Abstract
Medical marijuana remains a highly debated treatment regimen despite removal of state penalties against care providers prescribing the drug and patients treated with the drug in many areas of the USA. The utility of marijuana in specific medical conditions has been studied at length, but its effects on driving performance and risk of motor vehicle collision remain unclear. As with other medications that affect psychomotor function, the healthcare provider should be informed of the potential risks of driver safety prior to prescribing this psychotropic drug to give appropriate anticipatory guidance for appropriate use. The goal of this narrative review is to assess the current literature regarding marijuana as it relates to driving performance and traffic safety. With a foundation in the pharmacology of cannabinoids, we consider the limitations of testing cannabinoid and metabolite concentration. In addition, we will review studies on driving performance and epidemiological studies implicating marijuana in motor vehicle collisions. The increasing prevalence of medical marijuana laws in the USA suggests that clinicians should be aware of marijuana’s influence on public safety. Patients should abstain from driving for 8 h if they achieve a subjective “high” from self-treatment with smoked marijuana and should be aware of the cumulative effects of alcohol and other psychoactive xenobiotics.
Keywords: Marijuana, Traffic safety, Cannabinoids
Introduction
In 1999, the Institute of Medicine recommended that rigorous clinical trials be done to study the effectiveness of cannabis in treating chronic conditions [1, 2]. Since then, medical marijuana laws (MML) have been instituted in 20 states and Washington, D.C. (Table 1), and debate continues regarding the removal of state penalties for marijuana used for medical purposes. An important aspect of this debate is the association between drugs of abuse and traffic safety. Alcohol, opioids, and benzodiazepines have been irrefutably linked with motor vehicle collisions and mortality [3–5]. The consumption of cannabis has also been correlated with an increased risk of traffic accidents based on epidemiological studies [6]. In this article, we review the literature on medical marijuana and the impact medical marijuana laws have on traffic safety among the general population.
Table 1.
States that have enacted medical marijuana laws
| State | Year passed |
|---|---|
| Alaska | 1998 |
| Arizona | 2010 |
| California | 1996 |
| Colorado | 1996 |
| Connecticut | 2012 |
| Washington, DC | 2010 |
| Delaware | 2011 |
| Hawaii | 2000 |
| Illinois | 2013 |
| Maine | 1999 |
| Massachusetts | 2012 |
| Michigan | 2008 |
| Montana | 2004 |
| Nevada | 2000 |
| New Hampshire | 2013 |
| New Jersey | 2010 |
| New Mexico | 2007 |
| Oregon | 1998 |
| Rhode Island | 2006 |
| Vermont | 2004 |
| Washington | 1998 |
Adapted from Table 20 “Legal Medical Marijuana States and DC, Laws, Fees, and Possession Limits” in http://medicalmarijuana.procon.org/view.resource.php?resourceID=000881 (accessed 02/11/2014)
Methods
We conducted a literature search of MEDLINE and Google® Scholar. A combination of the following search terms were used: “marijuana”, “cannabis”, “pharmacology”, “toxicology”, “driving”, “traffic safety”, “psychomotor testing”, “driving simulation”, “medicinal marijuana”, and “per se drug limits”. Reference lists of retrieved articles were reviewed for additional studies not found by the above search method. Manuscripts were limited to English and German languages. No date range was set in the search engine, and articles were retrieved from January 1964 through May 2013. Articles were included in this review based on relevancy to the topics of cannabinoid pharmacokinetics, psychomotor effects of cannabinoid consumption, driving simulation and on-road driving performance under the influence of marijuana, and epidemiological studies with a focus on culpability in motor vehicle collisions. Articles that did not add to the information within these topic headings were excluded. The initial reservoir of articles was created by the first author using the above method to determine relevance to the subject and provided to the remaining authors, who then used a more focused search to clarify specific data.
Background
Marijuana has been used as an herbal remedy for thousands of years and is a common recreational drug in developed nations [7]. In the United States, marijuana has had a long and complicated history of state and federal regulation beginning with the Marihuana Tax Act of 1937 [8]. It was designated as a Schedule I substance according to the Controlled Substances Act of 1972. The principle requirements for placement as a Schedule I drug include a high potential for abuse, no widely accepted medical use, and a lack of acceptable safety for use of the drug. This scheduling of marijuana has been questioned, but marijuana has not been considered for rescheduling by the federal government.
Medical marijuana is used in the treatment of several chronic diseases, including refractory pain associated with cancer and chemotherapy, cachexia in end-stage AIDS, and severe muscle spasm in multiple sclerosis. The efficacy of inhaled and ingested cannabis for a variety of disease processes has been studied at length and will not be discussed here. Starting with California in 1996, many states have developed legislation to remove state punishments against prescribed medical marijuana for patients, healthcare providers, and caregivers [Table 1].
Opponents of medical marijuana laws argue that smoked marijuana is an addictive drug, with identifiable negative sequelae to the user. In its smoked form, marijuana may increase the risk of cancer due to tar content and other carcinogens [9, 10]. Smoked marijuana may also adversely affect pulmonary function and immune modulation, leading to increased risk of pulmonary infections [10]. Chronic use of cannabis may be associated with persistent cognitive delays [11]. Long-term cannabis use is thought to produce irreversible impairment in memory, attention, and the organization and integration of complex functions [12]. Importantly, these are all risks borne by the user.
State and federal governments have a duty to protect the entire population; driving under the influence of marijuana, as with any psychoactive drug, is an important consideration because it directly affects public safety. There is a large and growing amount of data regarding marijuana smoking and decreased performance on cognitive testing [13, 14]. Driver simulations and on-road driving assessments have shown variable degrees of impairment compared to neuropsychiatric or cognitive testing [15]. Epidemiologic studies consistently link marijuana with motor vehicle accidents, but causation is not entirely clear [16, 17]. Since medical marijuana has been available in some states for over a decade, there is an opportunity to research the influence of medical marijuana laws on traffic safety.
Marijuana Pharmacology, Pharmacokinetics, and Pathophysiology
Cannabinoids are the bioactive substances derived from the Cannabis sativa plant. Although there are numerous cannabinoids, most research has focused on cannabinol, cannabidiol, and tetrahydrocannabinol. Delta-9-tetrahydrocannabinol (also known as THC—the biologically active form of cannabis) was first isolated in 1964 by Gaoni et al [18]. Manuscripts describing the principle psychoactive cannabinoid receptors, named CB-1 and CB-2, were published in 1990 [19]. CB-1 receptors are G protein-linked, presynaptic receptors found primarily in the brain that inhibit the release of multiple neurotransmitters, including acetylcholine, GABA, dopamine, norepinephrine, and 5-hydroxytryptamine [20]. CB-2 receptors are also G protein-linked, but are found peripherally and are thought to be immune modulators. Positron emission tomography (PET) imaging has given investigators further clues for the site of action of smoked marijuana on brain function. O’Leary et al. demonstrated substantial reduction in blood flow to the temporal lobe when volunteers showed impaired performance of auditory attention tasks [21]. Marijuana smoking has also been shown to increase blood flow in the frontal lobes and lateral cerebellum at rest [22, 23].
