Abstract
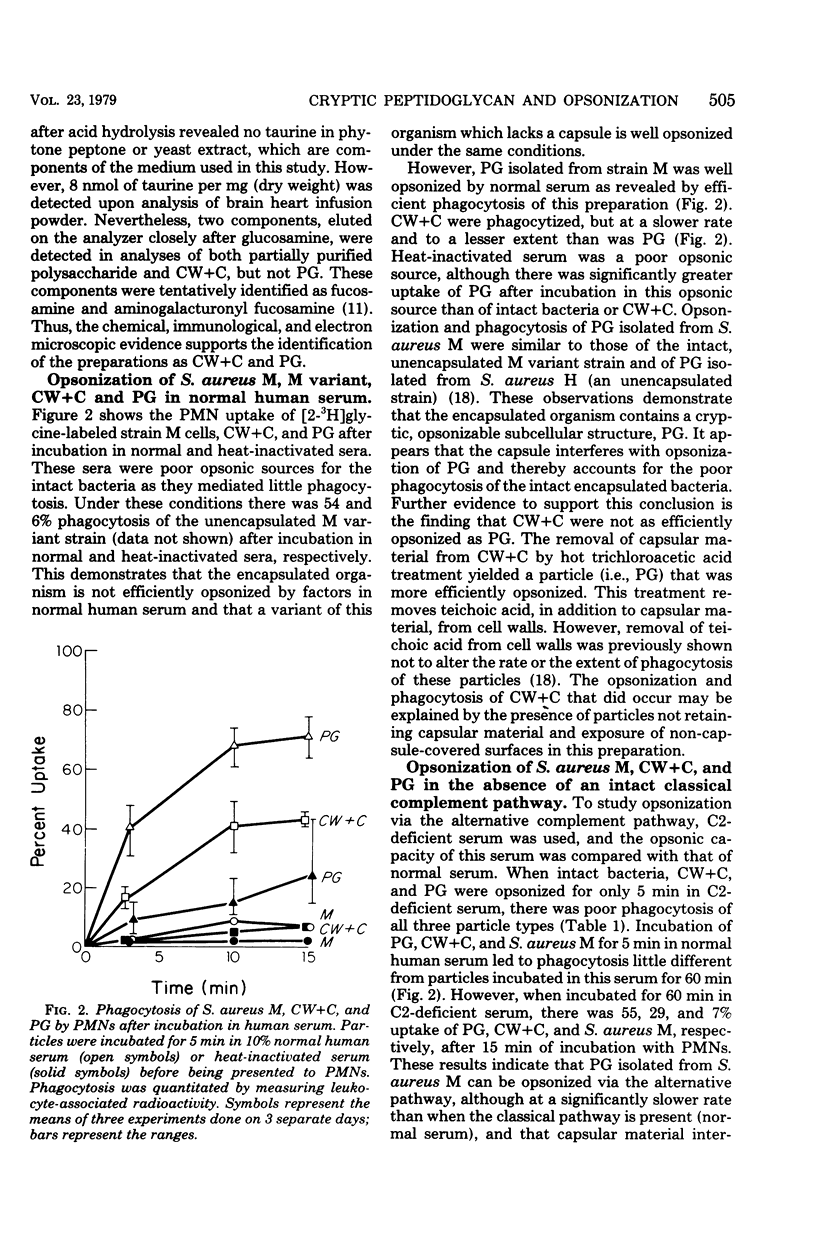
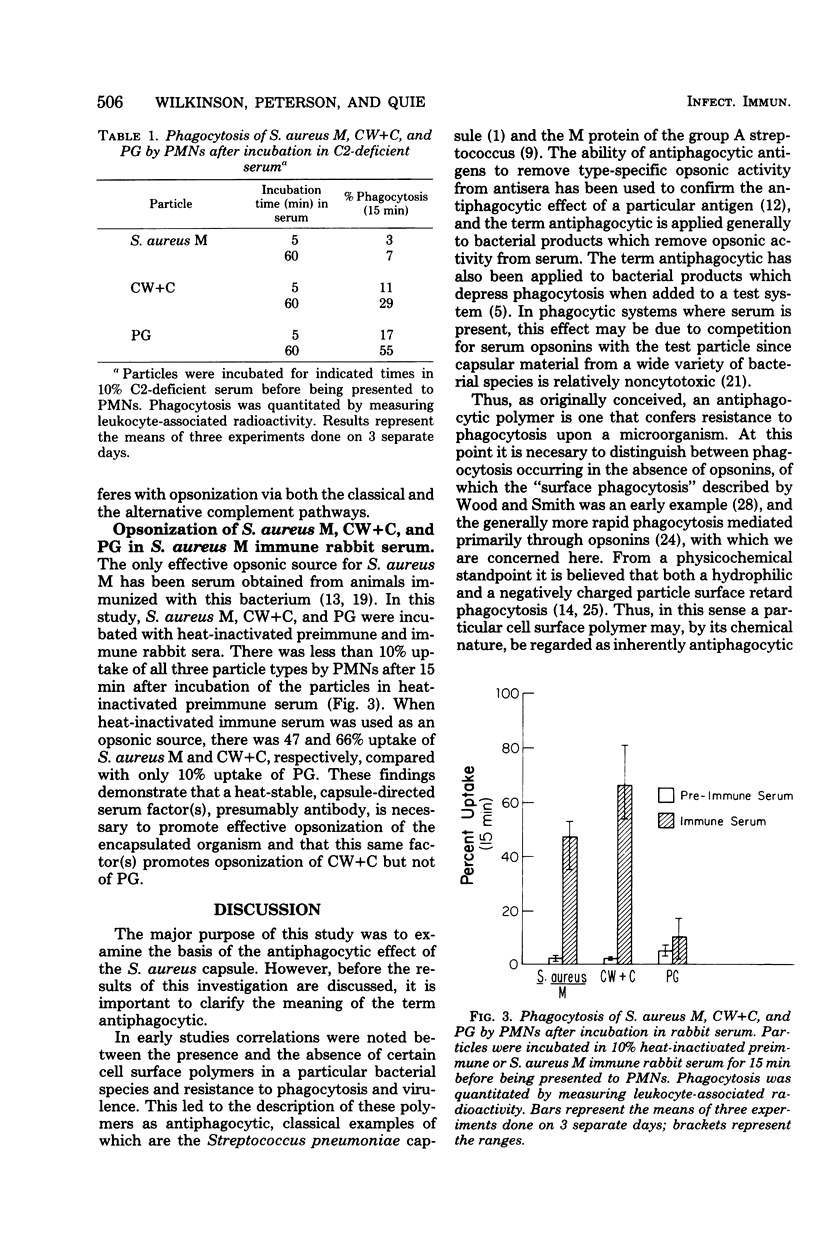
The antiphagocytic effect of the Staphylococcus aureus capsule is known to be related to its ability to interfere with opsonization by normal human serum. In this study, evidence is presented with isolated cell surface components which indicates that the capsule hinders opsonization by masking cell wall peptidoglycan. In contrast to intact, encapsulated S. aureus M cells, peptidoglycan particles isolated from the organism were efficiently opsonized by normal human serum and phagocytized by human polymorphonuclear leukocytes. Cell wall particles retaining capsular material were opsonized less efficiently than peptidoglycan. Studies comparing the opsonic capacities of normal, C2-deficient, and heat-inactivated sera led to the conclusion that both the classical and the alternative complement pathways contribute to the opsonization of peptidoglycan in normal human serum. It appears that the capsule interferes with opsonization via both of these complement pathways. Serum from rabbits immunized with S. aureus M had significant heat-stable opsonic activity for the intact organism and cell walls retaining capsular material, but not for peptidoglycan. A general model is proposed to explain how antiphagocytic cell surface polymers may inhibit bacterial opsonization and thereby impede natural immunity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- KEPPIE J., HARRIS-SMITH P. W., SMITH H. THE CHEMICAL BASIS OF THE VIRULENCE OF BACILLUS ANTHRACIS. IX. ITS AGGRESSINS AND THEIR MODE OF ACTION. Br J Exp Pathol. 1963 Aug;44:446–453. [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Braun D. G., Lackland H., Krause R. M. Immunochemical studies on the cross-reactivity between streptococcal and staphylococcal mucopeptide. J Exp Med. 1968 Aug 1;128(2):325–340. doi: 10.1084/jem.128.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Friend P. S., Dresner I. G., Yunis E. J., Michael A. F. Inherited deficiency of the second component of complement (C2) with membranoproliferative glomerulonephritis. Am J Med. 1977 May;62(5):765–771. doi: 10.1016/0002-9343(77)90881-6. [DOI] [PubMed] [Google Scholar]
