Abstract
Purpose
Methiopropamine use in Europe has been detected since January 2011, but there is limited information on its acute toxicity. Here, we describe a case of analytically confirmed methiopropamine acute toxicity.
Case report
A 27-year-old woman with no previous medical history was brought to the emergency department with palpitations, chest tightness, anxiety, nausea, vomiting and visual hallucinations following the use of a ‘Quicksilver’. Toxicological analysis of her urine collected at presentation to the ED detected methiopropamine at a concentration of 400 ng/mL. Other drugs were also detected but at a much lower concentration.
Conclusion
This is the first ever case report of analytically confirmed acute toxicity related to methiopropamine use. It confirms the potential for significant acute toxicity with cardiovascular, gastrointestinal and psychotic symptoms thus providing further information to help with managing these patients and allow legislative authorities to consider the need for its control.
Keywords: Methiopropamine, 1-(thiophen-2-yl)-2-methylaminopropane, MPA), Novel psychoactive substance, Toxicity, Recreational drug
Background
The variety of novel psychoactive substances (NPS) available and being used as recreational drugs has increased substantially in Europe, North America and around the world [1]. As there is no good single source of information on the toxicity of these drugs, triangulation of data from several different sources is needed to describe the range of acute toxicities associated with their use [2, 3]. One of the important components of this data triangulation is case reports of acute toxicity with analytical confirmation of the NPS in biological samples [2, 3].
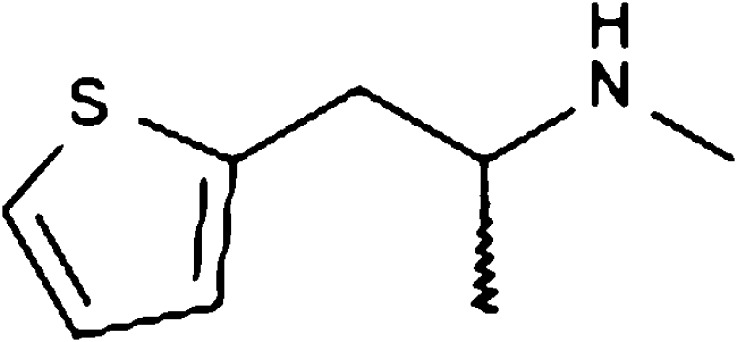
The NPS methiopropamine (1-(thiophen-2-yl)-2-methylaminopropane, MPA) is a methamphetamine analogue in which the benzene ring has been bioisosterically exchanged with a thiophene ring (Fig. 1).
Fig. 1.
Chemical structure of methiopropamine
It was first synthesised in 1942 [4] and first detected as a recreational drug in Europe in January 2011 when it was notified by Finland to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Early Warning System [5]. There is currently limited data available on the epidemiology of use of methiopropamine as it is not included in population or subpopulation surveys of drug use. There is evidence of use of methiopropamine in the UK, as it has been detected in pooled anonymous urine samples collected in both city centre environments (London) and festivals (North West of England) in 2012 [6–9].
There is no published data on the pharmacodynamics or toxicity of methiopropamine; the only information available on the effects of methiopropamine, both desired and adverse effects, is from anecdotal user reports on Internet discussion fora [10]. One needs to interpret these reports with caution, since there is variability in the content of NPS purchased from the Internet and high-street suppliers [11, 12]. We describe here the first case of analytically confirmed acute toxicity related to recreational use of methiopropamine.
Case Report
A 27-year-old woman with no previous medical history was brought to the Emergency Department (ED) by ambulance 21 h after oral ingestion of eight ‘Hawaiian baby seeds’ and nasal insufflation of 50 mg of ‘Quicksilver’ powder. The patient denied the use of alcohol or any other drugs and had not used recreational drugs or NPS previously. Both products were purchased from a market stall in central London.
After the use of the drugs, the patient had difficulty sleeping and had experienced intermittent palpitations and ‘chest tightness’. Nine hours after use, she developed nausea and vomited a total of 10 times; this was not associated with abdominal pain. During this time, she describes a general feeling of anxiety and euphoria with visual hallucinations (variations of her husband and other things coming out of the wall) lasting until 6 h prior to presentation.