The absorption, distribution, metabolism, and excretion of marijuana have been studied extensively. Understanding the pharmacology of marijuana will help to interpret epidemiologic studies reviewed later in this manuscript. Smoking marijuana causes an abrupt rise in THC serum concentrations, achieving peak concentrations within 3–10 min [24]. Peak serum concentrations are difficult to predict, however, for orally ingested marijuana. One study, using both hemp milk decoctions of 16.5 and 45.7 mg of THC and 20 mg dronabinol to study psychomotor performance, recorded peak serum THC concentrations occurring about 1 h after oral ingestion [25]. Delta-9-THC has a volume of distribution of 2.5–3.5 L/Kg, and is lipid soluble. Chronic use of marijuana leads to THC deposition in fatty tissue, with subsequent slow release as fatty tissue is turned over. This was first discovered in a rat model in which subcutaneous THC administration resulted in high concentrations of THC in body fat 2 weeks later [26].
The pharmacokinetics of smoked marijuana is highly variable among patients. The bioavailability of marijuana is 10–35 % when smoked in cigarette form and 5–20 % when ingested [24]. Pyrolysis of marijuana reduces the amount of cannabinoid available for absorption; the variable bioavailability of smoked marijuana cigarettes is thought to be related to individual smoking behavior including rate and depth of inhalation [27]. Vaporization of marijuana avoids the degradation of cannabinoids by pyrolysis and is an efficient vehicle for administration [28]. Dosing recommendations for medicinal marijuana, therefore, are highly variable. A commonly cited reference suggests that dosing is patient-specific, and should be titrated by the patient for desired results [29]. This individualized dosing strategy should be considered when interpreting studies on driving performance that use a fixed marijuana dose or those using ad libitum smoking with the goal of achieving a satisfactory “high.”
Cannabinoids are metabolized through hydroxylation and subsequent carboxylation. Delta-9-tetrahydrocannabinol is rapidly metabolized in the liver by the cytochrome P450 system [24]. The active metabolite of delta-9-THC, 11-hydroxy-delta-9-tetrahydrocannabinol (11-OH-THC), is further oxidized to 11-nor-9-carboxy-THC (THC-COOH), the most ubiquitous THC metabolite found in urine [30, 31]. Qualitative screens for this inactive metabolite may remain positive for weeks to months, depending on chronicity of marijuana use. Cannabinoid glucuronides, produced by phase II metabolism of THC metabolites, contribute to cannabinoid elimination and have also been used to identify marijuana exposure [32].
The euphoric effects of marijuana are well known to the general public, but the complete physiology of the psychoactive effects of marijuana is poorly understood. It is hypothesized that the inhibitory effect of the CB-1 receptor plays a crucial role [19]. Complex skills, such as driving a motor vehicle, are dependent on higher cognition and motor functions and may be influenced by marijuana.
Psychomotor Effects of THC
Psychomotor impairment is a major concern for any drug, prescribed or used illicitly. A review of over 200 studies on psychomotor performance testing found a high degree of variability among types of tests used and methods of performing these tests [33]. This review was described as “a methodological survey”, but unfortunately, there was no description of how these manuscripts were retrieved, and there were no inclusion or exclusion criteria expressed. Foltin et al. provided, however, an excellent source of psychomotor studies done between 1970 and 1991 [33]. Their compilation of results from 1,253 individual experiments on human performance in laboratory testing (driving simulation was excluded) found that stimulants (e.g., methylphenidate and amphetamine) generally did nothing or enhanced performance, while alcohol, benzodiazepines, and marijuana either did nothing or decreased performance on certain tasks [33]. There were no rigorous statistics performed on these results, likely due to the degree of variability among experiments. The aim of this paper was to demonstrate the variability in testing parameters when measuring psychomotor performance; this was not a meta-analysis of psychomotor testing to validate results across experiments. The authors concluded that future testing protocols should use placebo-control groups; uniformly train subjects prior to testing; test multiple drug doses; and test performance before, and several times after, drug administration [33]. In their review, these four criteria were met only 13 % of the time for studies on marijuana [33]. This serves as an excellent overview of psychomotor testing, and points out the limitations of these studies due to the lack of standardization.
Numerous studies have shown that acutely administered marijuana impairs cognition and affects psychomotor performance [13, 14]. Early studies focused mainly on the acute cognitive effects of smoking marijuana. In 1971, medical students with a history of recreational marijuana use were studied after smoking placebo, low-dose, and high-dose marijuana. The main conclusion was that marijuana adversely affected short-term memory [34]. A few years later, Borg et al. measured reaction time, temporal judgment, and word association after varied doses of smoked marijuana in five experienced adult marijuana smokers [35]. The subjects were sober from marijuana for at least 2 weeks prior to the start of the experiments. The researchers found significant dose-dependent impairment in all test scores. The simple reaction time test, in which the subject lifted a hand from one button to another button in response to a visual stimulus, demonstrated greater dose-response correlation than tests of temporal judgment or word association. A later study tested memory retrieval times after smoking a marijuana (10 mg THC content) or placebo cigarette [36]. The researchers tested the speed at which subjects differentiated letters displayed on a flashcard by same name (Aa), same case (AA), or different name (Ab). The goal of this study was to determine whether marijuana smoking affects the retrieval of long-term memory. The authors knew that, under normal conditions, reaction times were longer for determining that “A” and “a” have the same name than for determining that “A” and “A” is the same case. If marijuana smoking affected long-term memory retrieval, then the difference in reaction time for “same name” and “same case” should increase. The authors found that marijuana smoking lengthened reaction times for all tasks (p < 0.01 for all tests) and conclude that long-term memory retrieval was not impaired [36].
Marijuana administration has been reported to affect coordination and motor performance on a number of tasks. The psychomotor functions most commonly tested include body sway, hand steadiness, rotary pursuit, driving and flying simulation, divided attention, sustained attention, and the digit-symbol substitution test [1]. These metrics can be tested in a variety of ways. For example, a study by Liguori and colleagues combined psychomotor testing and driving simulation [37]. Ten marijuana users (seven men, three women) smoked one marijuana cigarette with a THC content of 0 (placebo), 1.77, and 3.75 % over 5 min. Two minutes later, they began a 60-min battery of tests including measurement of body sway and break latency in a driving simulator. Body sway was measured while subjects stood on a platform and stared at a pictured landscape. Movement of the landscape or platform itself caused body sway due to visual or proprioceptive cues, respectively. The high dose of marijuana significantly increased body sway, but the low-dose THC (1.77 % THC) did not. Driving simulation results did not correlate with body sway results, and will be discussed later in this review.
