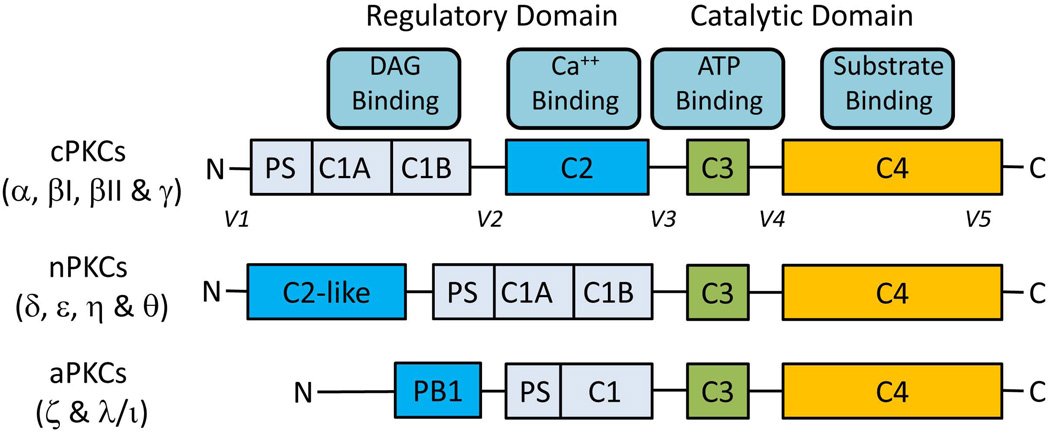
Fig. 1.
Primary structures of PKCs. There are four structurally conserved domains (C1-C4) in PKC isoforms divided into the N-terminal regulatory domain (C1-C2) and the C-terminal catalytic domain (C3-C4). The regulatory domain contains the binding sites for pseudosubstrate (PS), DAG (C1) and Ca++ (C2 or C2-like). The catalytic domain contains the binding sites of ATP (C3) and substrate (C4). C-regions (C1-C4) represent conserved domains and V-regions represent variable domains (V1-V5). The regulatory and the catalytic domains are separated by a flexible hinge domain (V3), which is cleaved by caspase-3 in apoptotic cells. Novel isoforms contain a C2-like domain which is unable to bind Ca++ and hence do not require Ca++ for activation. Atypical isozymes contain a variant of the C1 domain, which lacks the ligand-binding pocket for DAG and lacks C2 domain. As a result aPKCs are not regulated by DAG and Ca++; they are regulated by protein-protein interactions via PB1 domain. The intermolecular binding between PS and catalytic domain is highly regulated by membrane interactions, PKC conformation and phosphorylation.