On arrival in the ED, she was still experiencing nausea and dizziness, but her other symptoms had settled. She had a normal temperature (36.9 °C), heart rate (80 beats per minute) and blood pressure (109/77 mmHg). She was agitated but had a Glasgow Coma Score of 15/15; her pupils were dilated to 5 mm bilaterally and reactive to light. The rest of her physical examination was unremarkable, with normal tone, power and reflexes in all four limbs.
Venous blood gas analysis performed on room air (21 % oxygen) revealed no acid–base disturbance [pH 7.372, pvCO2; 5.86 kPa (44 mmHg), bicarbonate; 24.8 mmol/L, base excess; −0.7 mmol/L] and further blood tests including full blood count, renal and liver profile were all normal. The toxicology service reviewed the patient and she was treated with a single dose of 5 mg oral diazepam for agitation and IV fluid replacement in view of the previous significant vomiting. She was admitted to the Emergency Medical Unit (observation ward). The following morning her symptoms had settled and she was discharged with no sequelae 16 h after ED presentation and 37 h after the use of the drugs.
Toxicological Screening
Urine sample collected at the time of presentation to the ED was sent for toxicological analysis. The urine sample was initially pretreated with β-glucuronidase to cleave any glucuronic acid conjugates and was then prepared for analysis using solid phase extraction sample preparation techniques. The resulting prepared sample was then analysed using high performance liquid chromatography interfaced to high resolution accurate mass spectrometry operating in full scan mode.
Methiopropamine was detected in the urine at a concentration of 400 ng/mL; two methiopropamine metabolites (N-desmethyl and hydroxy N-desmethyl [13]) were also detected but it was not possible to quantify these. The only other substances detected were morphine 100 ng/mL; carboxy and dihdroxy metabolites of the naphthoylindole synthetic cannabinoid receptor agonists JWH-018 and JWH-019 (at concentrations of less than 5 ng/mL) and ergonovine at a concentration of less than 10 ng/mL.
Discussion
We report here a patient with symptoms of sleeplessness, nausea, vomiting, palpitations, chest tightness and visual hallucinations after insufflating a powder by the name of ‘quicksilver’ and ingesting ‘Hawaiian baby seeds’ (likely to be Argyreia nervosa containing ergoline alkaloids such as lysergic acid amide).
Searching on the Internet for ‘quicksilver, the only reference found related to NPS is for ‘the quicksilver aesthetic’ being methoxetamine [14]. No methoxetamine was detected in the urine on comprehensive toxicology analysis. The powder the patient insufflated was most likely methiopropamine which was detected in the urine at a concentration of 400 ng/mL. Metabolites of the synthetic cannabinoids JWH-018 and JWH-019 together with a low concentration of ergoline were also detected. It is possible that these contributed to the clinical picture, but methiopropamine was found at a much greater concentration.
There have been reports of methiopropamine use on Internet discussion fora from as early as May 2011 with users referring to it as ‘legal methamphetamine’ [10]. There is also confirmation of its use from detection in samples from portable urinals in the UK in 2012 [2, 6–9, 15]. It is available from a number of ‘research chemical’ websites as a powder with prices and volume ranging from £15 (US$25) for 1 g to 1 kg £3,250 (US$4,500) for 1 kg [16].
There is no data available on the pharmacodynamics of methiopropamine. The only pharmacokinetic data is on its metabolism which involves N-demethylation and hydroxylation at the side chain and the thiophene ring which is then followed by glucuronidation and/or sulphation [13]. The N-desmethyl derivative possesses some mono-amine oxide inhibitory properties [17].