Many of the tasks affected by marijuana smoking seem to be related to diminished attention and increased reaction time. Kurzthaler et al. performed a study in which sixty healthy volunteers smoked a marijuana or placebo cigarette and then performed several physical and psychological tests 15 min and 24 h later [38]. One of the tests used is called the Efficiency Test System. Subjects reviewed a column of numbers and letters, and crossed out every eighth “0”, followed by every eighth “1” and so on until time ran out. They concluded that perceptual motor speed and accuracy were decreased after smoking marijuana compared to placebo (p = 0.012) [38]. The authors were unable to find statistically significant differences in verbal or visual memory. A more recent study of memory and attention in cannabis intoxication used auditory stimuli of varied tone and position, and asked subjects to respond to only one type of stimulus by pushing a button [12]. They found that the more difficult the discrimination task, the poorer the performance of intoxicated subjects compared with controls [12]. A meta-analysis of 60 studies confirmed that acute marijuana intoxication impairs driving-related functions such as visual tracking and reaction times [39]. Such cognitive and motor impairments have been extrapolated to activities such as driving a vehicle or operating potentially dangerous equipment.
Similar findings on psychomotor dysfunction have been shown with oral cannabinoids [25]. In a controlled clinical study, Menetrey and colleagues demonstrated decreased performance on tracking tasks while subjects were under the influence of medium or high-dose oral THC (hemp milk containing 16.5 or 45.7 mg THC) or 20 mg dronabinol. “Roadsign testing” was described as visual matching of a road sign with its identical counterpart, as a measure of visual processing and short-term memory, and revealed statistically significant differences between placebo and marijuana (p < 0.0001) [25]. Of note, most of the participants reported a significant feeling of intoxication after drinking the strongest concoction of hemp milk. Subjects tended to be very aware of their impairment. After marijuana exposure the participants refused to drive when asked to accomplish several tasks (e.g., driving a friend to a party) [25].
Drug Testing
Appropriate cannabinoid measurement is important in studying dose-related effects of marijuana. From a legal standpoint, understanding the limitations of cannabinoid measurement as it relates to driver impairment is crucial to determining the value of per se drug limits. Marijuana metabolites commonly are found in urine drug screens of injured drivers, which suggest that the demographic of marijuana users is at higher risk of being involved in motor vehicle accidents. There is strong evidence that at least part of that increased risk is associated with young males, who are more prone to risk-taking behaviors. Researchers have found correlations between acute marijuana intoxication and blood THC concentrations, to varying degrees of success. This has led many researchers to weigh in on their suggestion for a per se cutoff value of THC blood concentrations for driving under the influence of cannabis cases. One review on the impairment of driving-related skills by alcohol or cannabis suggested a serum THC concentration of 7–10 ng/ml is equivalent to a blood alcohol concentration of 0.05 g/dL, the legal cutoff for many European countries [40]. While this concentration may seem reasonable as a “legal limit”, the pharmacokinetics of THC would suggest any serum concentration of THC could be considered indicative of intoxication [6]. Tetrahydrocannabinol concentrations are only measurable within the first 2 h of smoking marijuana, while the psychomotor effects may last 8 h or more. An undetectable THC concentration does not rule out driver impairment due to marijuana consumption. Research by Schwope and others on glucuronidated cannabinoids shows promise with regards to determining temporal relationships with smoking marijuana [32]. Further work is needed to validate these detection methods to prove driver intoxication. Field sobriety testing will likely remain an important aspect in evaluating driver fitness.
As our knowledge of cannabinoid pharmacokinetics expands, our methods to predict impairment and determine temporal relationships with cannabinoid concentrations have advanced. Moeller et al. first described a method for measuring serum THC and serum THC-COOH using gas chromatography/mass spectrometry in 1992 [41]. The researchers recorded serial measurements of serum THC and serum THC-COOH in 24 subjects after smoking marijuana cigarettes containing 300 μg/Kg of THC. They found serum THC peaked within 40 min, at which time serum THC-COOH had already formed and begun to accumulate. The rapid decline in THC compared to THC-COOH may be due to the increased lipophilicity of THC, which distributes throughout the body, while THC-COOH, a more polar and hydrophilic molecule, remains primarily in plasma. Huestis et al. were the first researchers to report the complete pharmacokinetic profile of smoked marijuana [42]. They measured THC, 11-OH-THC, and THC-COOH blood concentrations after subjects smoked a fixed amount of marijuana. Similar concentration curves were recorded in this study.
Investigators have tried to develop a THC limit for impairment by measuring performance skills related to driving. Ramaekers et al. measured performance in subjects after they were administered single doses of 0, 250 and 500 μg/kg THC by smoking [43]. Performance tests included measures of perceptual motor control, motor impulsivity, and cognitive function. Blood and oral fluid were collected throughout testing. A linear relationship with task performance impairment as a function of serum THC concentrations was demonstrated. They determined that 2–5 ng/mL serum THC could be a potential standard for measured impairment. Unfortunately, the rapid rise and decline in serum THC make it difficult to use as a marker of intoxication in the setting of real world driving. Serum must be drawn within 2 h of smoking marijuana to have a generally quantifiable concentration of THC; the psychomotor effects of marijuana, however, may exceed this time frame [43, 44]. Whereas an undetectable blood alcohol concentration would effectively rule out driving under the influence of alcohol (barring the effects from a hangover), an undetectable THC concentration does not similarly rule out impairment. Ramaekers et al. measured decreased performance compared to placebo up to 6 h after smoking marijuana [43]. In addition, the qualitative measurement of THC-COOH metabolite in urine does not correlate with either time of ingestion or active intoxication. This dilemma has led investigators to pursue alternative testing to determine marijuana intoxication and predict time of ingestion.
Several other methods of cannabinoid detection have been studied to help determine impairment. In the previously described Menetrey study, whole blood was collected at regular intervals to measure concentrations of THC, 11-OH-THC, and THC-COOH. They found the sum of THC and 11-OH-THC blood concentrations correlated with impairment more closely than THC concentration alone [25].
In an effort to correlate the use of marijuana with driving performance, Daldrup et al (1998) analyzed blood samples of drivers who had been arrested for erratic driving. The “Cannabis Influence Factor” (CIF) was calculated as the sum of active metabolites (THC and 11-OH-THC) divided by the concentration of inactive metabolites (THC-COOH). Coefficients were used to produce a line of best fit, which was in turn compared with police officers’ reports of driving errors. Symptoms of the acute cannabis effect such as lethargic or apathetic behavior, delayed understanding, tiredness, and mydriasis, but also swerving were found more frequently with increasing CIF [45].