- Krause R. M. Immunological activity of the peptidoglycan. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):136–150. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- LI I. W., MUDD S. THE HEAT-LABILE SERUM FACTOR ASSOCIATED WITH INTRACELLULAR KILLING OF STAPHYLOCOCCUS AUREUS. J Immunol. 1965 Jun;94:852–857. [PubMed] [Google Scholar]
- Liau D. F., Melly M. A., Hash J. H. Surface polysaccharide from Staphylococcus aureus M that contains taurine, D-aminogalacturonic acid, and D-fucosamine. J Bacteriol. 1974 Sep;119(3):913–922. doi: 10.1128/jb.119.3.913-922.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melly M. A., Duke L. J., Liau D. F., Hash J. H. Biological properties of the encapsulated Staphylococcus aureus M. Infect Immun. 1974 Aug;10(2):389–397. doi: 10.1128/iai.10.2.389-397.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagura H., Asai J., Katsumata Y., Kojima K. Role of electric surface charge of cell membrane in phagocytosis. Acta Pathol Jpn. 1973 May;23(2):279–290. doi: 10.1111/j.1440-1827.1973.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Nickerson D. S., Kazmierowski J. A., Dossett J. H., Williams R. C., Jr, Quie P. G. Studies of immune and normal opsonins during experimental staphylococcal infection in rabbits. J Immunol. 1969 May;102(5):1235–1241. [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Kim Y., Wilkinson B. J., Schmeling D., Michael A. F., Quie P. G. Dichotomy between opsonization and serum complement activation by encapsulated staphylococci. Infect Immun. 1978 Jun;20(3):770–775. doi: 10.1128/iai.20.3.770-775.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Selinger D. S., Reed W. P. Pneumococcal adherence to human epithelial cells. Infect Immun. 1979 Feb;23(2):545–548. doi: 10.1128/iai.23.2.545-548.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis (third of three parts). N Engl J Med. 1974 Apr 11;290(15):833–839. doi: 10.1056/NEJM197404112901506. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis: recognition and ingestion. Semin Hematol. 1975 Jan;12(1):83–116. [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. II. Contact angles and phagocytosis of encapsulated bacteria before and after opsonization by specific antiserum and complement. J Reticuloendothel Soc. 1972 Nov;12(5):497–502. [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Human polymorphonuclear leucocyte receptors for staphylococcal opsonins. Immunology. 1977 Aug;33(2):231–239. [PMC free article] [PubMed] [Google Scholar]



