopeptide assignment. Biochemistry. 2006;45:3357–3369. doi: 10.1021/bi052437v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukhopadhayay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. Sodium taurocholate cotransporting polypeptide is a serine, threonine phosphoprotein and is dephosphorylated by cyclic AMP. Hepatology. 1998;28:1629–1636. doi: 10.1002/hep.510280624. [DOI] [PubMed] [Google Scholar]
- 52.Choi JH, Murray JW, Wolkoff AW. PDZK1 binding and serine phosphorylation regulate subcellular trafficking of organic anion transport protein 1a1. Am J Physiol Gastrointest Liver Physiol. 2011;300:G384–G393. doi: 10.1152/ajpgi.00500.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anwer MS, Gillin H, Mukhopadhyay S, Balasubramaniyan N, Suchy FJ, Ananthanarayanan M. Dephosphorylation of Ser-226 facilitates plasma membrane retention of Ntcp. J Biol Chem. 2005;280:33687–33692. doi: 10.1074/jbc.M502151200. [DOI] [PubMed] [Google Scholar]
- 54.Noe J, Hagenbuch B, Meier PJ, St Pierre MV. Characterization of the mouse bile salt export pump overexpressed in the baculovirus system. Hepatology. 2001;33:1223–1231. doi: 10.1053/jhep.2001.24171. [DOI] [PubMed] [Google Scholar]
- 55.McClatchey AI, Fehon RG. Merlin and the ERM proteins--regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009;19:198–206. doi: 10.1016/j.tcb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujise A, Mizuno K, Ueda Y, Osada S, Hirai S, Takayanagi A, et al. Specificity of the high affinity interaction of protein kinase C with a physiological substrate, myristoylated alanine-rich protein kinase C substrate. J Biol Chem. 1994;269:31642–31648. [PubMed] [Google Scholar]
- 57.Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 58.Webster CRL, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP-mediated translocation of rat liver Ntcp. Am J Physiol. 1999;277:G1165–G1172. doi: 10.1152/ajpgi.1999.277.6.G1165. [DOI] [PubMed] [Google Scholar]
- 59.Dranoff JA, McClure M, Burgstahler AD, Denson LA, Crawford AR, Crawford JM, et al. Short-term regulation of bile acid uptake by microfilament-dependent translocation of ntcp to the plasma membrane. Hepatology. 1999;30:223–229. doi: 10.1002/hep.510300136. [DOI] [PubMed] [Google Scholar]
- 60.Wakabayashi Y, Kipp H, Arias IM. Transporters on demand: intracellular reservoirs and cycling of bile canalicular ABC transporters. J Biol Chem. 2006;281:27669–27673. doi: 10.1074/jbc.R600013200. [DOI] [PubMed] [Google Scholar]
- 61.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suda J, Zhu L, Karvar S. Phosphorylation of radixin regulates cell polarity and Mrp-2 distribution in hepatocytes. Am J Physiol Cell Physiol. 2011;300:C416–C424. doi: 10.1152/ajpcell.00467.2010. [DOI] [PubMed] [Google Scholar]
- 63.Amieva MR, Wilgenbus KK, Furthmayr H. Radixin is a component of hepatocyte microvilli in situ. Exp Cell Res. 1994;210:140–144. doi: 10.1006/excr.1994.1021. [DOI] [PubMed] [Google Scholar]
- 64.Kojima H, Sakurai S, Yoshiji H, Uemura M, Yoshikawa M, Fukui H. The role of radixin in altered localization of canalicular conjugate export pump Mrp2 in cholestatic rat liver. Hepatol Res. 2008;38:202–210. doi: 10.1111/j.1872-034X.2007.00209.x. [DOI] [PubMed] [Google Scholar]
- 65.Kojima H, Nies AT, Konig J, Hagmann W, Spring H, Uemura M, et al. Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. J Hepatol. 2003;39:693–702. doi: 10.1016/s0168-8278(03)00410-0. [DOI] [PubMed] [Google Scholar]
- 66.Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, et al. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet. 2002;31:320–325. doi: 10.1038/ng905. [DOI] [PubMed] [Google Scholar]