Manno et al. measured the serum concentrations of THC and metabolites in subjects who had smoked marijuana, and found that THC metabolism to 11-OH-THC was rapid, which also explains the rapid decline of THC in serum. In fact, serum THC dropped to 4.2 ng/mL by 1 h post-inhalation for the high-dose marijuana group. In contrast, serum THC-COOH concentrations did not peak until 2 h post-inhalation, and remained elevated for the 8 h that the subjects were monitored. The authors then developed models to predict time of marijuana exposure. Model 1 used serum THC concentration, and Model 2 used the ratio of serum THC-COOH concentration to serum THC concentration. Both models were measured for accuracy using 95 % confidence intervals. As time from ingestion increases, the accuracy of these models decreases and after 8 h, these models become inaccurate. In their study, Manno et al. also found that urine THC concentration fell below 2 ng/mL at 5 h after inhaled marijuana, suggesting that urine THC, rather than urine THC-COOH, may be used to detect recent marijuana use.
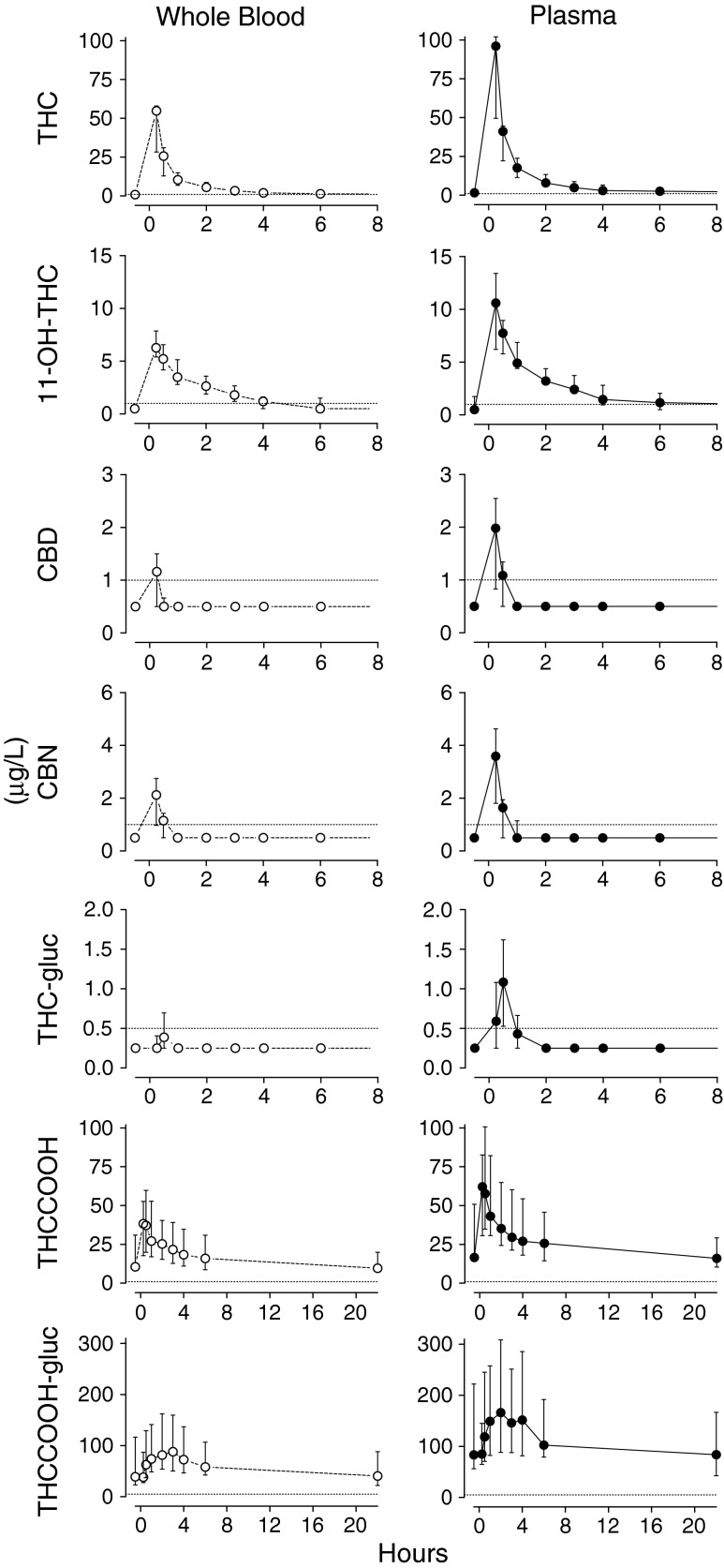
Measuring serum concentrations of THC and THC-COOH glucuronides may also prove useful in differentiating acute versus chronic smoking. Schwope et al. measured serum and whole blood concentrations of THC, THC-glucuronide, THC-COOH, THC-COOH-glucuronide, 11-OH-THC, cannabinol, and cannabidiol in ten subjects after smoking a marijuana cigarette ad libitum [32]. Using liquid chromatography and tandem mass spectrometry, the researchers quantified serum and whole blood concentrations of these cannabinoids and metabolites over 8 h (Fig. 1). They found that THC-COOH-glucuronide might act as an indicator of chronic marijuana use. The ratio of glucuronide/free THC-COOH may help predict acute or chronic marijuana use. These studies did not correlate concentration with degree of impairment (Fig 1).
Fig. 1.
Cannabinoid concentrations after smoking marijuana. Taken with permission from Schwope et al. [32]
Driver Simulation Studies and Actual Driving Performance
Studies measuring the psychomotor effects of marijuana have generally concluded that THC has a negative impact on coordination, visual function, and attention. Driving simulation studies, however, have had mixed results. Yesavage et al. showed that experienced airplane pilots had impaired flight simulator test performance even 24 h after use of marijuana [46]. A later study that included a control group failed to replicate this result, but did show evidence of impairment between 1–4 h after marijuana smoking [47]. Interestingly, drivers who use marijuana recreationally drive slower, have increased following distances, and overestimate their degree of intoxication; conversely, alcohol-intoxicated vehicle operators drive faster, have shorter following distances, and underestimate their degree of intoxication [15].
A major pitfall in determining fitness to drive while intoxicated is relying solely upon psychomotor testing. Experimental laboratory studies measure skills related to driving, but do not necessarily reflect performance in the real world [48]. Critical tracking tasks, stop signal tasks, and assessments of executive function such as “Tower of London” tests cannot fully reproduce the complexity of our experience on the road. Driving simulators, closed circuit driving courses, and on-road highway and urban driving assessments provide a much more comprehensive analysis of functional impairment due to marijuana use. An example of discrepancies between psychomotor testing and driving simulation results is the study by Liguori et al described earlier [37]. Subjects smoked varied concentrations of marijuana cigarettes and immediately began performance testing. The authors found a significant increase in body sway with the high concentration THC group. The driving simulator portion of the test measured the time it took to remove the foot from the accelerator and place it on the brake when a barrier is placed in front of the car. The authors reported the use of high-dose THC cigarettes marginally increased brake latency by a mean of 55 ms, but there was no statistically significant difference with controls (p < 0.10). The authors conclude by stating the brake latency measurement in high-dose THC smoking in this study was similar to break latency measured in subjects with a breath alcohol content of 0.05 mg/dL in a prior study. This conclusion is misleading somewhat, since the brake latency measurements in the marijuana study did not achieve significant difference with placebo. The study also points out that body sway did not predict driving simulator performance. It is important to remember, though, that driver simulation studies, like psychomotor testing, are not without bias. Test subjects are aware that they are being tested or observed, and may be more aware of impairments than in real life settings [49].