The only information available on the acute toxicity associated with methiopropamine use is from user reports on Internet discussion fora. Users report mostly insufflation and occasional oral, rectal or vapourised administration [18–22]. There are reports from nine isolated MPA users on Erowid, who also report several adverse symptoms [10]. One 31-year-old male user reported nasal insufflation of 20–30 mg lines of methiopropamine over several days; he reported initially ‘stimulation’ and also described the ‘lack of need to sleep.’ On the third day, he developed chest pain and a headache together with visual and auditory hallucinations [21]. Several other users only reported insomnia [23–25]. One user who took a total of 100 mg of methiopropamine rectally complained of insomnia despite five ‘sleeping pills’ [26]. She noted a high heart rate but no actual heart rate was given. She also complained of nausea, feeling feverish together with shaking and a cold sweat [26]. Another user reported nasal insufflation of a total of 45 mg methiopropamine and oral ingestion of 30 mg of methiopropamine. He reported an increase in heart rate and also complained of an uneasy feeling [27]. Two users reported gastrointestinal symptoms of ‘discomfort resembling trapped air’ and several episodes of vomiting [23, 28]. Two users did not report any adverse effects related to methiopropamine use, they reported a feeling of increased energy and talkativeness/chattiness [19, 20].
Conclusion
In summary, the most common adverse reports reported on Internet discussion fora related to methiopropamine was insomnia. Some users also experienced cardiovascular and others gastrointestinal symptoms and one had auditory and visual hallucinations. This anecdotal data together with our case of analytically confirmed case confirms the potential for significant acute toxicity including insomnia, nausea and vomiting, palpitations, chest pain and hallucinations. Methiopropamine is not controlled under the Misuse of Drugs Act, 1971 in the UK or under similar drug control legislation elsewhere in Europe or North America. More data is required on the epidemiology of use of methiopropamine and the pattern of toxicity associated with its use in order to determine whether control is appropriate.
References
- 1.2012 Annual report on the state of the drugs problem in Europe. http://www.emcdda.europa.eu/attachements.cfm/att_190854_EN_TDAC12001ENC_.pdf (last accessed 1st Nov Oct 2013)
- 2.Wood DM, Dargan PI. Novel psychoactive substances: How to understand the toxicity (harm) associated with the use of these substances. Ther Drug Monit. 2012;34(4):363–367. doi: 10.1097/FTD.0b013e31825b954b. [DOI] [PubMed] [Google Scholar]
- 3.Wood DM, Dargan PI. Understanding how data triangulation identifies acute toxicity of novel psychoactive drugs. J Med Toxicol. 2012;8(3):300–303. doi: 10.1007/s13181-012-0241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blicke FF, Burckhalter JH. ‘α-thienylaminoalkanes’. J Am Chem Soc. 1942;64(3):477–480. doi: 10.1021/ja01255a001. [DOI] [Google Scholar]
- 5.EMCDDA Europol (2012) Information exchange, risk assessment and control of new psychoactive substance. New psychoactive substances notified in 2011. Annual Report on the implementation of Council Decision 2005/387/JHA.
- 6.Archer JR, Dargan PI, Chan WL, Hudson S, Wood DM. (2013) Variability in recreational drugs and novel psychoactive substances detected in anonymous pooled urine samples from street pissoirs (street urinals) over time: a technique to monitor trends in drugs use. Clin Tox (Phila) In press
- 7.Wood DM, Archer JR, Measham F, Hudson S, Dargan PI. Detection of use of novel psychoactive substances by attendees at a music festival in the North West of England. Clin Toxicol (Phila) 2013;51:340–341. [Google Scholar]
- 8.Archer JR, Dargan PI, Chan WL, Hitchings A, Hudson S, Wood DM. Trend analysis of novel psychoactive substances detected in pooled urine samples from street urinals over six months. Clin Toxicol (Phila) 2013;51:666. [Google Scholar]
- 9.Wood DM, Archer JR, Chan WL, Hudson S, Dargan PI, (2013) Developing analysis of pooled urine samples as a technique to monitor the use of novel psychoactive substances in an urban environment. Published in Proceedings of the Nights 2013. Edited by Gamberini L, Varotto A, Zamboni L, Spagnoli A. First edition. Pages 131–134. ISBN: 978 88 6787 1077
- 10.Erowid: Methiopropamine. Available from https://www.erowid.org/chemicals/methiopropamine/ (Last Accessed 18th March 2014).