- 67.Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, et al. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131:878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fouassier L, Duan CY, Feranchak AP, Yun CH, Sutherland E, Simon F, et al. Ezrin-radixin-moesin-binding phosphoprotein 50 is expressed at the apical membrane of rat liver epithelia. Hepatology. 2001;33:166–176. doi: 10.1053/jhep.2001.21143. [DOI] [PubMed] [Google Scholar]
- 69.Li M, Wang W, Soroka CJ, Mennone A, Harry K, Weinman EJ, et al. NHERF-1 binds to Mrp2 and regulates hepatic Mrp2 expression and function. J Biol Chem. 2010;285:19299–19307. doi: 10.1074/jbc.M109.096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sekine S, Ito K, Saeki J, Horie T. Interaction of Mrp2 with radixin causes reversible canalicular Mrp2 localization induced by intracellular redox status. Biochim Biophys Acta. 2011;1812:1427–1434. doi: 10.1016/j.bbadis.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 71.Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horgan CP, McCaffrey MW. Rab GTPases and microtubule motors. Biochem Soc Trans. 2011;39:1202–1206. doi: 10.1042/BST0391202. [DOI] [PubMed] [Google Scholar]
- 73.Kelly EE, Horgan CP, McCaffrey MW. Rab11 proteins in health and disease. Biochem Soc Trans. 2012;40:1360–1367. doi: 10.1042/BST20120157. [DOI] [PubMed] [Google Scholar]
- 74.Zeigerer A, Gilleron J, Bogorad RL, Marsico G, Nonaka H, Seifert S, et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature. 2012;485:465–470. doi: 10.1038/nature11133. [DOI] [PubMed] [Google Scholar]
- 75.Goldenring JR, Smith J, Vaughan HD, Cameron P, Hawkins W, Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol. 1996;270:G515–G525. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- 76.Schonhoff CM, Thankey K, Webster CR, Wakabayashi Y, Wolkoff AW, Anwer MS. Rab4 facilitates cyclic adenosine monophosphate-stimulated bile acid uptake and Na(+)-taurocholate cotransporting polypeptide translocation. Hepatology. 2008;48:1665–1670. doi: 10.1002/hep.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wakabayashi Y, Dutt P, Lippincott-Schwartz J, Arias IM. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc Natl Acad Sci U S A. 2005;102:15087–15092. doi: 10.1073/pnas.0503702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and rab11-positive endosomes. Mol Biol Cell. 2004;15:3485–3496. doi: 10.1091/mbc.E03-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park SW, Schonhoff CM, Webster CR, Anwer MS. Rab11 facilitates cyclic-AMP- and tauroursodeoxycholate-induced MRP2 transloctaion [Abstract] Hepatology. 2013;58(4 suppl):843A. doi: 10.1152/ajpgi.00457.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pavarotti M, Capmany A, Vitale N, Colombo MI, Damiani MT. Rab11 is phosphorylated by classical and novel protein kinase C isoenzymes upon sustained phorbol ester activation. Biol Cell. 2012;104:102–115. doi: 10.1111/boc.201100062. [DOI] [PubMed] [Google Scholar]
- 81.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:1–28. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anwer MS, Stieger B. Sodium-dependent bile salt transporters of the SLC10A transporter family: more than solute transporters. Pflugers Arch Eur J Physiol. 2014;466:77–89. doi: 10.1007/s00424-013-1367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rao YP, Stravitz RT, Vlahcevic ZR, Gurley EC, Sando JJ, Hylemon PB. Activation of protein kinase C alpha and delta by bile acids: correlation with bile acid structure and diacylglycerol formation. J Lipid Res. 1997;38:2446–2454. [PubMed] [Google Scholar]
- 84.Rust C, Karnitz LM, Paya CV, Moscat J, Simari RD, Gores GJ. The bile acid taurochenodeoxycholate activates a phosphatidylinositol 3- kinase-dependent survival signaling cascade. J Biol Chem. 2000;275:20210–20216. doi: 10.1074/jbc.M909992199. [DOI] [PubMed] [Google Scholar]