Common methods of assessing driver impairment in simulation and on-road study environments include standard deviation of lateral position (SDLP), time driven out of lane (TOL), reaction time (RT), break latency, and standard deviation of headway (SDH). A commonly cited report from the National Highway Traffic Safety Administration by Robbe and O’Hanlon concluded that smoking a marijuana cigarette with a specific THC dose does not seriously affect driving performance as measured by SDLP and SDH [15]. First, they allowed subjects to continuously smoke marijuana until achieving a subjective desired effect or “high” and determined the preferred dose of these chronic marijuana users to be approximately 300 μg/Kg. Next, the investigators instructed a different set of subjects to smoke one cigarette of marijuana placebo or marijuana at THC concentrations of 100, 200, and 300 μg/kg over a 10-min period. Closed highway driver testing began 40 min after beginning the smoking of marijuana cigarette.
A prior experiment of similar design had been performed on alcohol consumption and driver performance. Blood alcohol content (BAC) correlated very closely with measured SDLP; the correlation was so consistent that researchers were able to derive an empirical equation to convert SDLP measurements to blood alcohol equivalencies. The investigators used BAC equivalencies to report their findings in regard to THC dosing and driver performance. When applied to THC’s effects in this study, the maximum mean change in lateral position on the road after 300 μg /kg did not exceed the equivalent BAC of 0.08 g/dL. SDLP variation was dose-dependent; BAC equivalents were between 0.04 g/dL and 0.07 g/dL for the three THC doses. Robbe et al. compared these results to a prior study on chronic benzodiazepine use in patients on a closed driving circuit, and determined THC’s effects on SDLP were markedly less than benzodiazepines [50]. They conclude that, “THC taken alone in doses preferred by its users does not seriously affect driving performance” [15]. Despite these conclusions, deviation of lateral position increased after smoking marijuana in a dose-related manner and mean speed was somewhat reduced following the higher THC doses. All THC doses increased mean following distance. This comprehensive report also included a city driving study that measured the drivers’ ability to operate a vehicle in urban traffic. Unlike the highway portion of the study, this city portion was restricted to100 μg/kg of THC and was compared to a group of drivers with a BAC of 0.04 g/dL. The modest dose of alcohol produced a significant impairment in driving performance, relative to placebo; THC did not. This portion of the study elegantly illustrated the key differences between alcohol and marijuana intoxicated drivers: alcohol impaired driving performance, but drivers did not perceive it—marijuana did not impair driving performance, but subjects thought it had. Marijuana related impairment was disproportionately higher in psychomotor testing than in real world performance.
Eight years later, the same group of researchers studied the driver impairment effects of marijuana and alcohol separately, and in combination, during normal traffic [51]. Subjects were given THC doses of 0, 100, and 200 μg/kg, with and without an alcohol dose sufficient for achieving blood alcohol concentrations (BAC) of 0.04 g/dL. Main outcome measures were standard deviation of lateral position (SDLP), time driven out of lane (TOL), reaction time (RT), and standard deviation of headway (SDH). Contrary to their prior results, performance impairment was minor after alcohol and moderate after both THC doses. Combining THC with alcohol dramatically impaired driving performance. A BAC of 0.04 g/dL combined with a THC dose of 100 and 200 μg/kg produced a rise in SDLP equivalent to that associated with a blood alcohol concentration of 0.09 g/dL and 0.14 g/dL, respectively. More recently, Downey et al. found that driving simulator performance was more impaired in drivers exposed to both THC and alcohol [52]. Interestingly, they found that regular cannabis users displayed more driving errors than non-regular cannabis users, which is contrary to prior evidence that chronic users have better compensatory mechanisms while driving [53, 54].
Results from a driving simulator study confirmed results from prior investigations that lapses in attention due to marijuana intoxication can be compensated for by decreasing speed [55]. Regardless of marijuana dose, there was an overall reduction in average speed on simulated driving. In these same simulator trials, participants reacted more slowly to a pulling-out event when they had taken the low dose of cannabis, suggesting a similar compensatory action for the effects of cannabis impairment.
Epidemiological and Culpability Studies
Epidemiologic analyses of traffic accidents in the setting of marijuana exposure demonstrate mixed results. One reason for these inconsistencies may be related to the demographic being studied; individuals with increased risk-taking behavior are more likely to be involved in traffic accidents and engage in recreational drug use. In addition, the pharmacokinetics of THC plays a crucial role in evaluating the literature regarding marijuana and traffic safety. Drug testing cannot be used yet to prove impairment. Lastly, other factors related to driver performance must be considered, such as sleep deprivation and co-ingestants.
The pharmacology of THC brings several challenges to epidemiologic studies of marijuana and traffic safety. The serum THC concentration peaks (at 10–20 min) before the psychomotor effects of marijuana peak (at 30 min). In addition, the psychomotor effects of marijuana can persist long after serum concentrations have fallen. Lastly, the elimination half-life is highly variable between chronic users (approximate 5 days) and infrequent users (approximately 1–3 days) [56]. Therefore, positive urine drug screens do not necessarily imply impairment. Epidemiologic studies can fall victim to this basic flaw. One study used urine THC-COOH to “confirm” driving under the influence of marijuana, leading to the subsequent arrest of drivers who tested positive for this metabolite in the urine [57].
The population at highest risk of fatal motor vehicle collision also happens to be the population with greatest use of recreational marijuana. A telephone survey conducted in late 2003 and early 2004 by The Canadian Addiction Survey (CAS) reported that those who drive after using cannabis and those who drive after drinking were predominantly male (>75 %) and more than half had never been married [58]. The driving under the influence of cannabis group was less likely than the drinking-driving group to drive daily (68.5 and 92.6 %, respectively). Those who drove after using cannabis were also an average of 11 years younger than those who drove after drinking (mean age 28.7 and 39.8 years, respectively). These demographics have been confirmed in several other studies, including a 10-year case-control study in Sweden, in which suspected drivers under the influence of drugs had positive urine screens for THC confirmed by measurable blood THC concentrations. Of those cases confirmed by blood THC concentrations, 94 % were male [59].
Epidemiologic studies on traffic safety must consider the population being studied. Asbridge et al. performed a meta-analysis of nine studies that measured recent cannabis use in drivers by analysis of whole blood or by self-report. The selected studies confirmed recent use by the presence of THC or 11-OH-THC in blood. The investigators determined that driving under the influence of cannabis was associated with a significantly increased risk of motor vehicle collisions compared with unimpaired driving. While this was a large meta-analysis with >49,000 subjects from nine studies, the authors failed to control for age and sex of the subjects. As discussed earlier, young males tend to be risk takers, leading to activities such as reckless driving and illicit drug use. Another confounding variable not accounted for is the time of day of occurrences, as the time most accidents occur is between midnight and 3 a.m. [60]. It has been shown that combining low-dose alcohol with moderate sleep restriction results in significant decrements to subjective alertness and performance [61]. The same may be true for marijuana use as well.