- 11.Ramsey J, Dargan PI, Smyllie M, Davies S, Button J, Holt DW, Wood DM. Buying legal recreational drugs does not mean you aren’t breaking the law. Q J Med. 2010;103:777–783. doi: 10.1093/qjmed/hcq132. [DOI] [PubMed] [Google Scholar]
- 12.Davies S, Wood DM, Smith G, Button J, Ramsey J, Archer R, Holt DW, Dargan PI. Purchasing Legal highs on the internet—is there consistency in what you get? Q J Med. 2010;103:489–493. doi: 10.1093/qjmed/hcq056. [DOI] [PubMed] [Google Scholar]
- 13.Welter J, Meyer MR, Wolf E, Weinmann W, Kavanagh P, Maurer HH. 2-Methiopropamine, a thiophene analogue of methamphetamine: studies on its metabolism and detectability in the rat and human using GC-MS and LC-(HR)-MS techniques. Anal Bioanal Chem. 2013;405:3125–3135. doi: 10.1007/s00216-013-6741-4. [DOI] [PubMed] [Google Scholar]
- 14.Bluelight: MXE/92 mg – Moderately experienced – The Quicksilver Aesthetic. Available from: http://www.bluelight.ru/vb/threads/620669-(MXE-92-mg)-Moderately-experienced-The-Quicksilver-Aesthetic (Last Accessed 18th March 2014)
- 15.http://globaldrugsurvey.com/run-my-survey/2012-global-drug-survey (Last Accessed 16thOct 2013)
- 16.Buy Research Chemicals UK: Methiopropamine. Available from: https://www.brc-finechemicals.com/methiopropamine.html (Last Accessed 18th March 2014)
- 17.Angelov D, O’Brien J, Kavanagh P. The syntheses of 1-(2-thienyl)-2-(methylamino) propane (methiopropamine) and its 3-thienyl isomer for use as reference standards Drug Test. Analysis. 2013;5:145–149. doi: 10.1002/dta.298. [DOI] [PubMed] [Google Scholar]
- 18.Erowid: Methiopropamine Effects. Available from: https://www.erowid.org/chemicals/methiopropamine/methiopropamine_effects.shtml (Last Accessed 18th March 2014)
- 19.Erowid: rectal administrations methiopropamine (MPA). Available from: http://www.erowid.org/experiences/exp.php?ID=99519 (Last Accessed 18th March 2014)
- 20.Erowid: A very sober stimulant – methiopropamine. Available from: http://www.erowid.org/experiences/exp.php?ID=93121 (Last Accessed 18th March 2014)
- 21.Erowid: Another addictive zinger – methiopropamine. Available from; http://www.erowid.org/experiences/exp.php?ID=89821 (Last Accessed 18th March 2014)
- 22.Bluelight: The methiopropamine N-methyl-1-(thiophen-2-yl)propan-2-amine (MPA) megathread V2. Available from: http://www.bluelight.ru/vb/threads/655417-The-Methiopropamine-N-methyl-1-(thiophen-2-yl)propan-2-amine-(MPA)-Megathread-V2?highlight=methiopropamine (Last Accessed 18th March 2014)
- 23.Erowid: Nothing to get excited aboue – methiopropamine. Available from: http://www.erowid.org/experiences/exp.php?ID=92297 (Last Accessed 18th March 2014)
- 24.Erowid: Great for staying awake – methiopropamine. Available from: https://www.erowid.org/experiences/exp.php?ID=92369 (Last Accessed 18th March 2014)
- 25.Erowid: Relief at last – methiopropamine. Available from: https://www.erowid.org/experiences/exp.php?ID=92416 (Last Accessed 18th March 2014)
- 26.Erowid: Sucks donkey shit – methiopropamine. Available from: https://www.erowid.org/experiences/exp.php?ID=93490 (Last Accessed 18th March 2014)
- 27.Erowid: Attentitive, focused, willing to work – methiopropamine. Available from: https://www.erowid.org/experiences/exp.php?ID=94912 (Last Accessed 18th March 2014)
- 28.Erowid: For study – methiopropamine. Available from: http://www.erowid.org/experiences/exp.php?ID=92674 (Last Accessed 18th March 2014)