Case-control studies of alcohol intoxication-related motor vehicle accidents have far clearer evidence to suggest a causal relationship than cannabis alone. A study reviewing 789 young male drivers killed in motor vehicle crashes in four counties in California between 1982 and 1983 found that alcohol was by far the most frequently found, and crash responsibility analysis provided evidence of causation. There was no evidence in this study to suggest marijuana contributed to the crashes [62]. Another study several years later determined there was no indication that cannabis by itself causes fatal crashes [63]. Interestingly, the responsibility rate for drivers with alcohol and THC in combination was 95 %, and the normalized relative risk for the combination was higher than alcohol by itself in the intoxication range. Perhaps the lack of self-awareness of impairment seen with alcohol intoxication prevents the driver from compensating for the psychomotor dysfunction of marijuana use. This was again echoed in another case-control study of injured drivers, where 17 % of injured drivers had positive urine drug screens for THC-COOH and 14 % of injured drivers had elevated blood alcohol concentrations [64]. Tetrahydrocannabinol alone was not associated with crash responsibility. The pitfall with this study is that it did not take into account the pharmacology of THC. By incorrectly assuming that positive urine drug screens for THC-COOH correlated with acute THC intoxication, the authors potentially overestimated the number of THC-intoxicated people (acute and chronic marijuana users), and therefore underestimated any effect of acute THC intoxication.
Post-mortem drug concentrations may not correlate with impairment at the time of an accident. A case-control study conducted on 3,398 fatally injured drivers to assess the effect of alcohol and drug use on culpability found that drivers with THC in their blood were slightly more likely to be found culpable than drug-free drivers, after adjusting for age, gender, and type of crash. Higher odds ratios were seen with higher concentrations of THC. The highest culpability rates were among drivers under 25 and over 65 years of age [65]. In fact, the authors report greater odds ratios for THC than blood alcohol concentrations of 0.1–0.15 mg/dL, a finding that does not correlate with previous driving simulation studies. This deviation from previously accepted notions of driving under the influence of alcohol compared with marijuana may suggest that post-mortem toxicology reports are not validated to infer intoxication with marijuana.
Conclusion
Consider the distinction between medical marijuana and recreational marijuana when interpreting the current compendium of literature on marijuana and traffic safety. Although marijuana is gaining acceptance as an alternative medication, there are no studies to our knowledge that associate the medical use of marijuana with driving impairment. A common research design used in psychomotor and driving simulation studies is to allow patients smoke marijuana ad libitum to achieve a subjective “high”. Patients prescribed marijuana for medical purposes are commonly instructed to smoke pro re nata to achieve symptom relief, so dosing will be inherently varied based on a multitude of factors (disease severity, tolerance, smoking behavior, and others). Regardless of the dose, research subjects tend to be aware of their impairment, and even overestimate it. Similarly, patients smoking marijuana as needed likely maintain this same self-awareness. We recommend that patients achieving a subjective “high” while smoking marijuana for medical purposes should abstain from critical tasks, such as driving.
To make a recommendation regarding a safe time course for driving after marijuana use, we looked at studies that evaluate psychomotor performance after marijuana exposure. Many studies only evaluated subjects over 2–3 h, which may imply that the effects of marijuana only last this long [15]. Others have concluded that smoking marijuana affects psychomotor performance for at least 24 h post-consumption; however, the translation of their results to on-road driving performance remains unclear [46, 66]. Yesavage et al. measured impairment of pilots in flight simulation after smoking marijuana, and found some degree of impairment in specific flying parameters at 24 h [46]. Heishman and colleagues demonstrated impairments in arithmetic and recall tasks the day after smoking marijuana [66]. A contrary study evaluated subject performance 1 day after smoking and found no residual effects from marijuana [67]. None of these 24-hour studies measured driving performance directly. As described earlier, Ramaekers, Moehler, and colleagues demonstrated that psychomotor testing performance is decreased for up to 5–6 h after smoking marijuana [43]. Although they did not measure past 6 h, the majority of impairment occurred in the first 2 h after smoking, gradually tapering down at hour six. Other researchers have concluded that the majority of psychomotor impairment appears to extinguish within 3–6 h [68–70]. Based on our interpretation of the strength of these various studies and observations, we conservatively recommend that patients abstain from driving for a minimum of 8 h after achieving a subjective “high” from marijuana use.
With more states adding ballot measures to institute medical marijuana laws, per se driving limits have become an important focus of research. Unfortunately, although an elevated serum THC concentration may suggest a greater likelihood of psychomotor disturbance, undetectable serum concentrations do not rule out impairment. Ethanol, unlike marijuana, has highly predictable pharmacokinetics and blood alcohol concentrations correlate closely with psychomotor disturbances. The degree of ethanol-induced impairment has been used as a standard by which other drugs can be compared. A common theme among studies is that alcohol has the most detrimental effects on driver safety while marijuana alone may have less impact on drivers’ ability. The degree of impairment with marijuana and alcohol use together, however, is cumulative. Other psychoactive medications may have a similar synergistic effect on driver performance when combined with marijuana. Patients should be warned of the dangers of combined use of medical marijuana with alcohol or other impairing drugs. Roadside psychomotor testing by drug recognition experts in law enforcement will continue to play a crucial role in driver impairment cases until a consensus on cannabinoid testing can be reached.
References
- 1.Institute of Medicine (1999) Marijuana and medicine: assessing the science base. The National Academies Press [PubMed]
- 2.Watson SJ, Benson JA, Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000;57(6):547–552. doi: 10.1001/archpsyc.57.6.547. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara SD, Zancaner S, Giorgetti R. Low blood alcohol concentrations and driving impairment. A review of experimental studies and international legislation. Int J Legal Med. 1994;106(4):169–177. doi: 10.1007/BF01371332. [DOI] [PubMed] [Google Scholar]
- 4.Movig KL, Mathijssen MP, Nagel PH, et al. Psychoactive substance use and the risk of motor vehicle accidents. Accid Anal Prev. 2004;36(4):631–636. doi: 10.1016/S0001-4575(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 5.Strand MC, Fjeld B, Arnestad M, Morland J. Can patients receiving opioid maintenance therapy safely drive? A systematic review of epidemiological and experimental studies on driving ability with a focus on concomitant methadone or buprenorphine administration. Traffic Inj Prev. 2013;14(1):26–38. doi: 10.1080/15389588.2012.689451. [DOI] [PubMed] [Google Scholar]
- 6.Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deitch R. Hemp—American history revisited, vital resource to contentious weed. New York: Algora Publishing; 2007. pp. 7–10. [Google Scholar]
- 8.Musto DF. The marihuana tax act of 1937. Arch Gen Psychiatry. 1972;26(2):101–108. doi: 10.1001/archpsyc.1972.01750200005002. [DOI] [PubMed] [Google Scholar]
- 9.Aldington S, Harwood M, Cox B, et al. Cannabis use and risk of lung cancer: a case-control study. Eur Respir J Off J Eur Soc Clin Respir Physiol. 2008;31(2):280–286. doi: 10.1183/09031936.00065707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace / Fondazione clinica del lavoro, IRCCS [and] Istituto di clinica tisiologica e malattie apparato respiratorio. Univ Napoli Secondo Ateneo. 2005;63(2):93–100. doi: 10.4081/monaldi.2005.645. [DOI] [PubMed] [Google Scholar]
- 11.Meier MH, Caspi A, Ambler A et al (2012) Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America [DOI] [PMC free article] [PubMed]
- 12.Solowij N (2006) Cannabis and cognitive functioning. Cambridge [u.a.]: Cambridge Univ. Press
- 13.Hall W (1994) The health and psychological consequences of cannabis use. Australian Government Publicaiton Service
- 14.Hollister LE. Health aspects of cannabis: revisited. Int J Neuropsychopharmacol. 1998;1(1):71–80. doi: 10.1017/S1461145798001060. [DOI] [PubMed] [Google Scholar]
- 15.Robbe HWJ, O'Hanlon JF (1993) Marijuana and actual driving performance. U.S. Department of Transportation, National Highway Traffic Safety Administration
- 16.Gerberich SG, Sidney S, Braun BL, Tekawa IS, Tolan KK, Quesenberry CP. Marijuana use and injury events resulting in hospitalization. Ann Epidemiol. 2003;13(4):230–237. doi: 10.1016/S1047-2797(02)00411-8. [DOI] [PubMed] [Google Scholar]
- 17.Li MC, Brady JE, DiMaggio CJ, Lusardi AR, Tzong KY, Li G. Marijuana use and motor vehicle crashes. Epidemiol Rev. 2012;34(1):65–72. doi: 10.1093/epirev/mxr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86(8):1646–1647. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- 19.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 20.Iversen L. Cannabis and the brain. Brain J Neurol. 2003;126(Pt 6):1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- 21.O'Leary DS, Block RI, Flaum M, et al. Acute marijuana effects on rCBF and cognition: a PET study. Neuroreport. 2000;11(17):3835–3841. doi: 10.1097/00001756-200011270-00047. [DOI] [PubMed] [Google Scholar]
- 22.O'Leary DS, Block RI, Koeppel JA, et al. Effects of smoking marijuana on focal attention and brain blood flow. Hum Psychopharmacol. 2007;22(3):135–148. doi: 10.1002/hup.832. [DOI] [PubMed] [Google Scholar]
- 23.Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE. Regional cerebral blood flow after marijuana smoking. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1992;12(5):750–758. doi: 10.1038/jcbfm.1992.106. [DOI] [PubMed] [Google Scholar]
- 24.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 25.Menetrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoids levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Delta9-THC. J Anal Toxicol. 2005;29(5):327–338. doi: 10.1093/jat/29.5.327. [DOI] [PubMed] [Google Scholar]
- 26.Kreuz DS, Axelrod J. Delta-9-tetrahydrocannabinol: localization in body fat. Science. 1973;179(4071):391–393. doi: 10.1126/science.179.4071.391. [DOI] [PubMed] [Google Scholar]
- 27.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther. 2007;82(5):572–578. doi: 10.1038/sj.clpt.6100200. [DOI] [PubMed] [Google Scholar]
- 29.Carter GT, Weydt P, Kyashna-Tocha M, Abrams DI. Medicinal cannabis: rational guidelines for dosing. IDrugs Investig Drugs J. 2004;7(5):464–470. [PubMed] [Google Scholar]
- 30.Lemberger L, Crabtree RE, Rowe HM. 11-hydroxy-9-tetrahydrocannabinol: pharmacology, disposition, and metabolism of a major metabolite of marihuana in man. Science. 1972;177(4043):62. doi: 10.1126/science.177.4043.62. [DOI] [PubMed] [Google Scholar]
- 31.Wall ME, Perez-Reyes M. The metabolism of delta 9-tetrahydrocannabinol and related cannabinoids in man. J Clin Pharmacol. 1981;21(8 suppl):178S–189S. doi: 10.1002/j.1552-4604.1981.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 32.Schwope DM, Karschner EL, Gorelick DA, Huestis MA. Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem. 2011;57(10):1406–1414. doi: 10.1373/clinchem.2011.171777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foltin RW, Evans SM. Performance effects of drugs of abuse: a methodological survey. Hum Psychopharmacol Clin Exp Hum Psychopharmacol Clin Exp. 1993;8(1):9–19. doi: 10.1002/hup.470080104. [DOI] [Google Scholar]
- 34.Dornbush RL, Fink M, Freedman AM. Marijuana, memory, and perception. Am J Psychiatry. 1971;128(2):194–197. doi: 10.1176/ajp.128.2.194. [DOI] [PubMed] [Google Scholar]
- 35.Borg J, Gershon S, Alpert M. Dose effects of smoked marihuana on human cognitive and motor functions. Psychopharmacologia. 1975;42(3):211–218. doi: 10.1007/BF00421258. [DOI] [PubMed] [Google Scholar]
- 36.Block RI, Wittenborn JR. Marijuana effects on the speed of memory retrieval in the letter-matching task. Int J Addict. 1986;21(2):281–285. doi: 10.3109/10826088609063457. [DOI] [PubMed] [Google Scholar]
- 37.Liguori A, Gatto CP, Robinson JH. Effects of marijuana on equilibrium, psychomotor performance, and simulated driving. Behav Pharmacol. 1998;9(7):599–609. doi: 10.1097/00008877-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Kurzthaler I, Hummer M, Miller C, et al. Effect of cannabis use on cognitive functions and driving ability. J Clin Psychiatry. 1999;60(6):395–399. doi: 10.4088/JCP.v60n0609. [DOI] [PubMed] [Google Scholar]
- 39.Bergaus G, Guo B. Medicines and driver fitness—findings from a meta-analysis of experimental studies as basic information to patients, physicians and experts. Australia: Paper presented at: alcohol, Drugs, and Traffic Safety-T95: Proceedings of the 13th International Conference on Alcohol, Drugs and Traffic Safety; Adelaide; 1995. [Google Scholar]
- 40.Grotenhermen F, Leson G, Berghaus G, et al. Developing limits for driving under cannabis. Addiction. 2007;102(12):1910–1917. doi: 10.1111/j.1360-0443.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- 41.Moeller MR, Doerr G, Warth S. Simultaneous quantitation of delta-9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH) in serum by GC/MS using deuterated internal standards and its application to a smoking study and forensic cases. J Forensic Sci. 1992;37:969–983. [PubMed] [Google Scholar]
- 42.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16(5):276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 43.Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Delta9-THC concentration in serum and oral fluid: limits of impairment. Drug Alcohol Depend. 2006;85(2):114–122. doi: 10.1016/j.drugalcdep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Manno JE, Manno BR, Kemp PM, et al. Temporal indication of marijuana use can be estimated from plasma and urine concentrations of delta9-tetrahydrocannabinol, 11-hydroxy-delta9-tetrahydrocannabinol, and 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid. J Anal Toxicol. 2001;25(7):538–549. doi: 10.1093/jat/25.7.538. [DOI] [PubMed] [Google Scholar]
- 45.Daldrup T (1998) Cannabis im Strassenverkehr—final report to the Ministry of the State of North Rhine-Westphalia. 1998. http://www.uni-duesseldorf.de/daldrup/file/cannabisbericht.pdf. Accessed 2 Aug 2013
- 46.Yesavage JA, Leirer VO, Denari M, Hollister LE. Carry-over effects of marijuana intoxication on aircraft pilot performance: a preliminary report. Am J Psychiatry. 1985;142(11):1325–1329. doi: 10.1176/ajp.142.11.1325. [DOI] [PubMed] [Google Scholar]
- 47.Leirer VO, Yesavage JA, Morrow DG. Marijuana, aging, and task difficulty effects on pilot performance. Aviat Space Environ Med. 1989;60(12):1145–1152. [PubMed] [Google Scholar]
- 48.Ramaekers JG, Berghaus G, van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73(2):109–119. doi: 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict Am Acad Psychiat Alcohol Addict. 2009;18(3):185–193. doi: 10.1080/10550490902786934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Laar MW, Volkerts ER, van Willigenburg AP. Therapeutic effects and effects on actual driving performance of chronically administered buspirone and diazepam in anxious outpatients. J Clin Psychopharmacol. 1992;12(2):86–95. [PubMed] [Google Scholar]
- 51.Ramaekers JG, Robbe HW, O'Hanlon JF. Marijuana, alcohol and actual driving performance. Hum Psychopharmacol. 2000;15(7):551–558. doi: 10.1002/1099-1077(200010)15:7<551::AID-HUP236>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 52.Downey LA, King R, Papafotiou K, et al. The effects of cannabis and alcohol on simulated driving: influences of dose and experience. Accid Anal Prev. 2012;50:879–886. doi: 10.1016/j.aap.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 53.Marks DF, MacAvoy MG. Divided attention performance in cannabis users and non-users following alcohol and cannabis separately and in combination. Psychopharmacology (Berlin) 1989;99(3):397–401. doi: 10.1007/BF00445566. [DOI] [PubMed] [Google Scholar]
- 54.Sutton LR. The effects of alcohol, marihuana and their combination on driving ability. J Stud Alcohol. 1983;44(3):438–445. doi: 10.15288/jsa.1983.44.438. [DOI] [PubMed] [Google Scholar]
- 55.Sexton BF TR, Brook-Carter N, Jackson PG, Wright K, Stark MM, Englehart K. The influence of cannabis on driving. In: department of Environment Transport and the Regions, Road Safety Division, ed. Berkshire: Transport Research Laboratory, Crowthorne; 2000. p. p110. [Google Scholar]
- 56.Delgado J. Marijuana. In: Shannon MW, Borron SW, Burns MJ, editors. Haddad and Winchester’s clinical management of poisoning and drug overdose. 7. Philadelphia: Saunders Elsevier; 2007. pp. 747–754. [Google Scholar]
- 57.Brookoff D, Cook CS, Williams C, Mann CS. Testing reckless drivers for cocaine and marijuana. N Engl J Med. 1994;331(8):518–522. doi: 10.1056/NEJM199408253310807. [DOI] [PubMed] [Google Scholar]
- 58.Beirness DJ, Davis CG. Driving under the influence of cannabis: analysis drawn from the 2004 Canadian addiction survey. Ottawa: Canadian Centre on Substance Abuse; 2006. [Google Scholar]
- 59.Jones AW, Holmgren A, Kugelberg FC. Driving under the influence of cannabis: a 10-year study of age and gender differences in the concentrations of tetrahydrocannabinol in blood. Addiction. 2008;103(3):452–461. doi: 10.1111/j.1360-0443.2007.02091.x. [DOI] [PubMed] [Google Scholar]
- 60.National Highway Traffic Safety Administration. Traffic safety facts 2010. In: U.S. Department of Transportation, Washington, D.C. http://www-nrd.nhtsa.dot.gov/Pubs/811659.pdf. Accessed 2 Aug 2013
- 61.Vakulin A, Baulk SD, Catcheside PG, et al. Effects of moderate sleep deprivation and low-dose alcohol on driving simulator performance and perception in young men. Sleep. 2007;30(10):1327–1333. doi: 10.1093/sleep/30.10.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams AF, Peat MA, Crouch DJ, Wells JK, Finkle BS. Drugs in fatally injured young male drivers. Washington, D.C: Public health reports; 1985. [PMC free article] [PubMed] [Google Scholar]
- 63.Terhune KW, Ippolito CA, Hendricks DL, Michalovic YG, Bogema, SC, Santinga P, Blomber R, Preusser DF (1992) The incidence and role of drugs in fatally injured drivers. U.S. Department of Transportation National Highway Traffic Safety Administration, Washington, D.C
- 64.Lowenstein SR, Koziol-McLain J. Drugs and traffic crash responsibility: a study of injured motorists in Colorado. J Trauma. 2001;50(2):313–320. doi: 10.1097/00005373-200102000-00019. [DOI] [PubMed] [Google Scholar]
- 65.Drummer OH, Gerostamoulos J, Batziris H, et al. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36(2):239–248. doi: 10.1016/S0001-4575(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 66.Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav. 1990;37(3):561–565. doi: 10.1016/0091-3057(90)90028-G. [DOI] [PubMed] [Google Scholar]
- 67.Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology (Berlin) 1994;115:340–349. doi: 10.1007/BF02245075. [DOI] [PubMed] [Google Scholar]
- 68.Huestis MA. Cannabis (marijuana)—effects on human behavior and performance. For Sci Rev. 2002;14:15. [PubMed] [Google Scholar]
- 69.Hollister LE, Gillespie HK, Ohlsson A, Lindgren J-E, Wahlen A, Agurell S. Do plasma concentrations of delta-9-tetrahydrocannabinol reflect the degree of intoxication? Clin Pharm. 1981;21(S1):171S–177S. doi: 10.1002/j.1552-4604.1981.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 70.Lemberger L, Weiss JL, Watanabe AM, Galanter IM, Wyatt RJ, Cardon PV. Delta-9-Tetrahydrocannabinol temporal correlation of the psychologic effects and blood levels after various routes of administration. N Engl J Med. 1972;286(13):685–688. doi: 10.1056/NEJM197203302861303. [DOI] [PubMed] [Google Scholar]